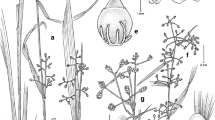
Abstract
Three new species in the genera Marasmiellus, Pusillomyces, and Gymnopus are described based on specimens found growing in the leaf litter of a mountain cloud forest relict from eastern Mexico. Distinctive macro- and micromorphological characters in combination with a phylogenetic analysis based on ITS sequences support their taxonomic identity and position in each of the above mentioned genera of the Omphalotaceae. Species here described form rhizomorphs. Morphological descriptions, including illustrations, photographs, and taxonomic discussions are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After recent description of the rhizomorph-forming Gymnopus nidus-avis [Pseudomarasmius nidus-avis (César, Bandala & Montoya) Petersen and Hughes 2020], new collections of marasmioid-gymnopoid fungi that produce flat rhizomorphs were found growing on leaf litter, in the same study site, a mountain cloud forest relict, a protected area by the Instituto de Ecología A.C. located near to Xalapa, Veracruz (East Coast of Mexico). The macro- and micromorphological characters exhibited by the studied fresh specimens, along with the results after a molecular phylogeny obtained, inform that the three new species here described have a taxonomic position in the Omphalotaceae Bresinsky, a family that includes other rhizomorph-forming species. The Omphalotaceae is undergoing a constant rearrangement by both the continuous application of molecular tools and by the additional information that is generated which includes data from formerly undocumented species or even new proposed genera (Moncalvo et al. 2002; Wilson and Desjardin 2005; Mata et al. 2007; Petersen and Hughes 2016, 2020; Oliveira et al. 2019). Accepting in Omphalotaceae an arrangement with a more strict concept of Gymnopus (Pers.) Roussel, as well as Marasmiellus Murrill, Mycetinis Earle, and Lentinula Earle, among others (Wilson and Desjardin 2005), and in correspondence with a recent phylogenetic conclusion on Omphalotaceae (Oliveira et al. 2019), the three species here described are recognized in Marasmiellus, Pusillomyces J.S. Oliveira, and Gymnopus, supported also with the morpho-anatomic characters that define such groups.
Several rhizomorph-forming species are known among the agarics, and their functional role in ecosystems is recognized by the harmful effect caused in other organisms (Hartig 1873; Seaver 1944; Morrison 2004; Dassanayake et al. 2009; Su et al. 2011) as well as their ecological importance such as traps for leaf litter, acting as storage of organic matter in the upper levels of the canopy, promoting biodiversity of arthropods and other life forms (Hedger 1990; Snaddon et al. 2012), or as sources of nest building material for some birds (Aubrecht et al. 2013; Chaparro and Ruiz 2014; Menezes et al. 2014; Gómez et al. 2014; César et al. 2018; Koch et al. 2018) and mammals (Prange and Nelson 2006) or even as decomposers that bind leaf litter particles and translocate nutrients from the discontinuous layers of plant debris in forest floors (Boddy 1999).
Despite the ecological importance of rhizomorphs and their interactions, the appropriate terminology to designate the different linear aggregates of hyphae has been used vaguely, even when the morpho-anatomic distinction between cords or mycelial strands and fungal rhizomorphs is clear: the mycelial cords or strands are formed by relatively loose hyphae in which the young hyphal branches grow and adhere to the oldest (Moore 1994), while in rhizomorphs, a highly organized apical growing point with clear apical dominance is present (Moore 1998). Additionally, rhizomorphs are uniquely characterized by a waterproof surface and a melanized rind that encloses a medullar cavity which transfers water and dissolved nutrients (Yafetto 2018).
The geographic range in which species of agarics that develop rhizomorphs are distributed is worldwide, except for Antarctica, and they practically occur in all ecosystems like coniferous, broad-leaved temperate, tropical and subtropical forests including mountain cloud forests (Antonín and Noordeloos 2010; Mešić et al. 2011; Petersen and Hughes 2019). The mountain cloud forest is characterized by being strongly threatened by anthropic activities throughout the world and at the same time for housing an important biodiversity of all biological groups in correspondence with the 0.14% of the Earth’s land surface in which these ecosystems are distributed (Scatena et al. 2010).
Materials and methods
Sampling and morphological study
As part of a field monitoring program developed weekly in the Santuario del Bosque de Niebla (SBN), samples of marasmioid-gymnopoid rhizomorph-forming species were collected in the rainy season of June 2016, 2018, and 2019. The SBN is a cloud forest relict having an extension of 30 ha of vegetation dominated by tree species of Quercus, Platanus, Carpinus, Liquidambar, and Clethra, among others and is a protected peri-urban area in the municipality of Xalapa, Veracruz, located at 1343 m.a.s.l. Fresh basidiomes were photographed and characterized morphologically, and their colors were annotated following Kornerup and Wanscher (1967) and Munsell (1994). Stipe and rhizomorphs width were measured using the × 20 lens and ocular micrometric rule of a Nikon E400 microscope. After drying, microscopical characters were observed with 3% potassium hydroxide (KOH), 1% Congo Red aqueous solution, and Melzer’s reagent (Largent et al. 1977). Thirty-five spores per specimens were measured, and an arithmetic mean of basidiospore length and width range was obtained and indicated with Xm; length/width ratio was calculated, and ranges are indicated with Qm. Collections were deposited in XAL herbarium (Thiers 2020). Line drawings were made using a Nikon E400 microscope with a drawing tube. Abbreviations: M., Marasmius; Ma., Marasmiellus.
