Abstract
High-performing LiAl x Mn2−x O4 (x = 0, 0.125, 0.25, 0.375, and 0.5) spinel cathode materials for lithium-ion battery were developed using a solution combustion method. The as-synthesized cathode materials have spinel cubic structure of LiMn2O4 without any impurity peak and accompanied with peak shift as doping with aluminium. LiAl0.375Mn1.625O4 (first cycle capacity = 113.1 mAh g−1) retains 85 % (96.2 mAh g−1), while pristine LiMn2O4 electrode (first cycle capacity = 135.8 mAh g−1) fades quickly and retains only 54 % (73.9 mAh g−1) after 50 cycles. The electrochemical performance of all the cathode samples prepared using the SCM is comparable to those reported for Al-doped LiMn2O4 spinel cathode materials. The experimental lattice parameter of LiAl x Mn2−x O4 was validated by ab initio calculations and correlated with the first cycle capacity of materials. The variation in lattice parameter as a result of Al doping greatly enhanced the cyclability of discharge capacity of the LiMn2O4 spinel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
LiMn2O4 spinel is a promising material for the positive (cathode) electrode in rechargeable lithium-ion batteries. It has attracted a lot of research interest because of its several advantages such as low cost, high abundance, low toxicity, simplicity of preparation, and high safety compared with other layered oxides such as LiCoO2 and LiNiO2 [1, 2]. However, the problem with spinel LiMn2O4 cathode material is its rapid capacity fading upon repeated charge/discharge cycling [3, 4]. Some of the causes for the capacity fade of spinel cathode materials when used as battery materials include two-phase unstable reaction [5], dissolution of spinel into the electrolyte, and decomposition of the electrolyte in the 4 V region [6] and Jahn–Teller distortion in the 3 V region [7]. One of the strategies to retain capacity is by substitution of small amount of Mn ions by dopant ions such as Al, Ni [3, 7–9], and microwave irradiation [14]. It is believed that the dopant ions occupy the octahedral 16d sites of Mn ions in the spinel lattice and stabilize the spinel structure from lattice distortion. Homogeneous dispersion of the Mn3+ substituting element in crystal lattice is crucial in the synthesis of doped LiMn2O4 cathode materials. Hence, generally, solution synthesis techniques are preferable in order to get the required homogeneously doped composition. Up to now, several solution synthesis methods have been used to synthesize pristine LiMn2O4 spinel structure cathode material for rechargeable lithium-ion batteries, such as sol–gel method [10], emulsion-drying method [11], and combustion method [12, 13]. However, there are few reports where Al-doped LiMn2O4 is obtained using the solution combustion method [14]. The solution combustion method (SCM) allows for rapid synthesis of highly substituted oxides in a one-step process. SCM simply involves the use of aqueous solution of metal precursors (typically nitrates serving as oxidizers) mixed with fuel (e.g. hydrazine, glycine, or urea) and heated to self-ignition to yield complex oxides. When compared to other solution methods, SCM possesses a unique advantage of the quasi-atomic dispersion of the component cations in liquid precursors, which facilitates synthesis of the crystallized powder with low particle size and high purity at low temperatures [15].
In this paper, we have synthesized LiMn2O4 spinel and its aluminium-doped counterparts (LiAl x Mn2−x O4, where x = 0.125, 0.25, 0.375, and 0.5) following the SCM using metal nitrates and urea as a fuel through an exothermic and self-sustaining chemical reaction. In addition, we report the correlation between the first cycle discharge capacity and lattice parameter of Al-doped spinel LiMn2O4 using experimental data analyses validated by ab initio first-principles calculations. The lithium intercalation energy of the pristine and aluminium-doped LiMn2O4 cathode materials are calculated using CASTEP code incorporated within Materials Studio software [16, 17].
2 Experimental
2.1 Materials and synthesis of LiAl x Mn2−x O4 spinel cathode materials
Similar to a previous study [18], the synthesis of LiAl x Mn2−x O4 spinel cathode materials for Li-ion battery was carried out in a furnace using 99.9 % pure Li(NO3), Mn(NO3)2 ·4H2O, Al(NO3)3 ·9H2O, and urea ((NH2)2CO) as starting materials. All the precursors were obtained from Sigma-Aldrich. The stoichiometric compositions of the redox mixtures for the combustion reaction are calculated using the total oxidizing and reducing valencies of the components to obtain the maximum energy released during the combustion process.
