Abstract
Purpose
To evaluate the lumbar nerve root alterations in patients with lumbar disc herniation sciatica using advanced multimodality MRI sequences and the correlations with clinical and neurophysiological findings.
Material and methods
We prospectively evaluated 45 patients suffering from unilateral lumbar radiculopathy due to disco radicular conflict. All patients underwent MRI examinations using a standard MRI protocol and additional advanced MRI sequences (DWI, DTI, and T2 mapping sequences). Relative metrics of ADC, FA, and T2 relaxation times were recorded by placing ROIs at the pre-, foraminal, and post-foraminal level, either at the affected side or the contralateral side, used as control. All patients were also submitted to electromyography testing, recording the spontaneous activity, voluntary activity, F wave amplitude, latency, and motor evoked potentials (MEP) amplitude and latency, both at the level of the tibialis anterior and the gastrocnemius. Clinical features (diseases duration, pain, sensitivity, strength, osteotendinous reflexes) were also recorded.
Results
Among clinical features, we found a positive correlation of pain intensity with ADC values of the lumbar nerve roots. The presence of spontaneous activity was correlated with lower ADC values of the affected lumbar nerve root. F wave and MEP latency were correlated with decreased FA values at the foraminal level and increased values at the post-foraminal level. The same neurophysiological measures correlated positively with pre-foraminal T2 mapping values and negatively with post-foraminal T2 mapping values. Increased T2 mapping values at the foraminal level were correlated with disease duration.
Conclusions
Evaluation of lumbar nerve roots using advanced MRI sequences may provide useful clinical information in patients with lumbar radiculopathy, potentially indicating active inflammation/myelinic damage (DTI, T2 mapping) and axonal damage/chronicity (DWI).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain caused by intervertebral disc (IVD) degeneration and/or lumbar disc herniation is one of the most prevalent musculoskeletal disorders associated with significant disability and economic impact. Magnetic resonance imaging (MRI) is the modality of choice for diagnosing degenerative changes in the intervertebral disc and evaluating the integrity of the affected nerve roots.
In recent years, several quantitative advanced MRI techniques have emerged and have been proved to be feasible and reproducible for evaluating the nerve roots. Among them, the ADC and DWI sequences are helpful techniques for evaluating restricted diffusivity in the affected nerve roots. Specifically, they provide image contrast based on differences in the magnitude of diffusion of water molecules within the affected nerve fibres. Interestingly, Eguchi et al. in 2010 already found a positive link between oedema, demyelination, and the increase in the mean ADC values due to radicular compression [1].
On the contrary, the DTI sequence analyses the three-dimensional shape of the diffusion and is mainly used to evaluate the axonal organization. Finally, T2 mapping is another well-established technique used for the ultrastructural evaluation of the collagen matrix of the articular cartilage, that has been recently proposed to evaluate the water and macromolecule content of the nerves.
In addition to the MRI, the Electromyography (EMG) represents another valuable tool for assessing the nerve conduction of the affected nerve roots, by recording the electrical activity produced by skeletal muscle.
The purpose of our study was to correlate the data obtained with the advanced MRI sequences with the main EMG findings and analyse the main stages of nerve root damage.
Material and methods
Patients
For this prospective cohort study, we recruited 45 patients (30 males, 15 females, mean age 59, range 49–70) presenting with L5–S1 discopathy at previous MRI examinations, who met the clinical and instrumental inclusion criteria for percutaneous paraganglionic CT-guided O2–O3 and received the clinical indication for EMG evaluation. These patients underwent a new MRI examination with the advanced sequences listed below.
Exclusion criteria for both groups included the following: age > 70 years, multiple level pathology, previous spine surgery, recent percutaneous interventional procedures, spinal trauma, surgery, or other treatments during the follow-up period. Written informed consent was obtained from all patients who participated in this study. All patients were submitted to clinical and imaging evaluation within 1–7 days before or after the MRI.
