Abstract
Introduction
Guidelines advocate the use of combined detection techniques to achieve optimal results for sentinel node (SN) biopsy. The fluorescent and radioactive (dual-) tracer ICG-99mTc-nanocolloid has been shown to facilitate SN biopsy in several indications. It was reported that an opto-nuclear probe permitted the detection of near-infrared fluorescence and gamma-rays. The aim of the current study was to evaluate this device in a large patient group and to test it in both open and laparoscopic surgery implications.
Methods
Thirty-three patients scheduled for SN biopsy with the dual-tracer were retrospectively analyzed. Pre-operative lymphoscintigraphy was performed in all patients; in 18 patients (55%), a SPECT/CT scan was also performed. Radioactive and fluorescent signatures in the SNs were assessed in vivo and ex vivo using the opto-nuclear probe.
Results
One or more SNs were identified in all patients (identification rate 100%). Planar lymphoscintigraphic images revealed 95 hot spots that were considered as SNs. This number increased to 103 SNs when SPECT/CT was used. During surgery, 106 SNs were excised. In vivo, the fluorescence mode of the opto-nuclear probe was able to locate 79 SNs (74.5%). When the gamma-ray detection option of the same probe was used, this number increased to 99 SNs (93.3%). Ex vivo analysis revealed fluorescence in 93.3% of the excised nodes and radioactivity in 95.2%.
Conclusions
This study underlines the feasibility of using the dual-tracer/opto-nuclear probe combination for SN resections. The use of the opto-nuclear technology has been extended to laparoscopic surgery. This study also underlines the fluorescence tracing can complement traditional radio-tracing approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sentinel node (SN) biopsy was introduced for melanoma and breast cancer in the last years of the twentieth century. Since then, this technique has also significantly improved the staging of other solid tumors (e.g. penile, vulvar and oral cavity) as well as applied in other malignancies like urological, gynaecological and gastrointestinal cancers. In all cases, the use of SN biopsy helps reduce the surgical morbidity caused by nodal dissection in patients free of lymphatic metastases [1,2,3,4,5,6].
Current guidelines advocate the use of combined detection techniques (blue dye + radiotracer) to achieve optimal intraoperative SN identification and reduce the risk of false negatives [7, 8].
However, the use of a radiotracer alone is now a frequent issue in clinical practice due to the inferior performance of blue dyes and their cumbersome staining of the resection margins [9–11]. Although this procedure is straightforward in the SN identification of single SNs that are clearly separated from the injection site, the limited spatial resolution of gamma tracing may complicate nodal identifications in other cases. There are two main issues that could hamper the intraoperative SN detection: (a) the complexity of lymphatic drainage and the presence of important and delicate structures surrounding the lymph nodes, and (b) the proximity of the radiotracer’s injection site may mask the activity coming from the SN due to the high radioactive background (shine-through phenomenon) [12, 13].
Near-infrared (NIR) fluorescence guidance using the dye indocyanine green (ICG) was introduced in the operating room to improve the sensitivity of dye detection and to support the optical intraoperative identification of SNs [14, 15]. While fluorescence imaging offers a superior spatial resolution, tissue-induced signal attenuation means that—similar to the blue dyes—the utility of the fluorescence technology is confined to superficial lesions. The development and implementation of dual-tracers, which contain both radioactive and fluorescent signatures, directly connects traditional radioguidance with intraoperative fluorescence guidance [16, 17]. The value of the dual-tracer ICG-99mTc-nanocolloid has been demonstrated for SN biopsy and, in this context, fluorescence guidance yields a superior optical SN identification compared to blue dye [18,19,20,21,22,23].
Surgical modalities have evolved as result of the clinical implementation of ICG-99mTc-nanocolloid. Some hybrid modalities have been created to permit detection of both radioactive and fluorescence signatures. [24,25,26,27,28] One of these devices is now commercially available. This so-called opto-nuclear probe supports acoustic tracing of both signatures put forward by the hybrid tracer. After positive phantom and ex vivo studies, the first in vivo evaluation of a prototype for open surgery was performed in nine patients (penile and head and neck cancer) [24]. The aim of this follow-up study was to evaluate the surgical guidance capabilities of the opto-nuclear probe in a variety of different clinical indications and in different surgical scenarios (open and laparoscopic surgeries).
