Abstract
The essential part of electrochemical devices, such as fuel cells and batteries, is the polymer electrolyte with good mechanical, thermal, and chemical stability. The search for a new proton-conducting membrane with easy processability, non-toxic, and low-cost has been growing rapidly. The bio-based polymer electrolytes are now receiving much attention due to the green environment. Among the commercially available biopolymers, iota-Carrageenan (I-Carrageenan) is one of the biopolymer with good film-forming nature and with good mechanical stability. I-Carrageenan-based biopolymer membranes doped with ammonium bromide (NH4Br) have been prepared using solution-casting technique, and distilled water is used as a solvent. The prepared I-Carrageenan-based biopolymer membranes have been characterized using FTIR, XRD, and AC impedance techniques. The complexation between the polymer and salt has been revealed by FTIR. The increase in the amorphous nature of the film due to the addition of salt has been confirmed by XRD. From AC impedance technique, the conductivity of pure I-Carrageenan has been found to be 1.46 × 10−5 S/cm. The addition of different wt% of NH4Br increases the conductivity and reaches the highest value of 1.08 × 10−3 S/cm for 20% NH4Br, and the conductivity decreases on further addition of NH4Br due to the formation of ion aggregates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes are widely studied as they have many advantages over liquid electrolytes such as flexibility, compactness, light weight, leak proof, and good film-forming property [1, 2]. Polymer electrolytes based on natural polymers such as chitosan [3], starch [4], and cellulose derivatives [5] are being widely investigated because of their abundance in nature, low production cost, biodegradability, and non-toxic nature [6]. Proton conduction in solid polymer electrolytes is investigated by many researchers. In general, proton conduction through polymer electrolyte is described by two mechanisms [7]. One is the hopping of a lone proton from one site to another site (Grotthuss mechanism) and the other is by the transfer of proton which is attached to a vehicle such as H3O+ (Vehicle mechanism). In polymer electrolytes doped with ammonium salt, the main contribution to ionic conduction is the proton that comes from NH4 + ion [8–10].
Carrageenan is the name representing a natural polymer extracted from red algae. It consists of linear sulfated polysaccharides of d-galactose and 3,6-anhydro d-galactose. It is a water-soluble polymer extensively used in food industries as stabilizer, thickener, emulsifier, and gelling agent [11, 12]. It is also used in pharmaceutical and cosmetic industries [12]. A few works have been reported in literature to make polymer electrolytes using Carrageenan. Polymer blends prepared from poly (3,4-ethylenedioxythiophene) (PEDOT) and κ-Carrageenan was reported by C A Ng et al. as a polymer electrolyte in dye-sensitized solar cell [13]. They reported a low efficiency of the solar cell using the prepared electrolyte in converting solar energy to electrical current. A conductivity value of 3.25 × 10−4 S/cm was reported for a polymer electrolyte based on kappa-Carrageenan and cellulose derivatives [14]. N. E. A. Shuhaimi reported a polymer-salt complex made from chitosan, κ-Carrageenan, and ammonium nitrate and achieved a conductivity value of 2.39 × 10−4 S/cm [15]. A polyelectrolyte complex based on carboxymethyl-kappa-Carrageenan was reported by Sonia Lefnaoui et al. for drug-delivery application [16]. In this present work, a polymer-salt complex based on iota-Carrageenan is prepared. Ammonium bromide is added as a dopant. Structural and conductivity studies are reported.
Experiment
Membranes of I-Carrageenan with different mol wt% of ammonium bromide (NH4Br) were prepared by solution-casting method. The amount of I-Carrageenan is fixed (1 g) for all films. Different mole weight percentages (0, 10, 20, and 30) of NH4Br were added to I-Carrageenan. Distilled water was used as a solvent. Each composition was thoroughly mixed using a magnetic stirrer, and the resulting homogenous solutions were transferred to petri disks. They were kept at room temperature to evaporate solvent, and dry films were obtained and used for performing different studies. X- ray diffraction (XRD) patterns of all the membranes were recorded at room temperature using X-ray powder diffractometer with Cu kα radiation (λ = 1.5405 Å) in a wide 2θ (Bragg angle) range (10 ≤ 2θ ≤ 90). FTIR (Fourier transform infrared) spectroscopy was performed at room temperature using Bruker spectrophotometer in the wave number range of 400–4000 cm−1. AC impedance spectroscopy was done with the help of HIOKI 3532 LCR meter in the frequency range from 42 to 1 MHz.