Clinical evaluation
All patients underwent clinical evaluation using the visual analogue score (VAS) for pain and the Oswestry Disability Index (ODI) questionnaire for pain and functionality. The ODI is a self-administered questionnaire that objectively evaluates the disability derived from the LBP. In particular, each question assesses the intensity of pain, the ability to lift and care for oneself, ability to work, sit, stand, travel, and have sexual intercourse. It also estimates the impact of the disease on social life and work. Each of the ten questions is scored from 0 to 5, giving a maximum score of 50.
In addition, time of onset, the side affected, referred pain, strength (MRC—Muscle Strength score 0–5), sex, age, increased or decreased ankle and patellar jerk reflex, and muscular trophism were also evaluated.
MRI protocol
All MRI examinations were performed using a 3T MRI scanner (MR 750W, GE Healthcare). The imaging protocol included an axial FSE T2-weighted sequence (FOV 17 cm, TR/TE/NE.
X 4000/108/4, matrix 256 × 192, slice thickness 5 mm, gap 1 mm), sagittal FSE-T2 and sagittal T2 fat-saturated sequences (FOV 26 cm, TR/TE/NEX 4000/108/4, matrix 256 × 192, slice thickness 5 mm, gap 1 mm), and a sagittal SE-T1sequence (TR/TE/NEX 500/20/2, matrix 256 × 192, slice thickness 5 mm, gap 1 mm).
-
SagT2 (body coil 48, FOV 30).
-
Cor T2 ideal (FOV 35 ST:1, S:0.1, Matrix 320 × 256 Nex: 2, body coil 48).
-
Ax T2 Map (FOV 22, Phase FOV 0.80 ST: 3.0, S: 0.0, TR: 1600, Matrix:256/192).
-
Ax T2 FRFSE (ST:3, S:0, TR:5811, TE:102, NEX: 2, Matrix: 288 × 224, scan time: 2:14).
-
Ax DTI (FOV 22, phase 0.8, TR: 11,084, TE: 96.6, B-value: 800, ST: 1.5, spacing: 0, n of slices: 39, scan time: 6:17).
-
Ax DWI (FOV 22, phase: 0.8, ST: 3, S: 0, TR: 6900, TE: 70, NEX: 6, B-value: 600, matrix: 128 × 128, scan time: 3:34).
Images were then loaded on a dedicated workstation, in which the sequences were processed with specific algorithms for statistical analysis.
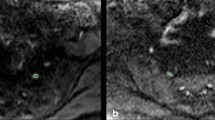
Mean values of ADC, FA, and T2 mapping were calculated by placing the ROIs of 5 mm at the preforaminal, intraforaminal, and postforaminal level of the affected root and the healthy one, as displayed in Fig. 1.
Intramuscular EMG recording
EMG recording was performed by an expert Neurophysiologist of the San Salvatore Hospital in L’Aquila.
Intramuscular EMG recording electrodes were inserted into the anterior tibialis muscle (L5, common peroneal nerve) and the gastrocnemius muscle (S1, internal sciatic popliteal nerve).
The selected measurements were as follows:
-
Spontaneous activity (index of acute injury; 0: absent, 1: mild, 2: moderate, 3: severe).
-
Voluntary activity (I = interference; optimal contraction, many motor units activated. T = Transition. SO = single oscillations; absent, mild, moderate, severe; enthity = extent of injury).
-
Large motor units/% Motor Unit Potential—MUP (SI = index of chronic injury).
Nerve conduction study
On the same nerves, a nerve conduction study was performed by the same neurophysiologists. Some important findings were acquired such as:
F-Wave: uses supramaximal stimulation of a motor nerve and recording of action potentials from a muscle supplied by the nerve. The action potential travels from the site of the stimulating electrode in the limb to the spinal cord's ventral horn and back to the limb in the same nerve that was stimulated. Increased latency time is correlated with myelinic damage, whereas reduced amplitude is linked to axonal injury. Both of them are representatives of acute radicular damage.