DNA extraction, PCR amplification, and sequencing
Extraction of genomic DNA of basidiomes was performed after César et al. (2018), and the nuc rDNA ITS (Internal Transcribed Spacer) was amplified using primers ITS1F and ITS4 (White et al. 1990; Gardes and Bruns 1993). The sequences of the amplified PCR products were obtained using a capillary sequencer. Once sequences were assembled and edited, they were deposited at GenBank database (Benson et al. 2017) with accession numbers indicated in Fig. 1.
Phylogenetic methods
Phylogenetic trees were generated according to Montoya et al. (2019). A dataset, using PhyDE v.0.9971 (Müller et al. 2010), was constructed with the sequences obtained in this study together with sequences of related taxa considered by Oliveira et al. (2019). The dataset was aligned with MAFFT online service (Katoh et al. 2019) and inconsistencies corrected manually. The evolutionary model was calculated using the IQ-Tree 2.0-rc1 (Nguyen et al. 2015; Kalyaanamoorthy et al. 2017) and the best-fit model selected using the Bayesian Information Criterion (BIC), the Akaike Information Criterion (AIC), and corrected AIC. This later was used to generate a phylogenetic tree with the maximum likelihood (ML) method, with a Nearest Neighbor Interchange (NNI) heuristic, and with the TIM2+F+I+G4 evolutionary model. A consensus tree was also generated calculating the Robinson-Foulds distance between the ML tree and the consensus tree, the branches being tested by means of Ultrafast Approach Bootstrap (UFBoot), SH-like Approximate Likelihood Ratio Test (SH-aLRT), Approximate Bayes test (aBayes), and Bootstrap Standard (BS). Another phylogenetic tree was also generated by Bayesian Inference (BI), using MrBayes v. 3.2.7 (Ronquist et al. 2012). The phylogenies from ML and BI analyses were displayed using FigTree v1.4.4 (Rambaut 2018). Only bootstrap values (BS) of ≥ 70% and Bayesian posterior probabilities (BPP) of ≥ 0.90 were considered and indicated on the tree branches (BS/BPP) of Fig. 1.
Results
Eight fresh collections of marasmioid-gymnopoid species were recovered from leaf litter at the study site. From each collection, ITS sequences (codes indicated in bold in Fig. 1) were obtained and included in a molecular phylogeny together with 252 sequences of species representing 11 different clades of the Omphalotaceae and with sequences of species of Marasmius Fr., Crinipellis Pat., Moniliophthora H.C. Evans, Stalpers, Samson & Benny, and Campanella Henn. as outgroup (Fig. 1); the alignment was deposited in the repository TreeBASE: Study ID 25993. Two sequences (specimens César 44 and 202) were clustered in a well-supported clade (88/100), representing an isolate species close to Ma. quercophilus (Pouzar) J.S. Oliveira and Ma. ramealis (Bull.) Singer. Three sequences (specimens César 49, 50, and 107) were nested and isolated in a supported clade (100/100) being a species sister to Gymnopus androsaceus (L.) Della Magg. & Trassin., G. portoricensis R.H. Petersen, G. neobrevipes R.H. Petersen and G. cremeostipitatus Antonín, and Ryoo & Ka along with other not formally described taxa of sect. Androsacei (Petersen and Hughes 2016). Three sequences (specimens César 43, 206, and 209) allow to recognize an isolated species related with Pusillomyces manuripioides, P. asetosus, and P. funalis with strong support (100/100), sharing characters with these species like a marasmioid habit and a pileipellis with incrusted hyphae and diverticulated elements. Because sequences of Mexican specimens were clustered in three different strongly supported isolated clades (Fig. 1) and considering that the specimens possess a distinctive set of morphological features, we recognize that they represent three new species which are proposed and described below.
Taxonomy
Marasmiellus diaphanus César, Bandala & Montoya, sp. nov.
Mycobank: MB 835526
Holotype: MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1343 m.a.s.l., gregarious on fallen leaves of Quercus, 6 June 2019, César 202 (XAL).
Diagnosis: Pileus 2–9 mm diam., white-translucent. Lamellae adnexed, distant, concolorous with pileus. Stipe 2.5–10 × 0.25–0.7 mm, filiform. Basidiospores 5.5–8 × 2.5–4.5 μm, subcylindrical to oblong. Pleurocystidia and caulocystidia absent. Cheilocystidia 16.5–29.5 × 10–13 μm, broadly clavate to subglobose. Rhizomorphs 3–15 × 0.25–0.5 mm, light brown to golden brown, flat, repent, and with ramifications, basidiomata not arising from them.
Gene sequence ex-holotype: MT232390.
Etymology. Diaphanus (Lat.): in reference to the white-transparent or diaphanous pileus.
