Abstract
To improve the cycle performance of spinel LiMn2O4 as the cathode of 4-V-class lithium secondary batteries, spinel phases LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) were successfully prepared using the sol–gel method. The spinel materials were characterized by powder X-ray diffraction (XRD), elemental analysis, and scanning electron microscopy. All the samples exhibited a pure cubic spinel structure without any impurities in the XRD patterns. Electrochemical studies were carried out using the Li|LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) cells. These cathodes were more tolerant to repeated lithium extraction and insertion than a standard LiMn2O4 spinel electrode in spite of a small reduction in the initial capacity. The improvement in cycling performance is attributed to the stabilization in the spinel structure by the doped metal cations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the recent trends in the lithium ion battery technology is focused on the characterization and development of high-voltage cathodes. Lithium transition-metal (V, Cr, Mn, Co, Ni) oxides, which are used as positive electrodes in secondary lithium batteries, have been extensively studied [1–4]. Among the various positive electrodes which have been used, spinel LiMn2O4 [5–7] offers considerable advantages over LiCoO2 [8] and LiNiO2 [9, 10] in terms of high cell voltage, a wide operating temperature, and long shelf life with much lower cost.
In spinel LiMn2O4, Li ions reside in the tetrahedral (8a) sites, Mn ions in the octahedral (16d) sites, and O2− ions in octahedral (32e) sites, respectively [11]. The oxygen ions in the octahedral sites form a closed-packed array in the spinel structure. The tetrahedral (8a) sites share faces with empty octahedral (l6c) sites and thus form 3D spaces. Li ions intercalate or de-intercalate through these spaces during electrochemical reaction [12].
Some problems for commercial application of LiMn2O4 are rechargeable capacity and poor cyclability of the charge–discharge process in the 4-V region. The major factors responsible for the capacity loss are as follows [10, 13, 14]: (1) the more stable one-phase structure in the low-voltage region transforms to an unstable two phase on the high-voltage region; (2) Mn3+ ions in LiMn3+Mn4+O4 cathode materials undergo a self-redox reaction to Mn2+ and Mn4+ at high voltages, which induces capacity loss due to a loss of the cathode; the Mn2+ so generated irreversibly dissolves in the electrolyte and increases in resistance of the cell; (3) the decomposition of the electrolyte solution at the electrode at high voltage causes lattice instability of LiMn2O4. This metal oxide shows cubic \({\text{Fd}}\overline {\text{3}} {\text{m}}\) symmetry at room temperature, with an average manganese valence of 3.5. The Mn exists in Mn4+ \(\left( {t_{2g}^3 e_g^0 } \right)\) and Jahn–Teller active Mn3+ \(\left( {t_{2g}^3 e_g^1 } \right)\) configurations. Several studies have been aimed at improving the material properties of LiMn2O4 and at improving its efficiency in maintaining electrochemical capacity over a large number of cycles without sacrificing initial reversible capacity and also its performance at room temperature [15, 16]. Doping the Mn (16d) sites with a trivalent cation or a cation with lower valence is a possibility, because it reduces the Mn3+ content and stabilizes the cubic structure in the face of Mn3+ Jahn–Teller distortion [17–23]. Guohua et al. [22] have shown that the substitution of M ions at the Mn site, Li(Mn, M)2O4 (M=Cr, Co and Ni), increases the average ionic valence of Mn and thus decreases the number of Jahn–Teller Mn3+ ions. Thackeray and co-workers [24] have pointed out that the substitution of a metal cation for Mn enhances the stability of spinels. In addition, Hosoya et al. [25] have suggested that the improvement of the cyclability refers to stronger M–O bonding of the MO6 octahedron of partially substituted LiM y Mn2 − y O4 (M=Cr, Co, Ni) in comparison with that of Mn–O in the parent LiMn2O4. Single-ion-doped LiMn2O4 spinels could not counteract all the factors responsible for the capacity loss. The effect of multiple cation-substituted LiMn2O4 has been reported and it has been pointed out that co-doping has a synergistic effect on the improvement of the cycling life of the materials as cathodes in lithium batteries [26–31].
In this work, we report the synthesis and characterization of double- and triple-metal-cation-doped spinel phases, wherein Mn has been partially replaced with monovalent lithium and other metal ions to improve the cycle performance of LiMn2O4 spinel materials.