Results and discussion
XRD study
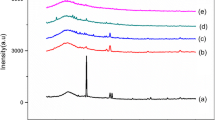
Figure 1 shows the XRD patterns of polymer (i-Carrageenan) membrane doped with different amount of NH4Br. Sharp diffraction peaks present in the undoped Carrageenan membrane at 31.70° and 45.66° may be due to the presence of inorganic impurities [17, 18]. Sharp peaks disappeared in XRD patterns of all doped films. A broad peak around 23° in all films shows the amorphous nature of the prepared films. Increase in the broadness of the peak with doping represents an increase in the amorphous nature. The appearance of small peaks in films of 20 and 30 mol % doping of NH4Br may be an indication of aggregate formation. Archana Sharma et al. also observed many crystalline peaks for the pure Carrageenan sample and a strong reduction in the peak height was seen after the addition of gelatin to prepare crosslinked cryogel [19]. Disappearance of peaks indicates that the interaction between the salt and polymer leads to a reorganization of the polymer structure and the polymer-salt complex becomes more amorphous [20, 21].
FTIR study
Figure 2a, b shows the FTIR spectra of undoped i-Carrageenan and doped polymer membranes in the range from 500 to 4000 cm−1. Frequencies of absorption peaks and their assignments are listed in Table 1. Interaction between the polymer and the ammonium salt is identified by the shift in the vibrational frequency for the doped polymer. The vibrational peak of O–H group centered at 3380 cm−1 for the undoped film is moved to 3387, 3386, and 3387 cm−1 for the film doped with 10, 20, and 30% NH4Br, respectively. There is no shift for the peak corresponding to O=S=O stretching. But the peak at 706 cm−1 corresponding to the sulfate on C-4 of galactose in the undoped membrane is moved to 718 cm−1 for all doped membranes, and the vibrational frequency of 803 cm−1 corresponding to C–O–SO3 on C2 of 3,6-anhydrogalactose has shifted to 801 cm−1 for all doped membranes. There is no shift in frequency found for C–O–C of 3,6-anhydro-o-galactose. The shift in wavenumber observed in the FTIR spectra of the doped membranes confirms the possible interaction of ammonium ion with the hydroxyl and sulfate group and the formation of polymer-salt complex [22].
Conductivity study
Figure 3 shows the Cole-Cole plot for all membranes recorded at room temperature. All the curve show a tilted spike which represents an equivalent circuit consisting of a resistor connected in series with a constant phase element [31]. The value of bulk resistance is extracted with the help of and thus the conductivity value is calculated by EQ software developed by Boukamp [32]. The absence of semicircle indicates that the ionic conductivity is mainly due to mobile ions. From the value of bulk resistance (Rb) extracted from EQ software, ionic conductivity has been calculated using the following equation.
l is the thickness of the membrane in centimeter and A is the contact area in square centimeter.
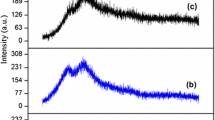
Conductivity of the pure I-Carrageenan membrane was calculated as 1.46 × 10−5 S/cm. Conductivity increases by the addition of ammonium bromide and reached a maximum value of 1.08 × 10−3 S/cm for the membrane doped with 20 mol wt% of NH4Br. Conductivity value decreases on further addition of ammonium salt. This may be due to the aggregate formation which is blocking the ion motion. Conduction spectra for the highest conducting membrane at different temperature are shown in Fig. 4. Conduction spectra show two distinct regions, a dispersion region at low frequencies and a frequency independent region at high frequencies. Dispersion effect is due to the ion blockage at the electrode-electrolyte interface. Plateau region corresponds to the bulk conductivity. DC conductivity values which are obtained by extrapolating the plateau region to zero frequency are in agreement with those obtained from the equivalent circuit model. Temperature-dependent conductivity was recorded for the film having the maximum conductivity at room temperature and is shown in Fig. 5. It shows a linear relation between ln σ and 1/T and obeys Arrhenius relation which is given by
σ 0 is the temperature independent constant, K is the Boltzmann constant, Ea is the activation energy, and T is the absolute temperature. Activation energy is calculated and is the lowest (0.1874 eV) for the Carrageenan membrane doped with 20% NH4Br.