Basidiomata marcescent. Pileus 2–9 mm diam., convex to plano-convex, slightly depressed at the disc; margin smooth to striate or weakly sulcate, radially forming bulges following the lamellae pattern, with a somewhat satiny or silky texture but not shiny, slightly rimose, pearly white (2.5Y/R 8/1) and almost translucent in mature specimens with faint creamy tinges at the disc (2.5Y/R 7/4). Context very thin (< 1 mm), soft, homogeneous, and concolorous with pileus. Lamellae adnexed, distant (15–18), concolorous with pileus (2.5Y/R 8/1), somewhat broad (1 mm approx.), margin smooth, transversal veins present in mature specimens, with attenuated lamellulae (14–17). Stipe 2.5–10 × 0.25–0.7 mm, central, cylindrical, but slightly tapering towards the base, smooth, solid, somewhat curved, whitish at the apex (2.5Y/R 8/1), golden brown (2.5Y 6/6, 5C4) downwards, becoming dark brown (2.5Y 3/3, 5F8) at the base, insititious and always arising from leaf litter. Rhizomorphs 3–15 × 0.2–0.5 mm, light brown (2.5Y 7/4) to golden brown (2.5Y 6/8) with some darker brown zones (2.5Y 3/2), silky bright, flat and repent, twisted at times, with ramifications, attached to leaf litter in which fruiting bodies emerge, binding 2–3 leaves around basidiomata. Odor and taste not distinctive; without reaction with KOH.
Basidiospores 5.5–8 × 2.5–4.5 μm, Xm = 6.5–7.5 × 3–3.6; Qm = 1.97–2.04, subcylindrical, somewhat oblong, hyaline, inamyloid, thin-walled. Basidia 16–29 × 5–8 μm, 4-spored, clavate, inamyloid, hyaline, thin-walled, clamped. Basidioles 15–25 × 5–9 μm, subcylindric to claviform. Pleurocystidia absent. Cheilocystidia 16–29 × 10–13 μm, broadly clavate to subglobose; in some, the apex is faintly irregular, moderately thick-walled (< 1 μm), hyaline, scarce. Pileipellis formed by compactly interwoven, inamyloid hyphae, which are periclinally arranged, cylindrical, non-gelatinized, thin-walled, clamped, hyaline, 3.5–5 μm diam.; with repent or semi-erect terminal elements which are irregular in form and bearing numerous appendages and short lateral outgrowths (< 1 μm long), reminding a Rameales-structure, thin walled, hyaline, inamyloid, not refringent, not incrusted. Pileus trama is composed of interwoven hyphae, 4–5 μm diam., cylindrical, slightly tortuous, thin-walled, hyaline, inamyloid. Hymenophoral trama subregular, composed of cylindrical hyphae, 4–6 μm diam., hyaline and thin-walled. Stipitipellis is a cutis composed of straight, cylindrical, thick-walled, inamyloid, light brown hyphae, 6–7.5 μm diam., septate, without caulocystidia although some terminal hyphae with obtuse apex could be present, not or faintly erect. Stipe trama regular with cylindrical, straight, thick-walled hyphae (< 1 μm), 4–5 μm diam., hyphae of the cortical layers 5–7 μm diam., inamyloid, hyaline or lightly pigmented, thick-walled, medullar layers with hyphae presenting the same diameter as in the cortical layers but tortuous and light brown. Clamp connections present and common.
Habitat. In mountain cloud forest, growing on humid leaf litter, under a relatively open canopy, on sloping ground dominated by Quercus. Arising directly from leaves, particularly on midribs and veins.
Additional specimens examined. MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1343 m.a.s.l., 7 June 2016, César 44 (XAL).
Remarks—In the molecular phylogeny here obtained (Fig. 1), Mexican specimens of Marasmiellus diaphanus appear closely related with strong support with Ma. quercophilus (Pouzar) J.S. Oliveira and Ma. ramealis (Bull.) Singer. Marasmiellus diaphanus in fact shares morphologic characters of taxonomic relevance (pileus and stipe colors, habit, and microscopic elements), although it is one of those species that are the exception in the genus for lacking well-developed caulocystidia which in Marasmiellus are present and well-developed (Oliveira et al. 2019). Marasmiellus quercophilus is distinguished from the Mexican taxon by its longer stipe [15–35 (− 50) mm length] with a white pileus having pinkish-brown or reddish-brown center, less distant lamellae (6–2) which are broadly adnate or emarginate, bigger basidiospores [(6.5–) 7–9 (− 10) × 3.5–6.5 μm] and has distinctive caulocystidia (Antonín and Noordeloos 2010). Marasmiellus ramealis has a broader pileus (2–20 mm width) with different disc colors (darker reddish brown or yellow brown) and a squamulose stipe up to 20 mm long (Antonín and Noordeloos 2010).
Other species morphologically resembling Marasmiellus diaphanus not included in the obtained phylogeny due to the lack of sequences are Ma. berkeleyi Singer, Ma. bisporiger Singer, Ma. defibulatus Singer, Marasmius pilgerodendri Singer, and M. polychaetopus Singer. Marasmiellus berkeleyi presents filamentous dermatocystidia, spores with a strongly developed oblique hilar appendage and long stipe hairs (50–100 μm) (Singer 1973). Marasmiellus bisporiger has different cheilocystidia (cylindrical to club-shaped), subcollariate lamellae, and clampless hyphae, the same as Ma. defibulatus that also has different cheilocystidia (dendroid-ramose to laterally diverticulated) and presents swollen elements up to 20 μm width in the Pileus trama (Singer 1973). The pileus of M. pilgerodendri is white but becomes brownish with age and has bigger spores (7–9.5 × 3–4 μm), cheilocystidia with numerous coarse diverticula, and dark and cylindrical rhizomorphs; this species grows on Pilgerodendron sp. and Fitzroya sp. leaves (both genera are not present in Mexico) (Singer 1965). Marasmius polychaetopus could be easily confused in size and colors with Ma. diaphanus but the presence of setiform caulocystidia, an insititious stipe arising directly from the rhizomorphs, not from the substrate, as well as its larger basidiospores (8–8.2 × 3.9–4.1 μm) (Singer 1965) distinguish it.