Experimental
The LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) powders were synthesized by the sol–gel method using citric acid as a chelating agent [32, 33]. Stoichiometric mixture of Mn(NO3)2·4H2O (Merck), Fe(NO3)3·9H2O (Merck), and the respective substituent compound [Al(NO3)3·9H2O (Riedel-de Haen), Co(NO3)2·6H2O (Surechem), Ni(NO3)2·6H2O (Merck)] was first dissolved in deionized water to give a saturated solution. To this solution, lithium nitrate (Riedel-de Haen) was added slowly with mild stirring. Finally, a saturated aqueous solution of citric acid was then added with stirring. In all the experiments, the molar ratio of chelating agent to the total metal ions was unity. The pH of the mixed solution was maintained between 6.5 and 7.0 by adding ammonium hydroxide solution. The solution was then heated at 80 °C with vigorous stirring for 4 h to remove excess ammonia and water. The resulting precipitate of metal citrate was dried in an air oven for 10 h at 100 °C. After drying, the precursors were decomposed at 300 °C for 6 h in air to eliminate organic contents. The decomposed powders were slightly ground and then calcined at 600–800 °C for 24 h in air.
The cation composition of the products was determined by flame atomic absorption spectrometer (AAS, Perkin Elmer 3110) after dissolving the powders in dilute nitric acid.
The valence of Mn was determined by chemical titration. The samples were dissolved in an excess of 20 ml Na2C2O4 and 2 ml H2SO4 at ∼65 °C (maintained by a water bath) to reduce all Mnn + to Mn2+ (2 < n ≤ 4), and then the excess C2O4 2− ions in the solution were determined by titration at 65 °C with a standard solution of KMnO4 [34].
The phase identification and the evaluation of lattice parameters of prepared samples were carried out by powder X-ray diffraction (XRD) using copper CuK α radiation (Bruker AXS D8). The diffractometer was equipped with a diffracted beam graphite monochromator. The diffraction data were collected at 40 kV and 40 mA over a 2θ range from 5° to 80° with a step size of 0.02° and a count time of 10 s/step.
The particle morphology of the products was examined by means of scanning electron microscopy (LEO 440) operated at 20 kV.
The electrochemical properties of the spinel products were investigated using two-electrode Teflon cells [25] consisting of a cathode, lithium foil anode, glass filter paper separator, and an electrolyte having 1 M LiClO4 in propylene carbonate. For the fabrication of the cathode, a mixture of 85 wt.% active material (LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1), 10 wt.% graphite as electronic conductor, and 5 wt.% PTFE as a binder was pressed on to a 13-mm-diameter stainless steel mesh current collector covered with aluminum foil under a pressure of 6 tons. The resulting disc was then dried at 120 °C under vacuum for 12 h. The charge–discharge tests were performed galvanostatically with a computer-controlled multi-channel battery tester at a constant current density of 0.300 mA/cm2 with cut-off potentials of 3.5–4.4 V versus Li/Li+ at 25 °C. All processes of assembling and dismantling the cells were carried out in an argon-filled dry glove box.
Results and discussion
The chemical composition and the oxidation state of Mn in spinel materials are listed in Table 1. The chemical composition of each sample was close to the targeted formula of LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1). The experimental oxidation state of Mn was determined by chemical titration and the theoretical one is calculated based on the experimental composition. It can be seen that there is a good agreement between the expected and observed values.
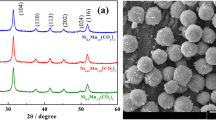
The powder XRD patterns of the LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) are shown in Fig. 1. All samples were identified as a single-phase spinel with a space group Fd3m in which the lithium ions occupy the tetrahedral sites (8a) and manganese and substituting metal ions reside at the octahedral (16d) sites [12]. This fact may indicate that the Mn site in LiMn2O4 is substituted fully by Fe–, Al–, Ni–, and Co–, respectively, and no other phase is formed. Metal substitution does not appear to change the basic LiMn2O4 structure. The cubic lattice parameters of LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1), calculated from the diffraction data, are given in Table 2. As compared with that of Li1.01Mn2O4, the lattice parameters of doped spinels are slightly decreased with increasing dopant content. This is due to the smaller size of the substituting ions Li+ (0.059 nm), Al3+ (0.0535 nm), Co3+ (0.055 nm), and Fe3+ (0.064 nm) as compared with the larger Mn+3 ion (0.066 nm) [35].