Accordingly, based on the stoichiometric ratios, appropriate masses of the precursor materials (see Table 1) were dissolved into 20 mL of deionized water in a 100-mL beaker and stirred at ambient temperature for about 30 min to obtain homogeneously mixed solution. After that, the precursor solution was introduced into a furnace, preheated at 500 °C, and the exothermic reaction took place and completed within 10 min to obtain a black powdered product. And then finally the product powder was calcined at 700 °C for 10 h to form spinel LiMn2O4 crystal structure. To investigate the effect of Al ion on the structural and electrochemical properties of LiMn2O4 cathode materials, Al-doped samples (with Al-ion concentration of 0.125, 0.25, 0.375, and 0.5) were prepared under atmospheric pressure and then calcined at 700 °C in air for 10 h. The voluminous and foamy combustion ash was easily milled to obtain the final LiAl x Mn2−x O4 cathode materials. The tap density of the as-synthesized LiMn2O4 materials, determined using the graduated measuring cylinder method, was about 1.41 g cm−3.
2.2 Equipment and procedure
The structural properties of the samples were investigated by X-ray diffraction analysis using a PANalytical X’Pert PRO PW3040/60 X-ray diffractometer with Fe-filtered Cu-Kα (λ = 1.5479026 nm) monochromated radiation source. Data were collected in the 2θ range of 10°–90° at a scan rate of 2 °/min. The morphology of the products was obtained using a high-resolution scanning electron microscope (JEOL, JSM-7600F, operated at an accelerating voltage of 5 kV). The lattice parameters of the synthesized spinels, obtained from the XRD, were further corroborated from ab initio calculations using CASTEP code [16, 17], which is a first-principles quantum mechanical programme based on the Hohenberg–Kohn–Sham density functional theory (DFT) [19–21], used within the generalized gradient approximation (GGA) formalism [22] to describe the electronic exchange–correlation interactions. We employed the spin-polarized PW91 [23] functional of GGA. Within CASTEP, maximum plane wave cut-off energy of 500 eV using Vanderbilt-type ultrasoft (US) pseudopotentials [24] and 3 × 3 × 3 Monkhorst–Pack [25] k-point mesh were applied on a conventional unit cell with P1 symmetry. Geometry optimization was conducted using the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method [26]. We have used this approach in our previous report for small Al content [27], while in this study we consider large Al content.
2.3 Electrochemical characterization
Coin cells of 2032 configuration were assembled using lithium metal as anode, Celgard 2400 as separator, 1 M solution of LiPF6 in 50:50 (v/v) mixture of ethylene carbonate (EC) and diethylene carbonate (DEC) as the electrolyte. The cathode was made through a slurry coating procedure from a mixture containing active material powder, conducting black and poly(vinylidene fluoride) binder in N-methyl-2-pyrrolidone in the proportion 80:10:10, respectively. The slurry was coated over aluminium foil and dried at 120 °C for 10 h in vacuum oven under vacuum condition. Then, 18-mm-diameter slurry-coated aluminium foils electrodes were punched out and used as cathode. Coin cells were assembled in an argon-filled glove box (MBraun, Germany) with moisture and oxygen levels maintained at <1 ppm. The cells were cycled at 0.2 C rate at room temperature with respect to corresponding theoretical capacities of LiAl x Mn2−x O4 spinel between 2.4 and 4.8 V in an MTI a multichannel battery tester.
3 Results and discussion
3.1 Morphology and structural characterization
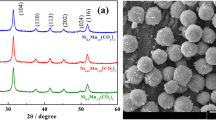
Figure 1a–d show typical SEM images of LiAl x Mn2−x O4 for x = 0, 0.125, 0.25, and 0.375 samples, respectively. It is clearly seen that the morphology of the as-synthesized powders is satisfactorily dispersed, reflecting the inherent nature of the combustion process (insets of Fig. 2b–d). The surfaces of the foams show a lot of voids and pores caused by the escaping gases during combustion reaction. The particles have regular shapes with well-defined faces, and the microstructure obtained indicates the high crystallinity of the as-synthesized product powder. Using the SEM images, the estimated particle size range for the compositions x = 0, x = 0.125, x = 0.25, x = 0.375, and x = 0.5, respectively, is 475 nm–2.2 µm, 200–525 nm, 200–800 nm, 300–800 nm, and 200–700 nm. The aluminium-doped samples are small in size compared with pristine LiMn2O4, in agreement with the literature report [14].
