Abstract
Twigs entangled by rhizomorphs of Marasmius crinisequi inside tea bushes are mostly devoid of leaves. This was found to be due to the emission of a defoliation-inducing volatile by the rhizomorphs, because when tea twigs were enclosed with rhizomorph sections of M. crinisequi, leaf fall occurred within 5 days. Leaves on control twigs remained healthy and attached. The volatile substances emitted from rhizomorphs were passed through acetone and the volatile compounds in acetone were analyzed by GC/MS. The emitted volatiles were identified as 3-oxo-β-ionol, 2,4,6-tri-ter-butyl-4-methyl-cyclohexadien-2,5-one and 2-phenyl-3,4,5,6-tetramethylpyridine. These compounds have not been reported from fungi or plants prior to this report. The significance of the production of a defoliation-inducing volatile by rhizomorphs of M. crinisequi in its acquisition of nutrients from tea leaves in the competitive environment is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marasmius is a worldwide distributed genus and its members are an important litter-decomposing agaric component of tropical ecosystems (Desjardin and Ovrebo 2006; Wannathes et al. 2009a,b). Some species of Marasmius produce abundant black, hair-like rhizomorphs as a means of exploration for colonization of twigs and leaves (Seaver 1944; Singer 1976; Redhead 1989). Rhizomorphs of several Marasmius species have been used as nesting material by various bird species (Foster 1976; Hedger 1990; McFarland and Rimmer 1996; Young and Zuchowski 2003; Freymann 2008; Chen et al. 2010) and even flying squirrels (Prange and Nelson 2006).
Horse-hair blight of tea (Camellia sinensis (L.) Kuntze) caused by Marasmius crinisequi Mull. ex Kalch. (=Marasmius equicrinis Mull.) is known to occur in India, Sri Lanka, Java, Australia, China, Japan and Taiwan (Petch 1923; Hara 1931; Sarmah 1960; Hu 1984; Chen and Chen 1990. Ezuka and Ando 1994; Dassanayake et al. 2009). M. crinisequi has been considered epiphytic on the tea bush, obtaining its food from the dead outer back of the older stems, and from the dead leaves and twigs in the tangle (Petch 1923; Sarmah 1960; Dassanayake et al. 2009).
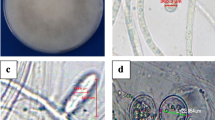
Decline of tea bushes associated with M. crinisequi (Fig. 1) has become serious in recent years in a number of tea farms in central Taiwan. The affected tea bushes became unthrifty and the production of harvestable tea leaves by these plants is greatly reduced (Hu 1984). During a survey conducted in the tea farms with decline problem, it was observed that twigs of lower branches entangled with rhizomorphs of M. crinisequi were devoid of leaves. It was, therefore, hypothesized that M. crinisequi rhizomorphs may have caused defoliation of affected twigs. Our study revealed the involvement of volatile substances produced by rhizomorphs of M. crinisequi in the defoliation of affected twigs. The chemistry of the released volatile substances were subsequently determined. Details of the study are reported herein.
Materials and methods
Detection of volatile substances released from rhizomorphs
Two-year-old cv. Chin Hsin Oolong tea plants each with three twigs were used. Rhizomorphs of M. crinisequi collected from declining tea bushes were cut to 3-cm sections with a pair of scissors, and 50 rhizomorph sections were fastened to each twig with a Parafilm band (5 mm wide) about 10 mm away from the main stem. Twigs similarly treated with Parafilm bands were used as controls. Each treated plant was sprayed with distilled water and enclosed in a plastic bag (27 cm diam., 37 cm high). Test plants were kept outdoors under shade with the temperature ranging from 28°C to 34°C. Plastic bags were removed after 5 days. Five plants were used for each treatment and the experiment was performed three times.
Chemical identification of volatile substances
For collection of volatile substances, approximately 80 g of rhizomorphs of M. crinisequi gathered from affected tea bushes was placed in a 5-l glass jar connected through Teflon tubing to a glass tube with a pointed tip immersed in 25 ml acetone in a 70-ml glass vial which was connected to a suction pump (Fig. 2). After equilibration of volatile substances, released from rhizomorphs in the jar at 24–26°C, was attained, the headspace gas was sucked through acetone at the flow rate of 8 ml min−1 for 3 h (Ioffe and Vitenberg 1984).
Analysis of the volatile substances in acetone was carried out using a Varian 4000 GC/MS system (Varian, Inc., Palo Ato, CA, USA Anonymous 1996a). The injection temperature was 250°C for 5 min. A non-polar VF-5-MS column (30 m × 0.25 mm i.d., 0.25 mm film thickness, Varian, Inc., Palo Ato, Ca, USA) was operated under the following conditions: 70°C for 3 min, 20°C min−1 to 180°C for 3 min, and 10°C min−1 to 230°C for 20 min. The MS was operated at 70 eV. Full scan of masses was from m/z 20 to 400 with a scan rate of 470 μ/s.