Methodology
Opto-nuclear probe
Two prototypes, one for open surgery and another for laparoscopy, opto-nuclear probes (Europrobe 3, Eurorad S.A., Eckbolsheim, France) with the combination of traditional gamma probe and a narrow-band 785 nm laser excitation source were used. The adjustable photomultiplier tube was set in 0.9 V (high-sensitivity mode) based on a previous experience [24].
The laparoscopic opto-nuclear probe, that fits in a 12–15 mm diameter laparoscopic trocar, was used in cervical cancer patients. In these specific cases, a dedicated laparoscope with near-infrared fluorescence imaging option (Karl Storz Image1 S™; Karl Storz, Tuttlingen, Germany) was also applied as an important part of our current SLN biopsy approach in cervical cancer in our institution [20].
Patients
Thirty-three patients scheduled for SN biopsy with the dual-tracer were retrospectively assessed for in vivo and ex vivo analysis. In all procedures, the opto-nuclear probe was used for intraoperative SN detection. There were 15 breast cancer patients, 7 melanoma patients, 2 oral cavity cases and 9 cervical cancer patients. The patients included in the current study presented several different pathologies from those 41 patients studied in a previous work (13 head and neck cancer, 23 penile cancer and 5 prostate cancer).
Informed written consent was obtained from all patients. The use of the tracer was approved by the IRB in a previously conducted study (HCB/2014/0404).
Hybrid tracer preparation and injection
ICG-99mTc-nanocolloid was prepared according to previously published protocols [17, 20, 29].
Patients attended the Nuclear Medicine Department the day prior to surgery. Dual-tracer administration was adapted to the type of lesion. Briefly, in melanoma and oral cavity tumor patients, a total dose of 148 MBq was split into four aliquots, 0.1 ml each, and intradermally administered surrounding tumor or biopsy scar. In breast cancer patients, a dose between 111 and 148 MBq in 0.3–0.5 ml was intratumorally administered. For cervical cancer patients, a dose of 111 MBq was injected periorificially into each quadrant of the cervix with four injections of 0.5 ml each).
Pre-operative imaging
Lymphoscintigraphy included an initial dynamic study (during 10 min after injection) in melanoma and oral cavity patients. In all patients, planar images (anterior and lateral views) were obtained at 30 and 120 min after tracer administration. In all the cervical cancer patients and the oral cavity patients a SPECT/CT scan (matrix 128 × 128 and 25 s per frame for SPECT) of the lymphatic draining anatomical region was performed after acquisition of the late planar image using a dual-head gamma camera (Infinia Hawkeye 4; GE Healthcare, Milwaukee, WI, USA). For melanoma and breast cancer patient indication of SPECT/CT was left to nuclear medicine physician decision based on the difficulty to identify the SN on planar images or to better depict its anatomical situation in cases with complex drainage. After SPECT/CT image reconstruction, volume-rendering images were generated using an OsiriX Dicom viewer (Pixmeo SARL, Geneva, Switzerland). Images were examined by two nuclear medicine physicians and discussed with the surgeon prior to surgery.
In the open surgery procedures the cutaneous projection of the SLN location was marked on the skin with indelible ink.
Surgery
After probe preparation (draping with sterile plastic bag in both, open and laparoscopic, procedures), the nuclear physician performed, intraoperatively, the measurements with the probe in open surgery cases. In laparoscopic procedures, the surgeons managed the laparoscopic opto-nuclear probe.
Incisions were placed based on skin markings that indicated the position of the SNs identified on lymphoscintigraphy. During the dissection the area defined in the pre-operative images was evaluated using the opto-nuclear probe. Initial fluorescence tracing lasted, approximately 30 s (in order to avoid a delay in the surgery time). After localization of the node or when no fluorescence signal could be clearly detected, the probe was switched to the gamma-tracing mode.
The fluorescent and radioactive activity of all the located nodes was assessed. Findings were scored for correct or incorrect prediction by the opto-nuclear probe. After excision of the SNs, ex vivo SN measurements were performed and evaluated by the surgeon and nuclear medicine staff as being radioactive (yes or no), and/or fluorescent (yes or no) (Fig. 1).
Pre-operative lymphoscintigraphy shows two faint hot spots in the right axilla of a breast cancer patient (upper row). After localization and excising of both SNs (middle row), ex vivo fluorescence and gamma-ray read-out was performed (lower row). Gamma tracing showed 30 cps in the first SN (3560 cps in the fluorescence modality “opto”). In the second case, the fluorescent component of the tracer (1040 cps) was of great help in order to ascertain a potential SN that may be missed by the gamma read-out (0 cps)
In all opto-nuclear determinations (except for in vivo laparoscopic approach), fluorescence tracing with the opto-nuclear probe was performed in ambient light. All measurements were repeated twice. Finally, surgeons and nuclear physicians were asked to rate the easiness of use and their impression about the device.