Figure 2a compares typical XRD pattern of each sample as a function of aluminium concentration. The examination of the diffraction patterns confirms that all recognizable reflection peaks including (111), (311), (222), (400), (331), (511), (440), (531), (533), (622), (551), and (731) can be clearly indexed to the single phase of the spinel cubic structure of LiMn2O4 (JCPDS File No. 88-1749) with space group Fd-3m, without any impurity peaks. The doped Al3+ mostly occupies octahedral Mn site (16d) and enhances the cyclability by stabilizing the spinel structure. However, there is optimal amount of Al-ion doping that increases the structural stability of LiMn2O4. This Al doping of spinel LiMn2O4 increases the mean Mn valence, but exceeding the critical concentration of Al might hinder the Li movement by occupying the Li site resulting in unacceptable low capacity. In our samples the optimized aluminium concentration to get a reasonable capacity and cyclability is x = 0.375. Figure 2b shows the peak shift of (111) plane towards higher angle for the samples LiMn2O4, LiAl0.25Mn1.75O4, and LiAl0.5Mn1.5O4 as the lattice structure of LiMn2O4 shrinks due to efficient Al doping.
Table 2 compares the lattice parameters (a-value/Å and unit cell volume/Å3) of the pristine and Al-doped spinel materials. The calculated values from the XRD follows same trend with the ab initio calculation. As the amount of aluminium is increased, the lattice parameter is decreased. This is expected considering that there is increase in the concentration of small Mn4+ (0.53 Å) ions in the spinel structure as big Mn3+ (0.645 Å) ions are substitutes by smaller Al3+ (0.53 Å) ions.
3.2 Electrochemical characterization
Generally, the theoretical capacity, C T, can be expressed as follows [28]:
where p and M denote the number of Mn(III) and the molecular weight of ion-doped LiMn2O4, respectively. Employing Eq. (1), the theoretical capacity of LiAl x Mn2−x O4 is found to be 148, 132.2, 115.6, 98.3, and 80.3 mAh g−1 for x = 0, x = 0.125, x = 0.25, x = 0.375, and x = 0.5, respectively. We have carried out the galvanostatic charge–discharge capacity performance testing of the cathode materials at 0.2 C rates with respect to their corresponding theoretical capacities. The representative first cycle discharge capacities of LiAl x Mn2−x O4 (x = 0, 0.125, 0.25, 0.375, and 0.5) are presented in Fig. 3A, B, C, D, and E, respectively. During the first cycle pristine LiMn2O4 delivers discharge capacity of 135.8 mAh g−1, LiAl0.125Mn1.875O4 gives 134.8 mAh g−1, LiAl0.25Mn1.75O4 about 111.5 mAh g−1, LiAl0.375Mn1.625O4 around 113.1 mAh g−1, and LiAl0.5Mn1.5O4 about 95.2 mAh g−1 which are comparable to their theoretical values. Our results are exhibiting superior capacity when compared to aluminium-doped LiMn2O4 cathode materials synthesized using sol–gel methods [29, 30]. Our LiMn2O4 cathode material using combustion method has relatively performed better (initial capacity of 135.8 mAh g−1 and retains 54 % after 50 cycle), as we observe the recent report by Zhang et al. [31] which gave them initial capacity of 118.6 mAh g−1 and 33.2 % capacity retention after 50 cycles. All the cathode samples prepared using the SCM are well-performing materials. Hence, synthesizing spinel using urea as fuel via SCM yields high-performing material.