M-Wave is an early response that occurs 3–6 ms after the onset of stimulation, generated by the direct nerve stimulus. In this case, increased latency correlates with the myelin damage on the distal nerve of the affected root, whereas the amplitude is inversely related to the axonal damage. F-wave/M-wave latency ratio: prolonged F-wave latency compared to m wave. The study of the M-wave was mainly conducted to exclude the trinocular damage of the nerves, and therefore to differentiate peripheral neuropathic pain from the central one.
Statistical analysis
MRI quantitative and qualitative parameters (ADC pre-, intra-, postforaminal; FA pre-, intra-, postforaminal; T2 mapping pre-, intra-, postforaminal) of the affected root were correlated with EMG parameters, considering as markers of acute radicular damage the F-wave amplitude reduction and the F-wave latency increase; markers of subacute radicular damage (14 days, 2 months) the spontaneous activity (fibrillation); markers of radicular chronic the presence of single oscillation during voluntary activity, large motor unit (denervation), M-wave latency increase and M-wave amplitude decrease.
A p value < 0.05 was considered significant. Pearson correlation was applied to evaluate the statistical correlation between the MRI, Clinical, and EMG parameters.
Results
Clinical parameters
Among the 45 patients selected, 30 were male and 15 females, with a mean age of 59 years (min: 49, max: 70). The mean disease duration was 105 days (min: 60, max: 150), and the mean VAS was 8.5 (min: 7, max: 10). The interested root was L5 in 35% of cases (16 patients) and S1 in 65% (29 patients), with the left side being affected in 50%. In 91% of cases, EMG confirmed the side affected at the MRI morphological evaluation, whereas 9% of subjects showed an opposite side at the EMG evaluation. In these patients, the ROIs were placed in the nerve root where the EMG parameters were abnormal.
Mean strength, on a scale from 1 to 5, was 4.4 (min: 4, max: 5), with 50% of them showing a reduction in the patellar and ankle jerk reflex and 25% (5 patients) reduction in muscular trophism.
EMG
EMG parameters were evaluated in the anterior tibialis muscle. In particular, spontaneous activity at rest was absent in all these patients. 75% of them presented with single oscillations during voluntary contraction, whereas 25% presented with interference; in addition, 50% showed large motor units. Mean m-wave latency in this group was 5.6 ms (min: 4.8 ms; max 6.5), while mean m-wave amplitude was 6.4 mV (min: 2.6 mV; max 12.7 mV). Mean F-wave latency was 56.17 ms (min: 54.4 ms; max 57.9 ms), whereas mean F-wave amplitude was 0.42 mV (min: 0.1 mV; max 0.9 mV). F-wave/m-wave latency ratio was 10.14 (min: 8.6; max: 12.6).
In patients with S1 root involvement (29 patients), EMG parameters were also evaluated in the gastrocnemius muscle. In particular, only one patient presented with spontaneous activity at rest. 65% of them presented with single oscillations during voluntary contraction, whereas 35% presented with interference; none showed large motor units. Mean m-wave latency in this group was 5.7 ms (min: 4.5 ms; max 6.6), while mean m-wave amplitude was 7.6 mV (min: 3.6 mV; max 11.7 mV). Mean F-wave latency was 45.17 ms (min: 30 ms; max 53.3 ms), whereas mean F-wave amplitude was 0.23 mV (min: 0.03 mV; max 0.7 mV). F-wave/m-wave latency ratio was 8.01 (min: 4.76; max: 9.48).
MRI evaluation
From the MRI analysis, be it at the L5 and S1level, there was a significant reduction in the mean ADC value on the side affected compared to the other side; in particular preforaminal values were 1.381 ± 0.316 versus 1.656 ± 0.604 × 10−3 mm2/s (p value = 0.031); intraforaminal 1.266 ± 0.221 versus 1.271 ± 0.310 × 10−3 mm2/s (p value = 0.238); postforaminal 1.193 ± 0.330 versus 1.264 ± 0.219 × 10−3 mm2/s (p value = 0.031).