Pathological analysis
SLNs were pathologically evaluated by histological examination with haematoxylin and eosin staining of 2-mm frozen serial slices. All 106 SNs analyzed were formalin-fixed and paraffin-embedded. Delayed pathological evaluation included haematoxylin and eosin plus immunohistochemical staining with a broad-spectrum cytokeratin (Dako®, Glostrup, Denmark).
Results
Patients
For patients’ characteristics, see Table SI1. The majority of patients were women (n = 29). Patient’s mean age was 52.7 ± 12.7 (median 50, range 30–76) years. Their median body mass index was 24.6 kg/m2 (range 19.7–35.4).
Overall results
One or more SNs were identified in all patients (identification rate 100%). The median number of harvested SNs was 3 (range 1–7; Table SI1).
Planar lymphoscintigraphic images showed 95 hot spots considered as SNs (23 for melanoma, 33 in breast cancer, 36 in cervical cancer and 3 for head and neck cancer patients). SPECT/CT was performed in 18 out of 33 patients. The number of visualized SNs increased to 103 although breast cancer patients were not explored with this imaging technique and the number of SN nodes remained equal than the planar images in this subgroup of patients).
Prior to incision, scanning with the opto-nuclear probe on the skin position where the nuclear medicine physician marked the location of the SN clearly detected radioactive activity in 24/24 patients. Prior to incision only a fluorescence signal could be detected in 1/24 patients. In this oral cancer cavity patient, a high focal uptake was identified in a very superficial left level II cervical node.
During surgery, 103 SNs were excised. Three additional nodes were resected following palpation and the surgeon defining them as suspicious (hard touch). This yielded a total of 106 resected SNs. In vivo, the fluorescence mode of the probe was able to locate 79/106 SNs (74.5%). When conventional gamma-ray detection was used 99/106 SNs (93.3%) were identified under the same conditions. In two breast cancer patients, the fluorescence signal allowed to identify SNs that only presented a faint radioactive signature (less than 5 cps).
In those cases where open surgery was performed (melanoma, breast and head and neck cancers), fluorescence tracing was able to in vivo locate 83% of SNs. The fluorescence based find-rate in vivo in the laparoscopic setting was 62%, substantially lower than the open surgery approach. In both cases radiotracing proved to be more reliable (see Table 1 and supplementary material).
In our series, the mean BMI was an acceptable 24.6 kg/m2 and did not seem to impair nodal identification, even not in patients with a higher BMI (cases 2, 13, 21, and 28). In some cases, however, the number of SNs identified by the fluorescence tracing in vivo was lower than for radiotracing (more than 2 SNs difference; cases 5, 10, 22, 23 and 24). The BMI was not significatively high, except in 2 cases (23 and 24).
After surgical removal, all samples were evaluated ex vivo to validate if the in vivo finding accurately presented the presence of both signatures. Ex vivo fluorescence identification in the SNs increased from 79 to 99 SNs (93.3%) and radioactivity increased from 99 to 101 SNs (95.2%). In two cases, 4 and 21, faint radioactive signatures meant fluorescence was the superior modality for the nodal identification.
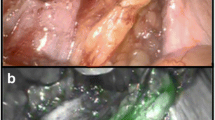
The Nuclear Medicine staff and surgeons that controlled the probes rated the utilization of the fluorescence tracing option as easy to use, comfortable and similar to a conventional gamma-ray detector probe. The fluorescence approach was judged as very sensitive when no tissue surrounds the SN. In this circumstance, especially when fat covers the SN (e.g. in laparoscopic approach), identifying the SN location using fluorescence tracing was complicated by tissue-induced signal attenuation and the difficulty of correctly positioning the optical fiber facing the SN (Figs. 2, 3). The gamma tracing ability of this modality was found to be equally reliable as conventional gamma probes.