The trend of the first cycle discharge capacity of the LiAl x Mn2−x O4 cathode materials in relation to the aluminium content is displayed in Fig. 4a. The first cycle discharge capacity of pristine LiMn2O4 and LiAl0.125Mn1.875O4 samples did not vary significantly. Also, as displayed in Fig. 4b, the calculated lattice parameter of LiAl0.125Mn1.875O4 (8.3228 Å) is almost equal to the lattice parameter of pristine LiMn2O4 (8.3232 Å). These experimental and theoretical results indicate that both pristine LiMn2O4 and small Al-content-doped LiAl0.125Mn1.875O4 samples exhibit high first cycle discharge capacity and larger lattice parameters compared with other high Al-content-doped samples. The larger the lattice parameter, the easier the Li ions can move more freely [32–34], thereby increasing the discharge capacity. As expected, beyond x = 0.125 (in our case for x = 0.25, 0.375, and 0.5) the first cycle discharge capacity of the doped LiMn2O4 cathode material becomes much less than that of the pristine LiMn2O4. The calculated lattice parameter for the samples with x = 0.25, x = 0.375, and x = 0.5 decreased to 8.28, 8.26, and 8.24 Å, respectively. A higher degree of Al doping can therefore be correlated with a decreased lattice parameter, which in turn leads to a lower discharge capacity. This finding indicates that the discharge capacity is not only correlated exclusively to the molecular weight of ion-doped LiMn2O4, but also to its lattice parameter.
Figure 5 compares the cyclability of the LiAl x Mn2−x O4 samples upon 50 repetitive cycles. The discharge capacity of LiMn2O4 and LiAl0.125Mn1.875O4 fades faster compared with the other samples. This figure depicts the effect of Al-ion content on the cycling performance of LiMn2O4. It can be seen that the curve of LiAl0.375Mn1.625O4 has a maximum cycling stability, which is about 1.6 times that of the cycling stability of undoped LiMn2O4 after 50 cycles. Our result agrees with Julien et al. [35] who reported that LiAl0.3Mn1.7O4 has a better cyclability than LiAl0.1Mn1.9O4. We surmise that the composition LiAl0.375Mn1.625O4 suppressed the effect of Jahn–Teller distortion and that the concentration of Mn3+ which induces Jahn–Teller distortion in the spinel is decreased.
Figure 6a represents the change in discharge capacity (50th cycle discharge capacity/first discharge capacity) for the LiAl x Mn2−x O4 cathode materials. Compared with the pristine LiMn2O4 cathode material, the LiAl0.125Mn1.875O4 composition did not improve the cyclability, whereas the other doped spinel compositions do retain their initial capacities for many cycles; for example, aluminium-doped compositions LiAl0.25Mn1.75O4, LiAl0.375Mn1.625O4, and LiAl0.5Mn1.5O4, respectively, retain 1.5, 1.6, and 1.3 times that of pristine LiMn2O4. It is apparent that the samples with Al-ion concentration of x = 0.375 exhibit higher capacity retention about 85 % after 50 cycles. The enhanced cyclability of the sample LiAl0.375Mn1.625O4 in the present study is primarily attributed to the optimum Al-ion doping which results in stabilizing the spinel structure. The observed stability trends are further validated by ab initio predictions in Fig. 6b, c. As shown in Fig. 6b, an increase in Al-dopant corresponds to more negative intercalation energy which implies easy Li-ion movement. On the other hand, this increase in Al yields higher average open-circuit voltage of up to approximately 4.8 when x = 0.5.
4 Conclusion
We have synthesized LiAl x Mn2−x O4 cathode materials for Li-ion battery using metal nitrates and urea as precursors by solution combustion method. The samples were characterized by SEM, XRD, and charge/discharge battery tester. The first cycle discharge capacity of LiAl0.125Mn1.875O4 is comparable to that of the pristine LiMn2O4, just as the values of their lattice parameter are essentially the same. In addition, the LiAl0.375Mn1.625O4 sample exhibited the more stable discharge capacity than the other samples. The variation in lattice parameter as a result of Al doping greatly enhanced the cyclability of discharge capacity of the LiMn2O4 spinel.