Pusillomyces spinulosus César, Bandala & Montoya, sp. nov
Mycobank: MB 835527
Holotype: MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1343 m a.s.l., gregarious on fallen leaves, 14 June 2019, César 206 (XAL).
Diagnosis: Pileus 1–6 mm diam., light cinnamon brown. Lamellae adnate, distant, light brown with fimbriate edge; Stipe 9–20 × 0.3–0.45-mm cylindrical, tomentose, lightly sticky. Basidiospores 5.5–9 × 2.5–4 μm, subcylindrical. Basidia 3–4 spored. Pleurocystidia absent. Cheilocystidia 13.5–23.5 × 7.5–11 μm, clavate to subclavate with diverticula 1–2 (− 3) × 0.5–1 μm. Caulocystidia 25–103 × 5–8 μm, setiform. Rhizomorphs 5–50 × 0.05–0.1 mm, golden brown to dark brown, flat and simple, basidiomata not arising from them. Growing on Quercus leaf litter.
Gene sequence ex-holotype: MT232386.
Etymology. Spinulosus (Lat.): in reference to the tiny spinous stipe surface.
Basidiomata marcescent. Pileus 1–6 mm diam., convex to plano-convex in mature specimens with a central depression, dry and slightly velvety; margin decurved, translucent striate and wavy or lobed following the arrangement of the lamellae, radially wrinkled when dry, light cinnamon brown (10 YR5/6, 6C6) to cream (2.5Y 7/3) with a slightly darker brown cinnamon disc (10YR 5/8, 6D6), a pale reddish-brown coloration (7.5YR 4/6) can be present in young specimens. Context thin (around 1 mm) and soft, concolorous with the pileus surface. Lamellae adnate, distant (9–10), light brown (2.5Y 8/2), somewhat broad and with fimbriate edge; truncate lamellulae (7–13) of three different sizes; without collar. Stipe 9–20 × 0.3–0.4 mm, central, cylindrical, filiform, insititious, straight or somewhat tortuous, solid, spinulose due to the presence of setiform caulocystidia, apex light cream (2.5Y7/3, 5B2) in young, turning olive brown (2.5Y5/6) in mature specimens, reddish brown (5YR 4/6, 7E7) in the basal zone and dirty mustard (2.5Y 6/8) in the middle, in some cases, entirely reddish brown (5YR 4/6); always arising from leaves, both from the veins and from other parts of the blade, without basal tomentum. Etiolated sterile stipes arising among whole developed basidiocarps with the same characters and colors but shorter than the normal stipes. Rhizomorphs 5–50 × 0.05–0.1 mm, fragile, flat, twisted, silky, and simple; adhered to the leaves or erect, stramineous, golden brown (2.5Y 5/6) to dark brown (7.5 YR 4/4, 6E7), binding no more than 3–4 leaves. Odor and taste not distinctive; without reaction with KOH.
Basidiospores 5.5–9 × 2.5–4 μm, Xm = 6.2–7.9 × 3.1–3.5; Qm = 1.95–2.26, subcylindrical, hyaline, inamyloid, thin-walled. Basidia 17–30 × 5–8 μm, 3–4 spored, clavate, inamyloid, hyaline, thin-walled. Basidioles 17.5–31.5 × 3.5–6.5 μm, cylindrical to clavate with narrow apex, thin-walled, hyaline. Pleurocystidia absent. Cheilocystidia 13.5–23.5 × 7.5–11 μm, clavate to subclavate, with a nodulose to more or less knobbed apex, with irregular, apically rounded appendages, 1–2 (− 3) × 0.5–1 μm, hyaline, thick-walled (< 1 μm thick). Pileipellis formed by interwoven hyphae, 4–6 μm diam., in a moderately compact, periclinally arrangement (but some anticlinally disposed), cylindrical, somewhat sinuous, non-gelatinized, thin-walled, inamyloid, some with refringent incrustations in a spiral pattern; terminal elements 17.5–24 × 9.5–20 μm, broad-claviform, appendiculate, with Rameales-structure, thin-walled, inamyloid, hyaline. Pileus trama of interwoven hyaline hyphae 4–5 μm diam., inamyloid, cylindrical, tortuous, thin-walled, without contents, some with refringent incrustations in spiral pattern. Hymenophoral trama regular, with cylindrical hyphae, 3–5 μm diam., without contents or incrustations. Stipitipellis is a cutis of hyaline, inamyloid, cylindrical, thin-walled hyphae with numerous caulocystidia. Caulocystidia 25–103 × 5–8 μm, setiform, brownish and hyaline, cylindrical or tapering towards the apex which is rounded, thick-walled (< 1 μm), some slightly tortuous. Stipe trama with cortical hyphae 4–5 μm diam., inamyloid, somewhat thick-walled (< 1 μm thick), light brown, straight smooth; medullar hyphae hyaline, similar to cortical hyphae. Clamp connections absent in all tissues.
Habitat. In mountain cloud forest, growing on Quercus leaf debris. Arising directly from leaves, from any part of the leaf blade.
Additional specimens examined. MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1343 m.a.s.l., 7 June 2016, César 43; 14 June 2019, César 209 (both at XAL).