Laparoscopic approach. Overall vision with the opto-nuclear probe positioned on the external iliac area (a) and a SN located over the grasper (b) and the fluorescent vision mode provided by the fluoroscope node (c). In this case, the use of fluorescence signal did not demonstrate a signal during in vivo scan, probably due to a combined tissue attenuation and erroneous positioning of the optical fiber in front of the SN. Gamma-ray lecture demonstrated a faint SN uptake (d). The importance of place the optical fiber (red arrow) right to the targeted tissue is crucial (e). When fluorescence option was ex vivo checked, with the optic fiber facing the SN, the tissue sample reached the limit of counts in the device (f)
Laparoscopic approach with the use of opto-nuclear probe (a). A faint fluorescent signal (1397 cps) was in vivo displayed on console (b). The increasing rate of fluorescence signal detection (10,936 cps) after ex vivo checking with the opto-nuclear probe (c) indicated that the tracer was not homogeneously distributed through the node, as demonstrated in the tissue sample after reading with near-infrared light laparoscope (d). This issue is important to demonstrate that learning curve is present and the probe must be slowly moved to reach the best signal
Pathological status
Of the 106 nodes resected, 5 of them showed macrometastases, two showed micrometastases and two presented isolated tumoral cells (Table 2). Positive nodes were isolated from 7 out of 33 (21.2%) patients. All metastatic-involved SNs presented both fluorescence and radioactivity.
The three suspicious nodes that were resected due to their hardness (and pathologically processed as SNs), were finally negative for metastasis.
Although follow-up time was scarce, no recurrences in pathologically negative SNs have been reported.
Discussion
The renewed technological diversification in the field of radioguided surgery has amongst other resulted in hybrid-concepts to be implemented in the clinic [30]. Hybrid-concepts combine two imaging signatures in a single tracer or modality. Next to the well know hybrid modalities such as SPECT/CT, PET/CT and PET/MRI activities in the areas of SPECT/optical and PET/optical are increasing. With the clinical introduction of the radioactive and fluorescent tracer ICG-99mTc-nanocolloid for SN identification, a platform has been generated for the development and clinical evaluation of surgical guidance technologies designed specifically to detect both signatures [17,18,19,20,21,22,23].
Previous studies demonstrated the in vivo feasibility of the opto-nuclear probe in 9 patients were the hybrid tracer ICG-99mTc-nanocolloid was used and in 21 breast cancer cases were separate injections of ICG and 99mTc-nanocolloid were applied [24, 31]. The current study expands these possibilities to other open-surgery approaches (melanoma and head and neck tumours), and explores the feasibility in laparoscopic approach (cervical cancer). Being familiar to the gamma probe technology, the adoption of this new device was judged positively by the surgeons and nuclear medicine staff (easiness of use, displaying panel and sound, sterile cover, etc.). The fact that fluorescence tracing could occur without dimming the lights in the operating theatre was also positively rated by the surgeons.
In line with the findings reported following the first in human introduction of the opto-nuclear probe, the current study underlines the viability of this modality and extends its use to the laparoscopic setting [24]. It, however, also confirms the previously reported limitations of fluorescence tracing in vivo, namely, the blockage of the fluorescence signal by tissues wherein the SNs were embedded. The effect asserted by attenuation by tissue was further underlined by the fact that prior to excision the fluorescence mode of the opto-nuclear probe was not able to reliably determine the fluorescence intensity. Also probe positioning is critical and the limited rotational freedom in laparoscopic procedures appears to limit this even further. This effect could explain the 62% fluorescence in vivo find rate in cervical cancer patients.
Logically, the time to in vivo identify a SN using the fluorescence-tracing mode was longer than the identification of the same node using the radio-tracing option. This is a direct result of the different detection sensitivities for the two respective signals: (1) fluorescence detection is limited by the size and off-center position of the optical fibers, as well as the signal attenuation asserted by tissue, (2) gamma-ray detection is more sensitive as a result of the larger detection surface, independence from illumination, and the ability of gamma photons to penetrate through tissues. As a result, in contrast to gamma tracing, the efficiency of fluorescence tracing is for a large part dependent on the user’s ability to quickly identify the areas most likely to harbor fluorescence. Although the opto-nuclear probe enabled the assessment of fluorescence within 30 s, the SN identification rate in vivo merely was 74.5% (gamma tracing achieved a 93.3% detection rate under the same conditions).
This indicates that the main value of fluorescence tracing is to complement the more traditional radioguidance concepts, but cannot replace it—as the use of the dual-tracer ICG-99mTc-nanocolloid was originally intended [32]. Hereby fluorescence can be used to confirm the superficial availability of the SNs. The additional value of fluorescence tracing was stressed by the two cases where in vivo fluorescence tracing was more reliable than radiotracing (Fig. 3).