Similarly, mean FA value was significantly reduced in the affected root compared to the other side. Preforaminal FA values were 0.356 ± 0.06 versus 0.406 ± 0.13 (p value = 0.8); intraforaminal 0.367 ± 0.17 versus 0.316 ± 0.06 (p value = 0.238); postforaminal 0.401 ± 0.11 versus 0.456 ± 0.07 (p value =).
Conversely, mean t2 mapping values were higher in the affected side compared to the contralateral. Mean T2 mapping values were in the preforaminal ROIs 62.2 ms ± 5.7 versus 61.12 ± 544 ms (p value = 0.07); intraforaminal ones 66.2 ms ± 5.4 versus 64.01 ± 4.58 ms (p value = 0.45); postforaminal 60.6 ms ± 11.7 versus 62.03 ± 2.53 ms (p value = 0.45). The main findings for each MRI sequence are reported in Table 1.
Morphologic MRI assessment demonstrated kinking of the affected root in 50% of cases, whereas healthy roots showed normal appearance and a diameter of the DRG of about 6 mm in all cases.
MRI and clinical parameters correlation
Disease duration and mean ADC values were inversely correlated. On the contrary, pain intensity significantly correlated with the mean ADC values. Finally, there were no significant correlations between FA and T2 mapping values and clinical parameters as well as between MRI parameters and strength, muscular tropism, and tendon reflexes. Table 2 synthesizes the main findings.
MRI and EMG parameters correlation
EMG parameters linked with acute damage of the nerve roots (f-wave amplitude reduction, f-wave latency growth) significantly correlated with the decrease in the mean ADC values. In addition, FA values positively increased with the M-wave amplitude, although this did not reach statistical significance. There was no correlation between T2 mapping values and the EMG parameters (Tables 3, 4, 5).
Subacute damage indicators: single oscillations (fibrillation) during spontaneous activity correlated with the mean ADC values increase. Similarly, the same appeared with the mean T2 mapping values.
Chronic damage indicators: a positive correlation between mean ADC values and abnormal voluntary activity and the large motor unit was observed, whereas a negative correlation was found with the mean FA values. In addition, a positive correlation was also found between abnormal voluntary activity, large motor unit, and T2 mapping values increase. An inverse correlation was found between M-wave latitude increase and mean ADC and T2values. Finally, M-wave amplitude reduction correlated with the mean ADC and T2 mapping values.
Discussion
Several reports have shown the feasibility and the reproducibility of quantitative advanced MRI techniques for evaluating the nerve root pathology [2,3,4,5,6,7,8]. Among them, the ADC and DWI sequences are helpful techniques for quantifying restricted diffusivity in the affected nerve roots.
From our MRI analysis, there was a significant reduction in the mean ADC values on the affected root compared to the healthy one: preforaminal 1.381 ± 0.316 versus 1.656 ± 0.604 × 10−3 mm2/s (p value = 0.031); intraforaminal 1.266 ± 0.221 versus 1.271 ± 0.310 × 10−3 mm2/s (p value = 0.238); postforaminal 1.193 ± 0.330 versus 1.264 ± 0.219 × 10−3 mm2/s (p value = 0.031). Nevertheless, these results were not consistent with those reported in the literature, with some supporting the data [7, 9, 10] and others rejecting them [1]. A possible explanation may depend on the different observation times of the studies. Indeed, as observed by MacDonald, the mean diffusivity value decreases between 6 h and 4 days since the injury, due to cytotoxic oedema, while it increases between the 1st and 4th week, due to vasogenic oedema and demyelination [11].
Besides, mean FA value showed lowest scores in the affected root compared to the healthy one: preforaminal 0.356 ± 0.06 versus 0.406 ± 0.13 (p value = 0.8); intraforaminal 0.367 ± 0.17 versus 0.316 ± 0.06 (p value = 0.238); postforaminal 0.401 ± 0.11 versus 0.456 ± 0.07 (p value = 0.03). This is likely associated with axonal injury and nerve root compression, in which the presence of local extracellular oedema and the reduced numbers of fibres results in increased extracellular space [6, 12, 13].