Results
When plastic bags were removed from the tea plants 5 days after exposure to rhizomorphs of M. crinisequi, some leaves fell from the twigs. Fallen leaves lost vigor and turned brown gradually. Leaf fall continued and none of the leaves remained on the treated twigs after 4 weeks (Table 1). Control plants remained healthy during the experiment. None of the rhizomorphs used formed adhesive mycelial discs to stick to the twigs, indicative of ability of the rhizomorphs to emit defoliation inducing volatiles.
Based on the GC/MS analysis, the volatile compounds released from M. crinisequi rhizomorphs were identified as 3-oxo-β-ionol, 2,4,6-tri-ter-butyl-4-methyl-cyclohexadien-2,5-one and 2-phenyl-3,4,5,6-tetramethylpyridine (Fig. 3). These constituents have more than 80% similarity with the known compounds in NIST standard reference database (Anonymous 1996b) (Table 2).
Discussion
This study shows that rhizomorphs of M crinisequi are able to induce defoliation of tea twigs by releasing volatile substances. Induction of defoliation by aerial rhizomorphs of M. crinisequi may explain the absence of leaves on most twigs entangled by rhizomorphs inside the affected tea bush (Petch 1923; Hara 1931; Hu 1984; Chen and Chen 1990). M. crinisequi is considered epiphytic and not plant pathogenic, obtaining its nutrients from dead bark, and from dead leaves and twigs of the tea bush (Petch 1923; Sarmah 1960; Dassanayake et al. 2009). Results from this study suggest that M. crinisequi employs a unique strategy in evading competitors during its acquisition of nutrients from dying leaves of the tea bush. The rhizomorphs of this fungus produces adhesive discs to stick on living leaves when crossing them during its exploration (Hedger 1990; Ko, unpublished observations). These leaves will fall from twigs due to defoliation-inducing volatiles emitted by the rhizomorph. The falling leaves will be captured by the fungus with its adhesive discs and suspended in the air. Since detached leaves are less disease resistance (Liu et al. 2007) and macromolecules will be degraded (Quirino et al. 2000), it will be easier for M. crinisequi to obtain nutrients from fallen tea leaves. Suspending fallen leaves in the air also can prevent the captured food source being eaten by soil animals (Coleman and Wall 2007) and/or colonized by other soil microorganisms (Alexander 1977). It is not known if emission of volatiles by rhizomorphs is common among fungi. Production of volatile antimicrobial compounds by endophytic Muscodor including Muscodor albus (Ezra et al. 2004), Muscodor crispans (Mitchell et al. 2008, 2010) and Muscodor yucatanensis (Macias-Rubalcava et al. 2010) in cultures has been reported in recent years. The volatile compounds produced by M. yucatanensis are also inhibitory to plant seed germination and root elongation (Macias-Rubalcava et al. 2010). Whether the volatile substances emitted from rhizomorphs of Marasmius are antimicrobial remains to by investigated.
The volatile substances produced by M. crinisequi and identified as 3-oxo-β-ionol, 2,4,6-tri-ter-butyl-4-methyl-cyclohexadien-2,5-one and 2-phenyl-3,4,5,6-tetramethylpyridine have not been reported from fungi or plants previously (Kaiser 2006; Pichersky et al. 2006) and thus are different to those produced by Muscodor species which are primarily alcohols, organic acids, esters, ketones and lipids (Macias-Rubalcava et al. 2010). Only a closely related 3-oxo-α-ionol has been detected in passion fruit (Winterhalter 1990), grapes (Strauss et al. 1987) and starfruit (Herderich et al. 1992). Since authentic compounds are not available, it is still not known which of the three volatile compounds detected in this study is involved in the induction of defilation. It appears to be a new kind of plant growth regulator. The mechanism of defoliation induction and possible application in agriculture for stimulation of flower production deserves further investigation. The functions of the other two volatile compounds also remains to be studied. It would also be interesting to know if the volatiles emitted by the rhizomorphs are involved in the selection by birds as the nesting material (Foster 1976; Hedger 1990; McFarland and Rimmer 1996; Young and Zuchowski 2003; Freymann 2008; Chen et al. 2010).
References
Alexander M (1977) Introduction to soil microbiology, 2nd edn. John Wiley and Sons, New York
Anonymous (1996a) Volatile organic compounds by gas chromatograph / mass spectrometry (GC / MS): capillary column technique, test methods for evaluating solid waste. United Sates Environmental Protection Agency Method 8260B
Anonymous (1996b). NIST / EPA / NIH mass spectral database. Standard References Database Program of the National Institute of Standards and Technology (NIST). Version 1.5 for PC. www.nist.gov.