Remarks—Pusillomyces spinulosus molecularly grouped in a well-supported clade (Fig. 1) close to P. manuripioides J.S. Oliveira, P. funalis (Har. Takah.) J.S. Oliveira, and P. asetosus (Antonín, Ryoo & Ka) J.S. Oliveira. Morphologically, the former is quite different, especially by its smooth hymenophore (Oliveira et al. 2019). Pusillomyces funalis has a dark reddish-brown pileus, slightly bigger basidiospores (6.5–8 × 4–5 μm) and shorter elements of the pileipellis (12–22 × 8–13 μm). In addition, the presence of rhizomorphs was not documented for P. funalis being recorded on twigs of Cryptomeria japonica (P. spinulosus was not found on twigs) and leaf litter of Carpinus tschonoskii and Quercus myrsinifolia, Asiatic tree species not present in Mexico (Takahashi 2002). Pusillomyces asetosus differs from Mexican species particularly by the lack of stipitipellis setae but also by its grayish-orange to brownish-orange pileus, the dark brown, shorter stipe (3–7 mm long) and the presence of Siccus-type broom cells in the pileipellis (Antonín et al. 2014).
Without available sequences, it is necessary to mention other Mexican species of Marasmius that superficially could resemble Pusillomyces spinulosus. Marasmius atroincrustatus Singer var. atroincrustatus exhibits a distinct garlic odor and different color variations of the lamellae (pale isabelline to dull creme), stipe (chestnut to nearly black), rhizomorphs (dark color), and the cheilocystidia have very long diverticula (2–12.5 μm) (Singer 1976). Marasmius chiapasensis Singer differs by its white lamellae, shorter caulocystidia (9–39 μm), the presence of fusoid-mucronate to ampullaceous cheilocystidia, and slightly smaller basidiospores (6–7.2 × 2.5–4 μm) (Singer 1976), while M. liquidambaris Singer differs in having obtuse and clavate to subcylindrical caulocystidia and clamped hyphae and grows on Liquidambar styraciflua leaves (Singer 1976) or Castanopsis acuminatissima (a native species from Southeast Asia and New Guinea) (Desjardin and Horak 1997).
Gymnopus brunneiniger César, Bandala & Montoya, sp. nov.
Mycobank: MB 8355282
Holotype: MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1343 m a.s.l., gregarious on fallen leaves of a deciduous tree species, 9 June 2016, César 49 (XAL).
Diagnosis: Pileus 2–14 mm diam., pale brown to pale brown orange. Lamellae free to adnate, subdistant, concolorous with pileus. Stipe 10–30 × 0.3–0.6 mm, central, filiform, insititious, smooth. Basidiospores 6.5–10 × 3–4.5 μm, subcylindrical, hyaline. Pleurocystidia and caulocystidia absent. Cheilocystidia 13–25.5 × 5–11.5 μm, clavate to subclavate, nodulose. Pileipellis is a cutis composed of smooth, simple hyphae with some segments having appendages and short lateral outgrowths. Clamp connections present. Rhizomorphs 10–250 × 0.2–0.5 mm, black, wiry, simple, repent 10–250 × 0.2–0.5 mm, black.
Gene sequences ex-holotype: MT232389.
Etymology. Brunneus (Lat.): brown and niger (Lat.): black, in reference to the pileus and stipe colors.
Basidiomata marcescent. Pileus 2–14 mm diam., convex to plano-convex, moderately broadly umbilicate when young, becoming slightly centrally depressed when mature, deflexed margin, smooth, not translucent but with radially arranged tortuous grooves in accordance with the lamellae and lamellulae, dry and somewhat rugulose, pale brown (10YR 7/4, 5B3) to pale brown orange (7.5 YR 5/6, 6C7) in the disc and grooves. Context thin (< 1 mm), soft, concolorous with pileus. Lamellae free to adnate, subdistant (12–18), concolorous with the pileus (10YR 7/4, 5B3), straight, somewhat broad (< 1 mm), with smooth edge, lamellulae (12–20) up to three different lengths, attenuate, other abrupt, transversal veins present. Stipe10–30 × 0.3–0.6 mm, cylindrical, filiform, central, insititious, smooth, shiny, firm, solid, becoming twisted and flat when dried, entirely black or dark reddish black (10R 2.5/1, 7F3). Sterile stipes or telepods present, arising near basidiomata with the same characters and colors as normal stipes. Rhizomorphs 10–250 × 0.2–0.5 mm, black, shiny, wiry, simple, repent, and attached to leaves, binding several leaves.
Basidiospores 6.5–10 × 3–4.5 μm; Xm = 8.5–9 × 3.5–3.6; Qm = 2.3–2.4, subcylindrical, hyaline, inamyloid, thin-walled. Basidia 16–24 × 4–8 μm, 4-spored, rarely 3-spored, clavate, thin-walled, hyaline, clamped. Basidioles 18.5–25 × 4.5–6.5 μm, cylindrical to clavate with subacute apex, hyaline, inamyloid, thin-walled, clamped. Pleurocystidia and caulocystidia absent. Cheilocystidia. 13–25.5 × 5–11.5 μm, clavate to subclavate, with a nodulose to more or less knobbed apex, with irregular, apically rounded appendages, 1–2 × 0.5–1 μm. Pileipellis is a cutis, composed of smooth, intertwined, simple, cylindrical, thin-walled, hyaline, hyphae 4–6 μm diam., dextrinoid, with segments having appendages and short lateral outgrowths (1–3 μm). Pileus trama is composed of interwoven, tortuous, cylindrical, thin-walled hyphae, 4–6 μm diam.; some dextrinoid otherwise inamyloid, smooth, and clamped. Hymenophoral trama subregular to irregular, composed of cylindrical, faintly tortuous hyphae, 5–6 μm diam., thin-walled, sometimes clamped, Stipitipellis is a cutis of parallel, thick-walled (< 1 μm), light brown, inamyloid hyphae, 3–5 μm diam. Stipe trama with parallel, tightly packed, hyaline, inamyloid, thick-walled, clamped hyphae 4–7 μm diam.; medullary hyphae hyaline undifferentiated except by coloration. Clamp connections present in all tissues.