Conclusions
This study underlines the feasibility of using the dual-tracer/opto-nuclear probe combination for SN resections. Thereby the use of the opto-nuclear technology has been extended to laparoscopic surgery. Critically, this study also underlines the fluorescence tracing can complement traditional radio-tracing approaches.
References
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE (2017) Sentinel Lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 35:561–564
Wong SL, Faries MB, Kennedy EB, Agarwala SS, Akhurst TJ, Ariyan C, Balch CM, Berman BS, Cochran A, Delman KA, Gorman M, Kirkwood JM, Moncrieff MD, Zager JS, Lyman GH (2018) Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American society of clinical oncology and society of surgical oncology clinical practice guideline update. Ann Surg Oncol 25:356–377
Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Jeffrey Lowery W, Rossi EC, Tanner EJ, Wolsky RJ (2017) Sentinel lymph node mapping and staging in endometrial cancer: a society of gynecologic oncology literature review with consensus recommendations. Gynecol Oncol 146:405–415
Oonk MHM, Planchamp F, Baldwin P, Bidzinski M, Brännström M, Landoni F, Mahner S, Mahantshetty U, Mirza M, Petersen C, Querleu D, Regauer S, Rob L, Rouzier R, Ulrikh E, van der Velden J, Vergote I, Woelber L, van der Zee AGJ (2017) European society of gynaecological oncology guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer 27:832–837
van der Poel HG, Meershoek P, Grivas N, KleinJan G, van Leeuwen FW, Horenblas S (2017) Sentinel node biopsy and lymphatic mapping in penile and prostate cancer. Urologe 56:13–17
Seim NB, Wright CL, Agrawal A (2016) Contemporary use of sentinel lymph node biopsy in the head and neck. World J Otorhinolaryngol Head Neck Surg 2:117–125
Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, Kunikowska J, Leidenius M, Moncayo VM, Uren RF, Oyen WJ, Valdés Olmos RA, Vidal-Sicart S (2013) The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging 40:1932–1947
Bluemel C, Herrmann K, Giammarile F, Nieweg OE, Dubreuil J, Testori A, Audisio RA, Zoras O, Lassmann M, Chakera AH, Uren R, Chondrogiannis S, Colletti PM, Rubello D (2015) EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur J Nucl Med Mol Imaging 42:1750–1766
Van den Berg NS, Buckle T, Kleinjan GI, Klop WM, Horenblas S, Van Der Poel HG, Valdés-Olmos RA, Van Leeuwen FI (2014) Hybrid tracers for sentinel node biopsy. Q J Nucl Med Mol Imaging 58:193–206
Sadeghi R, Alesheikh G, Zakavi SR, Fattahi A, Abdollahi A, Assadi M, Jangjoo A, Keshtgar M (2014) Added value of blue dye injection in sentinel node biopsy of breast cancer patients: do all patients need blue dye? Int J Surg 12:325–328
Guo J, Yang H, Wang S, Cao Y, Liu M, Xie F, Liu P, Zhou B, Tong F, Cheng L, Liu H, Wang S (2017) Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 15:196
Broglie MA, Stoeckli SJ (2011) Relevance of sentinel node procedures in head and neck squamous cell carcinoma. Q J Nucl Med Mol Imaging 55:509–520
Buscombe J, Saad Z (2015) Sentinel nodes: a promise half fulfilled. Clin Transl Imaging 3:169–170
Murawa D, Hirche C, Dresel S, Hünerbein M (2009) Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 96:1289–1294
Tagaya N, Aoyagi H, Nakagawa A, Abe A, Iwasaki Y, Tachibana M, Kubota K (2011) A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg 35:154–158
van Leeuwen FWB, Hardwick JCH, van Erkel AR (2015) Luminescence based imaging approaches in the field of interventional molecular imaging. Radiology 276:12–29
Brouwer OR, Buckle T, Vermeeren L, Klop WM, Balm AJ, van der Poel HG, van Rhijn BW, Horenblas S, Nieweg OE, van Leeuwen FW, Valdés Olmos RA (2012) Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J Nucl Med 53:1034–1040
Brouwer OR, van den Berg NS, Matheron HM, van der Poel HG, van Rhijn BW, Bex A, van Tinteren H, Valdés Olmos RA, van Leeuwen FW, Horenblas S (2014) A hybrid radioactive and fluorescent tracer for sentinel node biopsy in penile carcinoma as a potential replacement for blue dye. Eur Urol 65:600–609