Chen CM, Chen SF (1990) Diagnosis and control of tea bush disease. Shanghai Science and Technology Publication Company, Shanghai
Chen HH, Chen TY, Lin RS (2010) Nest Materials and Measurements of the Black-Naped Monarch (Hypothymis azurea) in Taiwan. Taiwan J Biodivers 12:167–175
Coleman DC, Wall DH (2007) Fauna: the engine for microbial activity and transport. In: Paul EA (ed) Soil microbiology, ecology, and biochemistry, 3rd edn. Academic, New York
Dassanayake N, Wanigasundara W, Balasuriya A, Amaratunge M (2009) A field assessment of the factors affecting horse hair blight (Marasmius equicrinis) in tea in the Ratnapura District. J Agric Sci 4:59–66
Desjardin N, Ovrebo CL (2006) New species and new records of Marasmius from Panamá. Fungal Divers 21:19–39
Ezra D, Hess WM, Strobel GA (2004) New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 150:4023–4031
Ezuka A, Ando Y (1994) Tea disease in Japan. Japan Plant Protection Association, Tokyo
Foster MS (1976) Nesting biology of the long-tailed Manakin. Wilson Bull 88:400–420
Freymann BP (2008) Physical properties of fungal rhizomorphs of marasmioid basidiomycetes used as nesting material by birds. Ibis 150:395–399
Hara K (1931) The disease of Tea Bush. Japan Mycological Society, Shizuoka
Hedger J (1990) Fungi in the tropical forest canopy. Mycologist 4:200–202
Herderich M, Neubert C, Winterhalter P, Schreier P, Skouroumounis GK (1992) Identification of C13-norisoprenoid flavour precursors in starfruit (Averrhoa carambola L.). Flavour Fragr J 7:179–185
Hu CC (1984) Horse-hair blight, new disease of tea bush caused by Marasmius equicrinis Mull in Taiwan. Taiwan Tea Res Bull 3:1–4
Ioffe BV, Vitenberg AG (1984) Headspace analysis and related methods in gas chromatography. John Wiley and Sons, Hoboken
Kaiser R (2006) Flowers and fungi use scents to mimic each other. Science 311:806–807
Liu G, Kennedy R, Greenshields DL, Peng G, Forseille L, Selvaraj G, Wei Y (2007) Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol Plant-Microbe Interact 20:1308–1319
Macias-Rubalcava ML, Hernandez-Bautista BE, Oropeza F, Duarte G, Gonzalez MC, Glenn AE, Hanlin RT, Anaya AL (2010) Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J Chem Ecol 36:1122–1131
McFarland KP, Rimmer CC (1996) Horsehair fungus, Marasmius androsaceus, used as nest lining by birds of the subalpine spruce-fir community in the northeastern United States. Can Field-Nat 110:541–543
Mitchell AM, Strobel GA, Hess WM, Vargas PN, Erza D (2008) A record of Muscodor crispans: a noval endophyte from Ananas annassoides in the Bolivian Amazon. Fungal Divers 31:37–43
Mitchell AM, Strobel GA, Moore E, Robison R, Sears J (2010) Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 156:270–277
Petch T (1923) The disease of Tea Bush. Macmillan and Company, London
Pichersky E, Nod JP, Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:806–807
Prange S, Nelson DH (2006) Use of fungal rhizomorphs as nesting material by Glaucomys volans (southern flying squirrels). Southeast Nat 5:355–360
Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5:278–282
Redhead SA (1989) A biogeographically overview of the Canadian mushroom flora. Can J Bot 67:3003–3062
Sarmah KC (1960) Disease of tea and associated crop in northeast India. India Tea Assoc Memo 26:48–50
Seaver FJ (1944) The horse-hair fungi. Mycologia 36:340–342
Singer R (1976) Marasmieae (Basidiomycetes – Tricholomataceae). Flora Neotrop Monogr 17:1–347
Strauss CR, Wilson B, Williams PJ (1987) 2-oxo-α-ionol, vomifoliol and reseoside in Vistis vinifera fruit. Phytochemistry 26:1995–1997
Wannathes N, Desjardin DE, Lumyong S (2009a) Four new species of Marasmius section Globulares from Northern Thailand. Fungal Divers 36:155–163
Wannathes N, Desjardin DE, Hyde KD, Perry BA, Lumyong S (2009b) A monograph of Marasmius (Basidiomycota) from Northern Thailand based on morphological and molecular (ITS sequences) data. Fungal Divers 37:209–306
Winterhalter P (1990) Bound terpenoids in the juice of purple passion fruit (Passiflora edulis Sima). J Agric Food Chem 38:450–455
Young BE, Zuchowski W (2003) First description of the nest of the Silvery-fronted Tapaculo (Scytalopus argentifrons). Wilson Bull 115:91–93
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, H.J., Thseng, F.M., Chen, J.S. et al. Production of volatile substances by rhizomorphs of Marasmius crinisequi and its significance in nature. Fungal Diversity 49, 199–202 (2011). https://doi.org/10.1007/s13225-010-0084-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-010-0084-7