Habitat. In mountain cloud forest, growing on fallen leaves of several deciduous tree species. Arising from any part of the leaf blade.
Additional specimens examined. MEXICO. Veracruz: Municipality of Xalapa, Santuario del Bosque de Niebla, Instituto de Ecología A.C., 1365 m a.s.l., 9 June 2016, César 50; 25 June 2018, César 107 (both at XAL).
Remarks—In the molecular phylogeny here obtained (Fig. 1), G. brunneiniger appears isolated in a well-supported clade, close to G. androsaceus and related species. Macroscopically, G. androsaceus has resemblance with the Mexican species by the basidiome color and habit, but it differs by its longer stipe (24–60 mm), a pileipellis with lobed or branched broom cells, the absence of sterile stipes and growing mostly on litter of conifers (Antonín and Noordeloos 2010). Gymnopus cremeostipitatus differs by its pale cream pileus color and the presence of caulocystidia, and it grows on leaves of Camellia japonica (Antonín et al. 2014). Gymnopus neobrevipes is distinguished by its habit, with a shorter stipe (0.5–6 mm long) and a pileipellis with different elements. Gymnopus portoricensis particularly differs by its pinkish-cinnamon pileus, a shorter stipe (1–2.5 mm), pleated or fold-like lamellae, pileal hairs, and cheilocystidia with irregular lobes (Petersen and Hughes 2019).
Marasmius cyrillaedis Dennis and Setulipes afibulatus Antonín are other morphologically similar species to Gymnopus brunneiniger. However, M. cyrillaedis is different by its smaller pileus (up to 4 mm), shorter basidiospores (5–8 × 2.5–3.2 μm), pileipellis “…of interwoven versiform elements, not in hymeniform arrangement, these often swollen to 7 μm diameter and some like the cheilocystidia…,” and having chestnut colored rhizomorphs, tapering upwards, 14–45 × 0.15–0.5 mm (Singer 1976). Setulipes afibulatus varies in the lack of clamp connections, pileipellis with incrusted hyphae bearing broom cells, and the presence of caulocystidia (Antonín 2003).
Discussion
Most recent circumscription of the Omphalotaceae by Oliveira et al. (2019) place molecularly related species, possessing a white or yellow pileus and a stipe with white or clear apex and dark base and microscopically distinguished by having cheilocystidia, a pileipellis with coralloid or diverticulated terminal elements and lacking both pleurocystidia (or very rare) and caulocystidia to Marasmiellus, a set of features found for the new Mexican Ma. diaphanous. Pusillomyces is characterized by marasmioid basidiomata, with a smooth hymenophore or well-developed lamellae, a filiform stipe, the presence of rhizomorphs, diverticulated elements in the pileipellis, caulocystidia present or absent, and the lack of clamp connections (Oliveira et al. 2019). With the addition of the new proposed P. spinulosus, currently are four species known in the genus. It is possible that after an appropriate revision of the morphologic features combined with sequencing, several other species in Marasmius will be combined in this genus. Gymnopus sect. Androsacei where G. brunneiniger grouped with strong support (Fig. 1), embracing marasmioid taxa having filiform stipe, cheilocystidia like broom cells of the Siccus type, diverticulate hyphae in the pileipellis and saprophytic growing habit on leaves. Recently two new genera, Paramycetinis and Pseudomarasmius, were added to the Omplalotaceae by Petersen and Hughes (2020). The former groups are the species of one of the Mycetinis subclade of Petersen and Hughes (2016) and part of the Gymnopanella clade of Oliveira et al. (2019), with two species Paramycetinis austrobrevipes and P. caulocystidiatus. Pseudomarasmius, mentioned to differ from Marasmius by the diverticulate hyphae present in the pileipellis and by the clampless hyphae, embraces the species of the Pallidocephalus clade (Oliveira et al. 2019) with eight species considered by Petersen and Hughes (2020); four of them, P. efibulatus from New Zealand, P. obscurus from Costa Rica, P. patagonianus from Chile, and P. quercophiloides from China, were newly described, while P. glabrocystidiatus from Korea (Antonín et al. 2014), P. nidus-avis from Mexico (César et al. 2018), P. pallidocephalus from USA (Gilliam 1975), and P. straminipes from USA (Peck 1873) were newly combined to this genus.