X-ray diffraction patterns of LiM x Mn2 − x O4 (M= Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) powders. a Li1.01Mn1.97O4, b Li1.05Mn1.96O4, c Li1.04Fe0.049Mn1.93O4, d Li1.05Fe0..96Mn1.88O4, e Li1.03Co0.145Mn1.81O4, f Li1.05Fe0.048Al0.098Mn1.81O4, g Li1.05Fe0.048Ni0.098Mn1.81O4, h Li1.04Fe0.049Co0.097Mn1.80O4
SEM photographs of substituted spinel LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) powders are presented in Fig. 2. The micrographs show that the particle size becomes smaller and the size distribution becomes much narrower with substituting of metal ions for the Mn3+. The smallest particle size and narrowest particle size distribution are observed for the triple-doped spinels. Since electrochemical lithium intercalation and deintercalation are, in general, limited by the rate of diffusion, the surface morphology and particle size distribution are the important factors for cycling performance of the Li ion batteries. Smaller grain size and narrower particle size distribution can favor the lithium ion mobility in the particles by reducing the ion diffusion pathway [36]. Therefore, it is expected that triple-doped spinels can display better electrode properties.
The discharge profiles for the samples synthesized by sol–gel method are shown in Fig. 3. The cells were first activated by charging up to 4.4 V and then discharging to 3.5 V at room temperature. The initial discharge capacity of cells was reduced by doping. Since the deintercalation of Li+ from the spinel structure must be electrically compensated by the oxidation of Mn3+ to Mn4+, it suggests that even for the substituted spinel phases only the amount of Mn3+ contributes to the charge capacity. So the initial capacity of LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) is limited by the initial amount of Mn3+ in the 16d sites [12].
The variation of discharge capacity as a function of cycle number for the LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) electrodes is given in Fig. 4. In Table 3, the theoretical capacities C 0, the first discharge capacities C 1, the discharge capacities at n = 30, C 30, and the percentages of capacity deterioration at the 30th cycle (C 1 − C 30) × 100 / C 1 under the standard of C 1 are summarized. As shown in Fig. 4 and Table 3, the initial discharge capacity of an electrode, based on Li1.01Mn1.97O4, was 110 mAh/g, which decreased rapidly to 86.2 mAh/g after 30 cycles. The capacity loss observed with the Li1.01Mn1.97O4 is about 21.6% after 30 cycles. The monovalent lithium ion- and other metal cation (M=Fe, Al, Ni, Co)-doped spinels display better cycle performance in terms of cycle life compared with Li1.01Mn2O4. In addition, cycling performance in the double-doped spinels (LiM x Mn2 − x O4 M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15), gradually decline; on the other hand, triple-doped spinels (LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) exhibit better capacity retention on cycling.
The significant improvement in the cycle performance can be explained by postulating that the doped metal ions enhance the stability of the octahedral sites in spinel skeleton structure because the bonding energy of doped metal oxygen Al–O, Ni–O, and Co–O is stronger than that of Mn–O [22, 37, 38]. Therefore, the spinel structure becomes more tolerant to repeated charge–discharge by doping of Li–Fe–Al, Li–Fe–Ni, and Li–Fe–Co. From the analysis of our results and those of earlier reports, we suggest that the Jahn–Teller distortion caused by Mn3+ was suppressed by the partial metal substitution. Another reason for the decreased capacity fade for the double- and triple-doped spinel materials is a result of their reduced particle size compared to that for the single-doped and undoped LiMn2O4 spinels. It has been known that a decrease in the mean particle size has increased the cyclability of the cathode material since the mechanical stability of small particles is more than that of large particles [39, 40]. From Fig. 4, it can be seen that the cycle performance was increased with triple-metal substitution.
Conclusions
The LiM x Mn2 − x O4 (M=Li, Fe, Co; x = 0, 0.05, 0.1, 0.15) and LiFe0.05M y Mn1.95 − y O4 (M=Li, Al, Ni, Co; y = 0.05, 0.1) have been prepared by the sol–gel method. All of the powders were identified as a single spinel phase with Fd3m space group. It has been demonstrated that cathodes based on substituting a part of Mn with Li–Fe–Al, Li–Fe–Ni, and Li–Fe–Co have good cycle life in spite of decrease in initial capacity. The improvement in cycling properties might be attributed to stabilization of spinel structure. Triple doping seems to have a synergetic effect on the improvement of the cycling life.