Finally, mean T2 values were higher in the affected root, namely: preforaminal 62.2 ms ± 5.7 versus 61.12 ± 5.44 ms (p value = 0.07); intraforaminal 66.2 ms ± 5.4 versus 64.01 ± 4.58 ms (p value = 0.45); postforaminal 60.6 ms ± 11.7 versus 62.03 ± 2.53 ms (p value = 0.45) due to the higher water content (oedema) in the affected root [14, 15].
We observed an indirect correlation between disease duration and the mean ADC values, due to the probable oedema reduction in the advanced stages of the disease. On the contrary, pain significantly correlated with the mean ADC values, confirming its predictive role as an imaging biomarker in the acute stages of the illness.
Perhaps the most clinically relevant findings are those related to the EMG evaluation.
One unanticipated result was that EMG parameters of acute damage of the affected root (F-wave reduction, F-wave latency increase) significantly correlated with the mean ADC values. This may be due to cytotoxic oedema in this stage and, therefore, to the isotropic diffusivity restriction, as observed by MacDonald [11]. No significant correlation was found between the T2 mapping values and the EMG parameters, probably because cytotoxic oedema is prevalent in this stage, and it did not determine any T2 mapping changes.
Subacute damage (14 days, 2 months) is characterized by single oscillation (fibrillation) at the spontaneous activity at rest. This parameter significantly correlated with the mean ADC values, supporting the idea that vasogenic oedema and/or demyelination with increased diffusivity are prevalent in this stage. Another critical association was found between spontaneous activity and the T2 mapping values, which progressively increase with intraradicular oedema development. There was no relationship between spontaneous activity and mean FA values.
Chronic damage is characterized by EMG evidence of denervation and axonal injury, such as single oscillations during voluntary activity, large motor unit, increase in the M wave latency, and reduction in m wave amplitude. There was a significant correlation between mean ADC values and abnormal voluntary activity and large motor unit, whereas there was an inverse correlation with the FA values. This was supported by in-vivo studies on animals that found the ADC increase and FA reduction with the histological and functional ultrastructural modification, namely: demyelination, axonal density reduction, and extracellular space increase [16]. Similarly, there was a significant correlation between abnormal voluntary activity, large motor unit, and the rise of the t2 mapping values, underlying the strong relationship between these EMG parameters and intraradicular oedema.
In the chronic stages, we found an M wave amplitude reduction associated with M wave latency augmentation. This may be due to the Wallerian degeneration that extends slowly and distally to the axonal damage, involving the peripheric nerve [17].
An inverse correlation was observed between M wave latency raise and the mean ADC values, which was the same as the t2 mapping values. This may be associated with the progressive intraradicular oedema reduction in the chronic stages. We observed the same trend for the M wave amplitude reduction that significantly correlated with the ADC and T2 mapping values. Fig. 2 summarizes the main changes in the advanced MRI sequences during the clinical course of lumbar radiculopathy.
Conclusions
Using advanced MRI sequences, evaluation of lumbar nerve roots may provide useful clinical information in patients with lumbar radiculopathy, potentially indicating active inflammation/myelinic damage (DTI, T2 mapping) and axonal damage/chronicity (DWI).