The three new species proposed here form rhizomorphs, which presumably help them to exploit organic resources of the forest ground, clustering leaf litter and debris. Of the 16 reported genera in Omphalotaceae (He et al. 2019; Petersen and Hughes 2020), 7 include species that form rhizomorphs (Gymnopus, Marasmiellus, Mycetinis, Paragymnopus J.S. Oliveira, Paramycetinis R.H. Petersen, Pseudomarasmius R.H. Petersen & K.W. Hughes, and Pusillomyces). Other genera reported to develop such feature are Crinipellis Pat., Marasmius Fr., and Moniliophtora H.C. Evans, Stalpers, Samson & Benny, inserted in family Marasmiaceae Roze ex Kühner, and Armillaria (Fr.) Staude, Cryptomarasmius T.S. Jenkinson & Desjardin, Gloiocephala Massee and Manuripia Singer, members of Physalacriaceae Corner, both families phylogenetically close to Omphalotaceae.
Data availability
The sequences generated in this study are available in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under the accession numbers explained in the text. The ITS alignment of the phylogeny obtained in this study was deposited in the TreeBASE as Study ID 25993 (https://www.treebase.org/).
References
Antonín V (2003) New species of marasmioid genera (Basidiomycetes, Tricholomataceae) from tropical Africa - II. Gloiocephala, Marasmius, Setulipes and two new combinations. Mycotaxon 88:53–78
Antonín V, Noordeloos ME (2010) A monograph of marasmioid and collybioid fungi in Europe. IHW Verlag, Eching
Antonín V, Ryoo R, Ka KH (2014) Marasmioid and gymnopoid fungi of the Republic of Korea. 7. Gymnopus sect. Androsacei. Mycol Prog 13:703–718. https://doi.org/10.1007/s11557-013-0953-z
Aubrecht G, Huber W, Weissenfofer A (2013) Coincidence or benefit? The use of Marasmius (horse-hair fungus) filaments in bird nests. Avian Biol Res 6:26–30. https://doi.org/10.3184/175815512X13531739538638
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2017) GenBank. Nucleic Acids Res 45(Database issue):D37–D42. https://doi.org/10.1093/nar/gkw1070
Boddy L (1999) Saprotrophic cord-forming fungi: meeting the challenge of heterogeneous environments. Mycologia 91:13–32. https://doi.org/10.2307/3761190
César E, Bandala VM, Montoya L, Ramos A (2018) A new Gymnopus species with rhizomorphs and its record as nesting material by birds (Tyrannideae) in the subtropical cloud forest from eastern Mexico. MycoKeys 42:21–34. https://doi.org/10.3897/mycokeys.42.28894
Chaparro S, Ruiz JM (2014) Anidación del Hormiguerito de Cherrie (Myrmotherula cherriei) en Colombia, con una revisión de los nidos y huevos en Myrmotherula. Ornitol Colomb 14:136–144
Dassanayake N, Wanigasundara W, Balasuriya A, Amaratunge M (2009) A field assessment of the factors affecting horse hair blight (Marasmius equicrinis) in tea in the Ratnapura District. J Agric Sci 4:59–66. https://doi.org/10.4038/jas.v4i2.1645
Desjardin DE, Horak E (1997) Marasmius and Gloiocephala in the South Pacific Region: Papua New Guinea, New Caledonia, and New Zealand Taxa. In: Petrini O, Petrini LE, Horak E (eds) Taxonomic monographs of Agaricales II. J. Cramer, Morlenbach
Gardes M, Bruns D (1993) ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Gilliam MS (1975) New North American species of Marasmius. Mycologia 67:817–844. https://doi.org/10.1080/00275514.1975.12019811
Gómez VH, Arriaga SL, Capello S, Rosique E, Cifuentes J (2014) Nidos de aves elaborados con material fúngico: un dato no registrado en México. Ornitología Neotropical 25:107–111
Hartig R (1873) Vorlaufige Mitteilung über den Parasitismus von Agaricus melleus und dessen Rhizomorphen. Botanische Zeitung 31:295–297
He MQ, Zhao RL, Hyde KD et al (2019) Notes, outline and divergence times of Basidiomycota. Fungal Divers 99:105–367. https://doi.org/10.1007/s13225-019-00435-4
Hedger J (1990) Fungi in the tropical forest canopy. Mycologist 4:200–202
Kalyaanamoorthy S, Minh BQ, Wong TKF, Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Koch RA, Lodge DJ, Sourell S, Nakasone K, McCoy AG, Aime MC (2018) Tying up loose threads: revised taxonomy and phylogeny of an avian-dispersed Neotropical rhizomorph-forming fungus. Mycol Prog 17:989–998. https://doi.org/10.1007/s11557-018-1411-8
Kornerup A, Wanscher JH (1967) Methuen handbook of colour. Methuen Co, London
Largent D, Johnson D, Watling R (1977) How to identify mushrooms to genus III: microscopic features. Mad River Press, Michigan
Mata JL, Hughes KW, Petersen RH (2007) An investigation of Omphalotaceae (Fungi: Euagarics) with emphasis on the genus Gymnopus. Sydowia 58:191–289
Menezes JC, Barbosa TC, Prezoto F (2014) Previously unreported nesting associations of the yellow-olive fly catcher (Tolmomyas sulphurescens) (Aves: Tyrannidae) with social wasps and bees. Ornitologia Neotropical 25:363–368
Mešić A, Tkalčec Z, Deng CY, Li TH, Pleše B, Ćetković H (2011) Gymnopus fuscotramus (Agaricales), a new species from southern China. Mycotaxon 117:321–330. https://doi.org/10.5248/117.321