References
Tarascon JM, Guyomard D (1993) Electrochimica Acta 38:1221
Winter M, Besenhard JO, Spahr ME, Novak P (1998) Adv Mater 10:725
Aurbach D (2000) J Power Sources 89:206
Scrosati B, Panero S, Reale P, Satolli D, Aihara Y (2002) J Power Sources 105:161
Thackeray MM, Kock A, Rossouw MH, Liles D, Bittihn R, Hoge D (1992) J Electrochem Soc 139:363
Tarascon JM, Wang E, Shokoohi FK, Mckinnon WR, Colson S (1991) J Electrochem Soc 138:2859
Pasquier A, Blyr A, Cressent A, Lenain C, Amatucci G, Tarascon JM (1999) J Power Sources 81–82:54
Reimers JN, Dahn JR (1992) J Electrochem Soc 139:2091
Ohzuku T, Ueda A, Nagayama M (1991) J Electrochem Soc 140:1862
Prado G, Fournes L, Delmas C (2000) Solid State Ion 138:19
Pistoia G, Zane D, Zhang Y (1995) J Electrochem Soc 142:2551
Wakihara M (2001) Mater Sci Eng R33:109
Xia Y, Kumada N, Yoshio M (2000) J Power Sources 90:135
Mukerje S, Thurston TR, Jisrawi MN, Yang XQ, McBreen J, Daroux ML, Xing XK (1998) J Electrochem Soc 145:466
Liu W, Kowal K, Farrington GC (1998) J Electrochem Soc 145:459
Miura K, Yamada A, Tanaka M (1996) Electrochimica Acta 41:249
Robertson AD, Lu SH Averill WF, Howard WF (1997) J Electrochem Soc 144:3500
Robertson AD, Lu SH, Averill WF, Howard WF (1997) J Electrochem Soc 144:3505
Pistoia G, Antonini A, Rosati R, Bellitto C, Ingo GM (1997) Chem Mater 9:1443
Ein-Eli Y, Howard WF Jr. (1997) J Electrochem Soc 144:L205
Sanchez L, Tirado JL (1997) J Electrochem Soc 144:1939
Guohua L, Ikuta H, Uchida T, Wakihara M (1996) J Electrochem Soc 143:178
Pasquier AD, Blyr A, Courjal P, Larcher D, Amatucci B, Tarascon JM (1999) J Electrochem Soc 146:428
Gummow RJ, Kock A, Thackeray MM (1994) Solid State Ion 69:59
Hosoys M, Ikuta H, Wakihara M (1998) Solid State lonics 111:153
Bonino F, Panero S, Satolli D, Scrosati B (2001) J Power Sources 97–98:389
Hwang BJ, Santhanam R, Hu SG (2002) J Power Sources 108:250
Manuel Stephan A, Renganathan NG, Gopukumar S, Subramanian V, Bobba R (2004) Solid State Ion 175:291
Alcantara R, Jaraba M, Lavela P, Lloris JM, Perez Vicente C, Tirado JL (2005) J Electrochem Soc 152:A13
Rojas RM, Amarilla JM, Pascual L, Rojo JM, Kovacheva D, Petrov K (2006) J Power Sources 160:529
Şahan H, Göktepe H, Patat Ş (2008) Inorganic Materials 44:420
Hwang BJ, Santhanam R, Liu DG (2001) J Power Sources 101:86
Hwang BJ, Santhanam R, Liu DG, Tsai YW (2001) J Power Sources 102:326
Hong YS, Han H, Kim K, Campet G, Choy H (2001) Solid State Ion 139:75
Taniguchi I (2005) Mater Chem Phys 92:172
Julien CM, Zaghib K (2004) Electrochimica Acta 50:411
Sun YK, Oh B, Lee HJ (2000) Electrochimica Acta 46:541
Myung ST, Komaba S, Kumagai N (2001) J Electrochem Soc 148:A482
Manev V, Ebner W, Thompson W, Dow S (1999) US Patent 5961949
Choi HJ, Lee KM, Kim GH, Lee JG (2001) J Am Ceram Soc 84:242
Acknowledgments
This study was financially supported by the Research Foundation of Erciyes University (Kayseri, Turkey). The authors thank Mr. I. Akşit for the SEM observation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Göktepe, H., Şahan, H., Patat, Ş. et al. Enhanced cyclability of triple-metal-doped LiMn2O4 spinel as the cathode material for rechargeable lithium batteries. Ionics 15, 233–239 (2009). https://doi.org/10.1007/s11581-008-0265-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-008-0265-5