van den Berg NS, Brouwer OR, Schaafsma BE, Matheron HM, Klop WM, Balm AJ, van Tinteren H, Nieweg OE, van Leeuwen FW, Valdés Olmos RA (2015) Multimodal surgical guidance during sentinel node biopsy for melanoma: combined gamma tracing and fluorescence imaging of the sentinel node through use of the hybrid tracer indocyanine green-Tc-nanocolloid. Radiology 275:530–537
Paredes P, Vidal-Sicart S, Campos F, Tapias A, Sánchez N, Martínez S, Carballo L, Pahisa J, Torné A, Ordi J, Carmona F, Lomeña F (2017) Role of ICG-(99m)Tc-nanocolloid for sentinel lymph node detection in cervical cancer: a pilot study. Eur J Nucl Med Mol Imaging 44:1853–1861
Stoffels I, Dissemond J, Pöppel T, Schadendorf D, Klode J (2015) Intraoperative fluorescence imaging for sentinel lymph node detection: prospective clinical trial to compare the usefulness of indocyanine green vs technetium Tc 99m for identification of sentinel lymph nodes. JAMA Surg 150:617–623
Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ, Frangioni JV, van de Velde CJ, van Leeuwen FW, Vahrmeijer AL (2013) Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg 100:1037–1044
Christensen A, Juhl K, Charabi B, Mortensen J, Kiss K, Kjær A, von Buchwald C (2016) Feasibility of real-time near-infrared fluorescence tracer imaging in sentinel node biopsy for oral cavity cancer patients. Ann Surg Oncol 23:565–572
van den Berg NS, Simon H, Kleinjan GH, Engelen T, Bunschoten A, Welling MM, Tijink BM, Horenblas S, Chambron J, van Leeuwen FW (2015) First-in-human evaluation of a hybrid modality that allows combined radio- and (near-infrared) fluorescence tracing during surgery. Eur J Nucl Med Mol Imaging 42:1639–1647
KleinJan GH, Hellingman D, van den Berg NS, van Oosterom MN, Hendricksen K, Horenblas S, Valdes Olmos RA, van Leeuwen FW (2017) Hybrid surgical guidance: does hardware integration of γ- and fluorescence imaging modalities make sense? J Nucl Med 58:646–650
KleinJan GH, van den Berg NS, van Oosterom MN, Wendler T, Miwa M, Bex A, Hendricksen K, Horenblas S, van Leeuwen FW (2016) Toward (hybrid) navigation of a fluorescence camera in an open surgery setting. J Nucl Med 57:1650–1653
Hellingman D, Vidal-Sicart S, de Wit-van der Veen LJ, Paredes P, Valdés Olmos RA (2016) A new portable hybrid camera for fused optical and scintigraphic imaging: first clinical experiences. Clin Nucl Med 41:e39–e43
Bugby SL, Lees JE, Perkins AC (2017) Hybrid intraoperative imaging techniques in radioguided surgery: present clinical applications and future outlook. Clin Transl Imaging 5:323–341
van Leeuwen AC, Buckle T, Bendle G, Vermeeren L, Valdés Olmos R, van de Poel HG, van Leeuwen FW (2011) Tracer-cocktail injections for combined pre- and intraoperative multimodal imaging of lymph nodes in a spontaneous mouse prostate tumor model. J Biomed Opt 16:016004
Valdés Olmos RA, Vidal-Sicart S, van Leeuwen FW (2016) Crossing technological frontiers in radioguided intervention. Eur J Nucl Med Mol Imaging 43:2301–2303
Poumellec MA, Dejode M, Figl A, Darcourt J, Haudebourg J, Sabah Y, Voury A, Martaens A, Barranger E (2016) Sentinel node detection using optonuclear probe (gamma and fluorescence) after green indocyanine and radio-isotope injections. Gynecol Obstet Fertil 44:207–210
van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW (2011) Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol 60:826–833
Acknowledgements
We deeply thank Eurorad for supporting the clinical evaluation by providing an opto-nuclear device.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vidal-Sicart, S., Seva, A., Campos, F. et al. Clinical use of an opto-nuclear probe for hybrid sentinel node biopsy guidance: first results. Int J CARS 14, 409–416 (2019). https://doi.org/10.1007/s11548-018-1816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-018-1816-5