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin S, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, Miller OK (2002) One hundred and seventeen clades of Euagarics. Mol Phylogenet Evol 23:357–400
Montoya L, Caro A, Ramos A, Bandala VM (2019) Two new species of Lactifluus (Fungi, Russulales) from tropical Quercus forest in eastern Mexico. MycoKeys 59:27–45. https://doi.org/10.3897/mycokeys.59.38359
Moore D (1994) Tissue Formation. In: Gow N, Gadd GM (eds) The growing fungus. Chapmann and Hall, London, pp 423–457
Moore D (1998) Fungal morphogenesis. Cambridge University Press, Cambridge
Morrison DJ (2004) Rhizomorph growth habit, saprophytic ability and virulence of 15 Armillaria species. For Pathol 34:15–26. https://doi.org/10.1046/j.1439-0329.2003.00345.x
Müller J, Müller K, Neinhuis C, Quandt D (2010) PhyDE – Phylogenetic Data Editor, version 0.9971. http://www.phyde.de
Munsell (1994) Munsell soil color charts. Macbeth, New Windsor
Nguyen LT, Schmidt HA, Haeseler A, Minh BQ (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Oliveira JJ, Vargas-Isla R, Cabral TS, Rodrigues DP, Ishikawa NK (2019) Progress on the phylogeny of the Omphalotaceae: Gymnopus s. str., Marasmiellus s. str., Paragymnopus gen. nov. and Pusillomyces gen. nov. Mycol Prog 18:713–739. https://doi.org/10.1007/s11557-019-01483-5
Peck CH (1873) Descriptions of new species of fungi. Bull Buffalo Soc Nat Sci 1:41–72
Petersen RH, Hughes KW (2016) Micromphale sect. Perforantia (Agaricales, Basidiomycetes); expansion and phylogenetic placement. MycoKeys 18:1–122. https://doi.org/10.3897/mycokeys.18.10007
Petersen RH, Hughes KW (2019) Two additional species of Gymnopus (Euagarics, Basidiomycotina). Mycokeys 45:1–24. https://doi.org/10.3897/mycokeys.45.29350
Petersen RH, Hughes KW (2020) Two new genera of gymnopoid/marasmioid euagarics. Mycotaxon 135:1–95. https://doi.org/10.5248/135.1
Prange S, Nelson DH (2006) Use of fungal rhizomorphs as nesting material by Glaucomys volans (Southern Flying Squirrels). Southeast Nat 5:355–360
Rambaut A (2018) FigTree v1.4.4 software. Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Scatena FN, Bruijnzeel LA, Bubb P, Das S (2010) Setting the stage. In: Bruijnzeel LA, Scatena FN, Hamilton LS (eds) Tropical Montane Cloud Forests: Science for Conservation and Management. Cambridge University Press, Cambridge
Seaver FJ (1944) The horse-hair fungi. Mycologia 36:340–342
Singer R (1965) Monographic studies on South American Basidiomycetes, especially those of the east slope of the Andes and Brazil. 2. The genus Marasmius in South America. Sydowia 18:106–358
Singer R (1973) The genera Marasmiellus, Crepidotus, and Simocybe in the Neotropics. Beihefte zur Nova Hedwigia 44:1–517
Singer R (1976) Marasmieae (Basidiomycetes- Tricholomataceae) Flora neotropica 17. The New York Botanical Garden Bronx, New York
Snaddon JL, Turner EC, Fayle TM, Khen CV, Eggleton P, Foster WA (2012) Biodiversity hanging by a thread: the importance of fungal litter-trapping systems in tropical rainforests. Biol Lett 8:397–400. https://doi.org/10.1098/rsbl.2011.1115
Su HJ, Thseng FM, Chen JS, Ko WH (2011) Production of volatile substances by rhizomorphs of Marasmius crinisequi and its significance in nature. Fungal Divers 49:199–202. https://doi.org/10.1007/s13225-010-0084-7
Takahashi H (2002) Four new species of Crinipellis and Marasmius in eastern Honshu, Japan. Mycoscience 43:343–350. https://doi.org/10.1007/S102670200050
Thiers B (2020) [continuously updated] Index Herbariorum: a global directory of public herbaria and associate staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih [accessed 14 January 2020]
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Wilson AW, Desjardin DE (2005) Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 97:667–679. https://doi.org/10.3852/mycologia.97.3.667
Yafetto L (2018) The structure of mycelial cords and rhizomorphs of fungi: A mini-review. Mycosphere 9:984–998. https://doi.org/10.5943/mycosphere/9/5/3
Acknowledgments
We recognize the support given by the strategic project INECOL-20035-30890 to study the macrofungi in the Santuario del Bosque de Niebla. E. César is grateful for the postdoctoral Instituto de Ecología A.C. (INECOL) grant. Thanks are given to Biol. D. Ramos (INECOL) by the assistance in the field and in the laboratory. We acknowledge the support given by the Consejo Nacional de Ciencia y Tecnología (CONACYT) (225382) to the Laboratorio de Presecuenciación, Red Biodiversidad y Sistemática, INECOL.
Funding
This research was supported by the Instituto de Ecología A.C. project 20035-30890.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Zhu-Liang Yang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
César, E., Montoya, L., Bandala, V.M. et al. Three new marasmioid-gymnopoid rhizomorph-forming species from Mexican mountain cloud forest relicts. Mycol Progress 19, 1017–1029 (2020). https://doi.org/10.1007/s11557-020-01608-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-020-01608-1