References
Eguchi Y, Ohtori S, Yamashita M et al (2010) Clinical applications of diffusion magnetic resonance imaging of the lumbar foraminal nerve root entrapment. Eur Spine J 19:1874–1882. https://doi.org/10.1007/s00586-010-1520-9
Perri M, Grattacaso G, Di Tunno V et al (2015) MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2–O3 discolysis. Neuroradiol J 28:198–204. https://doi.org/10.1177/1971400915576658
Perri M, D’Elia M, Castorani G et al (2020) Assessment of lumbar disc herniaton using fractional anisotropy in diffusion tensor imaging along with conventional T2-weighted imaging. Neuroradiol J 33:24–31. https://doi.org/10.1177/1971400919891288
Bruno F, Palumbo P, Tommasino E et al (2019) Evaluation of intervertebral disc using T2 mapping sequences in patients undergoing O2–O3 chemiodiscolysis: an instrumental study with clinical correlation. Neuroradiology. https://doi.org/10.1007/s00234-019-02308-8
Zhang J, Zhang F, Xiao F et al (2018) Quantitative evaluation of the compressed L5 and S1 nerve roots in unilateral lumbar disc herniation by using diffusion tensor imaging. Clin Neuroradiol 28:529–537. https://doi.org/10.1007/s00062-017-0621-9
Miyagi R, Sakai T, Yamabe E, Yoshioka H (2015) Consecutive assessment of FA and ADC values of normal lumbar nerve roots from the junction of the dura mater. BMC Musculoskelet Disord 16:4–9. https://doi.org/10.1186/s12891-015-0576-4
Li J, Wang Y, Wang Y et al (2016) Study on lumbosacral nerve root compression using DTI. Biomed Rep 5:353–356. https://doi.org/10.3892/br.2016.734
Wu W, Liang J, Chen Y et al (2017) Microstructural changes are coincident with the improvement of clinical symptoms in surgically treated compressed nerve roots. Sci Rep 7:1–9. https://doi.org/10.1038/srep44678
Eguchi Y, Ohtori S, Yamashita M et al (2011) Diffusion-weighted magnetic resonance imaging of symptomatic nerve root of patients with lumbar disk herniation. Neuroradiology 53:633–641. https://doi.org/10.1007/s00234-010-0801-7
Takashima H, Takebayashi T, Yoshimoto M et al (2013) Efficacy of diffusion-weighted magnetic resonance imaging in diagnosing spinal root disorders in lumbar disc herniation. Spine 38:998–1002. https://doi.org/10.1097/BRS.0b013e31829862d3
Mac Donald CL, Dikranian K, Bayly P et al (2007) Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 27:11869–11876. https://doi.org/10.1523/JNEUROSCI.3647-07.2007
Delgado-López PD, Rodríguez-Salazar A, Martín-Alonso J, Martín-Velasco V (2017) Lumbar disc herniation: Natural history, role of physical examination, timing of surgery, treatment options and conflicts of interests. Neurocirugia 28:124–134. https://doi.org/10.1016/j.neucir.2016.11.004
Liheng M, Guofan X, Balzano RF et al (2021) The value of DTI: achieving high diagnostic performance for brain metastasis. Radiol Med 126:291–298. https://doi.org/10.1007/S11547-020-01243-6
Shen S, Wang H, Zhang J et al (2016) Diffusion weighted imaging, diffusion tensor imaging, and T2* mapping of lumbar intervertebral disc in young healthy adults. Iran J Radiol 13:1–9. https://doi.org/10.5812/iranjradiol.30069
Sato T, Eguchi Y, Norimoto M et al (2020) Diagnosis of lumbar radiculopathy using simultaneous MR neurography and apparent T2 mapping. J Clin Neurosci 78:339–346. https://doi.org/10.1016/j.jocn.2020.04.072
Morris et al (2012) (2015) 基因的改变NIH public access. Gerontology 61:515–525. https://doi.org/10.1002/nbm.2902.Diffusion
Chaudhry V, Cornblath DR (1992) Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve 15:687–693. https://doi.org/10.1002/MUS.880150610
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruno, F., Marrelli, A., Tommasino, E. et al. Advanced MRI imaging of nerve roots in lumbar radiculopathy due to discoradicular conflict: DWI, DTI, and T2 mapping with clinical and neurophysiological correlations. Radiol med 127, 1270–1276 (2022). https://doi.org/10.1007/s11547-022-01550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01550-0