The contribution to the total ionic conductivity as a result of charge transport comes from both anion and cation. Introducing large and heavy anion can improve the cationic conductivity which is important to consider in proton conductors. In our previous study on PAN + NH4Br polymer electrolyte system, it was shown that mobility and diffusion coefficient of cation are much higher than that of anion and increase with the conductivity [33]. Hence, the ionic conduction is predominantly by proton in this type of polymer electrolyte.
SEM study
Figure 6a, b shows the SEM image of the pure Carrageenan and the Carrageenan doped with 20 mol wt% of NH4Br, respectively. Doped film shows more roughness compared to the pure membrane. From SEM images, it is seen that the addition of NH4Br strongly affects the surface morphology of the membrane. Some pores and grain like structures are visible in the doped membrane. Appearance of pores may be due to the evaporation of the solvent [34]. Formation of grain like structures may be due to presence of added salt interacting with the polymer host [35].
Linear sweep voltammetry study
Electrochemical stability of the highest conducting polymer electrolyte is evaluated by linear sweep voltammetry (LSV) with two electrode system. A linearly varying potential, from 0 to 5 V at the scanning rate of 1 mV/s, is applied and the change in current is recorded. Stainless steel electrodes are used. One is used as a working electrode. Both reference and the counter electrode are connected together. The measurement was done at room temperature. The electrolyte is stable up to 2.1 V as shown in Fig. 7. This result shows that the prepared electrolyte is suitable for the construction of a proton battery which is having the working voltage around 1 V. Increase in voltage beyond 2.1 V causes a current to begin to flow as a result of the decomposition of the polymer electrolyte.
Conclusion
Polymer electrolytes based on I-Carrageenan complexed with NH4Br have been prepared by solution-casting method. Broad peak observed in XRD pattern shows the amorphous nature of prepared membranes. FTIR study confirms the complex formation between the polymer and the added salt. The maximum conductivity of value 1.46 × 10−5 S/cm has been obtained for the film doped with 20 mol wt% of NH4Br. Arrhenius nature of ionic conductivity is observed. SEM analysis reveals the effect of the addition of salt on the surface morphology of the membrane. Electrochemical stability window of 2.1 V was measured for the membrane of the highest ionic conductivity.
References
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195:4554–4569
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paper like polymer super capacitors. Nano Lett 10:4025–4403
Tan W, Majid SR, Khiar ASA, Arof AK (2006) Ionic conductivity of chitosan membranes and application for electrochemical devices. Polym Adv Technol 17:523–527
Ahmad Khiar AS, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16:123–129
Selvakumar M, Krishna Bhat D (2008) LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110:594–602
Bai H, Li Y, Zhang H, Chen H, Wu W, Wang J, Liu J (2015) Anhydrous proton exchange membranes comprising of chitosan and phosphorylated graphene oxide for elevated temperature fuel cells. J Membr Sci 495:48–60
Alloina F, Iojoiu C (2010) Composite polymer electrolytes for electrochemical devices. In: Sequeira CAC, Santos DMF (eds) Polymer electrolytes fundamentals and applications. Woodhead Publishing Limited, Cambridge, pp. 129–175
Selvasekarapandian S, Hirankumar G, Kawamura J, Kuwata N, Hattori T (2005) 1H solid state NMR studies on the proton conducting polymer electrolytes. Mater Lett 59:2741–2745
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting κ-carrageenan-chitosan electrolytes. Funct Mater Lett 1:195–201
Srivastava N, Chandra S (2000) Studies on a new proton conducting polymer system poly(ethylene succinate) + NH4ClO4. Eur Polym J 36:421–433
Liang L, Ni R, Yang S, Mao S (2014) Carrageenan and its applications in drug delivery. Carbohydr Polym 103:1–11
Campo VL, Kawano DF, da Silva DB Jr, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym 77:167–180