References
A.S. Arico, P. Bruce, B. Scrosati, J.M. Tarascon, W. Van Schalkwijk, Nat. Mater. 4, 366 (2005)
H. Şahan, H. Göktepe, Ş. Patat, A. Ülgen, J. Alloys Compd. 509, 4235 (2011)
A. Yuan, L. Tian, W. Xu, Y. Wang, J. Power Sources 195, 5032 (2010)
M. Prabu, M. Reddy, S. Selvasekarapandian, G.S. Rao, B. Chowdari, Electrochim. Acta 88, 745 (2013)
W. Zhou, S. Bao, B. He, Y. Liang, H. Li, Electrochim. Acta 51, 4701 (2006)
L. Xiao, Y. Zhao, Y. Yang, Y. Cao, X. Ai, H. Yang, Electrochim. Acta 54, 545 (2008)
T. Yi, Y. Zhu, R. Zhu, L. Zhou, P. Li, J. Shu, Ionics 15, 177 (2009)
R. Thirunakaran, A. Sivashanmugam, S. Gopukumar, R. Rajalakshmi, J. Power Sources 187, 565 (2009)
D. Guo, B. Li, Z. Chang, H. Tang, X. Xu, K. Chang, E. Shangguan, X. Yuan, H. Wang, Electrochim. Acta 134, 338 (2014)
C.J. Curtis, J. Wang, D.L. Schulz, J. Electrochem. Soc. 151, A590 (2004)
S. Myung, S. Komaba, N. Kumagai, J. Electrochem. Soc. 148, A482 (2001)
K. Lee, H. Choi, J. Lee, J. Mater. Sci. Lett. 20, 1309 (2001)
C. Peng, H. Bai, Q. Li, Y. Guo, J. Huang, C. Su, J. Guo, Int. J. Electrochem. Sci. 10, 3885 (2015)
F.P. Nkosi, C.J. Jafta, M. Kebede, L. le Roux, M.K. Mathe, K.I. Ozoemena, RSC Adv. 5, 32256 (2015)
K.C. Stella, A.S. Nesaraj, Iran. J. Mater. Sci. Eng. 7, 36 (2010)
M. Segall, P.J.D. Lindan, M. Probert, C. Pickard, P. Hasnip, S. Clark, M. Payne, J. Phys. Condens. Matter 14, 2717 (2002)
S.J. Clark, M.D. Segall, C.J. Pickard, P.J. Hasnip, M.I.J. Probert, K. Refson, M.C. Payne, Z. Kristallogr. 220, 567 (2005)
M.A. Kebede, N. Kunjuzwa, C.J. Jafta, M.K. Mathe, K.I. Ozoemena, Electrochim. Acta 128, 172 (2014)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
W. Kohn, L.J. Sham, Phys. Rev. 140, A1133–A1138 (1965)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
J.P. Perdew, Y. Wang, Phys. Rev. B 45, 13244 (1992)
J.P. Perdew, J. Chevary, S. Vosko, K.A. Jackson, M.R. Pederson, D. Singh, C. Fiolhais, Phys. Rev. B 46, 6671 (1992)
D. Vanderbilt, Phys. Rev. B 41, 7892 (1990)
H.J. Monkhorst, J.D. Pack, Phys. Rev. B 13, 5188 (1976)
T.H. Fischer, J. Almlof, J. Phys. Chem. 96, 9768 (1992)
M.A. Kebede, M.J. Phasha, N. Kunjuzwa, L.J. le Roux, D. Mkhonto, K.I. Ozoemena, M.K. Mathe, Sustain. Energy Technol. Assess. 5, 44 (2014)
T. Kakuda, K. Uematsu, K. Toda, M. Sato, J. Power Sources 167, 499 (2007)
S.J. Bao, Y.Y. Liang, W.J. Zhou, B.L. He, H.L. Li, J. Power Sources 154, 239 (2006)
R. Thirunakaran, A. Sivashanmugam, S. Gopukumar, C.W. Dunnill, D.H. Gregory, J. Mater. Process. Technol. 208, 520 (2008)
H. Zhang, Y. Xu, D. Liu, X. Zhang, C. Zhao, Electrochim. Acta 125, 225 (2014)
Y. Huang, R. Jiang, S.J. Bao, Y. Cao, D. Jia, Nanoscale Res. Lett. 4, 353 (2009)
G. Amatucci, J.M. Tarascon, J. Electrochem. Soc. 149, K31 (2002)
T. Tsumura, A. Shimizu, M. Inagaki, Solid State Ion. 90, 197 (1996)
C. Julien, S. Ziolkiewicz, M. Lemal, M. Massot, J. Mater. Chem. 11, 1837 (2001)
Acknowledgments
We thank the CSIR for supporting this work. MK would like to acknowledge the NRF for supporting this work financially.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kebede, M.A., Phasha, M.J., Kunjuzwa, N. et al. Solution-combustion synthesized aluminium-doped spinel (LiAl x Mn2−x O4) as a high-performance lithium-ion battery cathode material. Appl. Phys. A 121, 51–57 (2015). https://doi.org/10.1007/s00339-015-9311-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9311-0