Ng CA, Camacho DH (2015) Polymer electrolyte system based on carrageenan-poly(3,4- ethylenedioxythiophene) (PEDOT) composite for dye sensitized solar cell. IOP Conf Series: Materials Science and Engineering. doi:10.1088/1757-899X/79/1/012020
Rudhziah S, Rani MSA, Ahmad A, Mohamed NS, Kaddami H (2015) Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind Crop Prod 72:133–141
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting κ-carrageenan–chitosan electrolytes. Funct Mater Lett 1:195–201
Lefnaoui S, Moulai-Mostefa N (2014) Polyelectrolyte complex based on carboxymethyl-kappa-carrageenan and Eudragit RL 30D as prospective carriers for sustained drug delivery. Chem Eng Res Des 97:165–174
Prasad K, Kaneko Y, Kadokawa J (2009) Novel gelling systems of k-, i- and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol Biosci 9:376–382
Martins JT, Cerqueira MA, Bourbon AI, Pinheiro AC, Souza BWS, Vicente AA (2012) Synergistic effects between k-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll 29:280–289
Sharma A, Bhat S, Vishnoi T, Nayak V, Kumar A (2013) Three-dimensional supermacroporous carrageenan-gelatin cryogel matrix for tissue engineering applications. BioMed Res Int. doi:10.1155/2013/478279
Chen J, Peng T, Fan K, Xia J (2011) Iodine-free quasi solid-state dye-sensitized solar cells based on ionic liquid and alkali salt. J Mater Chem 21:16448–16452
Agarwala a S, Thummalakunta LNSA, Cook CA, Peh CKN, Wong ASW, Ke L, Ho GW (2011) Co-existence of LiI and KI in filler-free, quasi-solid-state electrolyte for efficient and stable dye-sensitized solar cell. J Power Sources 196:1651–1656
Frech R, Huang W (1995) Conformational changes in diethylene glycol dimethyl ether and poly(ethylene oxide) induced by lithium ion complexation. Macromolecules 28(4):1246–1251
PaGcalsu V, Popescu V, Popescu GL, Dudescu MC, Borodi G, Dinescu AM, Moldovan M (2013) Obtaining and characterizing alginate/k-carrageenan hydrogel cross-linked with adipic dihydrazide. Adv Mater Sci Eng 2013:1–12
Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll 23(7):1903–1909
Rochas C, Lahaye M, Yaphe W (1986) Sulfate content of carrageenan and agar determined by infrared spectroscopy. Bot Mar 29:335–340
Prado-Fernandez J, Rodriguez-Vazquez JA, Tojo E, Andrade (2003) JM quantitation of κ-, ι- and λ-carrageenans by mid-infrared spectroscopy and PLS regression. Anal Chim Acta 480:23–37
Chopin T, Whalen E (1993) A new and rapid method for carrageenan identification by FT IR diffuse reflectance spectroscopy directly on dried, ground algal material. Carbohydrate Research Res 246:51–59
Jumaah FN, Mobaraka NN, Ahmad A, Ramli N (2013) Characterization of Ų -carrageenan and its derivative based green polymer electrolytes. AIP Conference Proceedings 1571:768
Elsupikhe RF, Shameli K, Ahmad MB, Ibrahim NA, Zainudin N (2015) Green sonochemical synthesis of silver nanoparticles at varying concentrations of κ-carrageenan. Nanoscale Res Lett 10:302
Brychcy E, Malik M, Drożdżewski P, Król Ż, Jarmoluk A (2015) Physicochemical and antibacterial properties of carrageenan and gelatine hydrosols and hydrogels incorporated with acidic electrolyzed water. Polymers 7(12):2638–2649
Karthikeyan S, Sikkanthar S, Selvasekarapandian S, Arunkumar D, Nithya H, Kawamura J (2016) Structural, electrical and electrochemical properties of polyacrylonitrile-ammonium hexaflurophosphate polymer electrolyte system. J Polym Res 23:51
Boukamp BA (1986) A package for impedance/admittance analysis. Solid State Ionics 18–19:136–140
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Vinoth Pandi D, Nithya S, Sanjeeviraja C (2015) Electrical conductivity characterization of polyacrylonitrile-ammonium bromide polymer electrolyte system. J Solid State Electrochem 19:987–999
Wang Y-J, Pan Y, Chen L (2005) Ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with Li 1.3Al 0.3Ti 1.7(PO 4) 3 salt. Mater Chem Phys 92:354–360
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim Acta 55:1475–1482
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karthikeyan, S., Selvasekarapandian, S., Premalatha, M. et al. Proton-conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23, 2775–2780 (2017). https://doi.org/10.1007/s11581-016-1901-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1901-0