Abstract
Purpose
A radioactive feeding tube was used to achieve both nutrition and brachytherapy for the treatment for malignant esophageal obstruction. We report the safety and effectiveness of this technique.
Methods
We conducted a retrospective analysis of 16 consecutive patients who employed this technique from January 2015 to March 2018. The radioactive feeding tube was made by binding the 125I seed chain on the feeding tube. Under fluoroscopic guidance, the tube was inserted into the obstructed esophagus, with the seed chain crossing over the segment of malignant esophageal obstruction. Technical success rate, dysphagia score, procedure time and complications were analyzed. Kaplan–Meier analysis was used to analyze the survival time.
Results
The radioactive feeding tube was easy to prepare. The technical success rate was 100%, without serious complications such as bleeding or infection. The median procedure time of tube insertion was 44.0 min. The Kamofsky score and Neuhaus dysphagia grading were significantly improved after tube insertion (p < 0.01). On esophageal radiography, the contrast agent passed through the narrow area smoothly. Complete remission (n = 1) and partial remission (n = 13) of local tumor were obtained in 14 patients, and the local tumor control rate was 87.5% (14/16). During follow-up, four patients survived with no obvious clinical symptom and 10 patients died of cancer. The median survival was 12.0 months.
Conclusion
Preparation of the radioactive feeding tube is simple and easy. The insertion of this kind of tube achieves parenteral nutrition and brachytherapy simultaneously and is safe and effective in dysphagia palliation of malignant esophageal stricture. The radiological-radiotherapeutic procedure could be an alternative tool in the case of refusing other treatments by the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer often shows high rates of morbidity and mortality, and poor prognosis, because half of patients are unable to be resected at the time of diagnosis [1, 2]. About half of patients need palliative treatment to relieve dysphagia caused by malignant obstruction. Self-expanding metal stent (SEMS) placement is the main palliative treatment for patients with advanced esophageal cancer complicated by malignant obstruction [3,4,5,6,7]. Different kinds of SEMS are used clinically [8,9,10,11,12]; unfortunately, and some patients cannot tolerate esophageal stenting for a variety of reasons. These patients include those with a Kamofsky score less than 60, patients who are prone to stent migration due to severe cardiac curvature, patients with severe dyspnea due to airway compression after placement of esophageal stents, and cervical esophageal cancer with fear of foreign somatosensory sensation after stent placement, and so on. In this study, a radioactive feeding tube was placed under fluoroscopic guidance, and a radioactive seed chain was placed across the occlusive segment of the esophagus for brachytherapy for the treatment for malignant obstruction caused by the esophageal cancer. We aimed to report the safety and effectiveness of the radioactive feeding tube in the palliation of esophageal malignant obstruction.
Materials and methods
Patient selection
This study was approved by the institutional ethics committee of our Hospital. Informed consent was obtained from all patients. From January 2015 to March 2018, all patients who underwent insertion of a radioactive feeding tube for malignant esophageal obstructions were retrospectively analyzed. Exclusion criteria included inability to obey follow-up and/or expected to survive no more than 3 months. We suggest that a nasal feeding tube is suitable for the following patients: (1) the thickness of esophageal carcinoma tumor is less than 3 mm; (2) the Karnofsky score of the patients is less than 60 and the patient cannot tolerate other anti-tumor treatment; (3) the patient has cancer of the esophagogastric junction and is prone to stent migration; (4) the patients has cervical esophageal carcinoma and is concerned about severe foreign sensation of stents; (5) patients shows tumor recurrence or proliferation in the ends of the stent after implantation; (6) there is tumor recurrence after radiotherapy or surgical resection; (7) the patients is unable to receive or refuse surgical treatment for various reasons.
Radioactive feeding tube
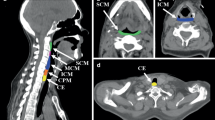
A computed tomography (CT) scan was performed routinely to show the tumor lesion site, obstruction degree and length (Fig. 1a). Digestive endoscopy was performed before tube insertion or during follow-up if necessary (Fig. 2). Preoperative esophagography was performed to measure the length of tumor occlusion and to estimate the length of seed chain and the number of seeds needed (Fig. 3a). A 3F catheter was used to contain 125I seeds, which were pushed into the catheter one by one by using a seed implantation gun. There is no filling material between the seeds. Then, both ends of catheter were sealed off to form the 125I seed chain. According to the length of the esophageal lesion, the two 125I seed chains were placed parallel to the gastrointestinal feeding tube. The feeding gastrointestinal feeding tube is made of medical polyurethane. (Product Code 11010, Beijing L&Z Medical Technology Development Co., Ltd., Beijing, China). The size of tube is about 130 cm in length and 4.5 mm in diameter. Parameters of 125I radioactive seeds (Atom High Tech, Beijing, China) are as follows: size 4.5 × 0.8 mm, mean photon energy 27–35 keV, half-life 60.1 days and tissue penetration 1.7 cm. The radioactivity of each seed is 0.80 mCi, and initial dosage rate is 7 cGy/h. According to the length of esophageal lesions, two seed chains were placed parallel to the gastrointestinal feeding tube, fixed with tape, and then fixed on the feeding tube with surgical suture. The seeds were fixed on both sides of the nasal feeding tube with a distance of 4 mm and an angle of 180°.
The insertion procedure of a radioactive feeding tube. a Anteroposterior esophagography shows severe stenosis in the upper esophagus. b A 5F single-curved catheter was introduced and passed through the lesion via a hard guide wire. c The delivery system of the radioactive feeding tube was introduced along the stiff guide wire. d The seed chain of the feeding tube was placed across the occlusive segment of the esophagus to perform continuous low-dose brachytherapy and esophagography confirmed that the tube was in good position
Feeding tube insertion
All procedures were performed under local anesthesia and fluoroscopic guidance with a floor-based flat panel Angio system (Artis Zeego, Siemens Healthineers, Erlangen, Germany). The patients were supine on the DSA examination table, with ECG monitoring and oxygen inhalation. Esophagography was performed with oral water-soluble iodine contrast agent to show the lesion site and the degree of esophageal stricture of esophagus (Fig. 3a). A 5F single-curved catheter (Cook Corporation, Bloomington, USA) was introduced with the guidance of a 0.035-in. hard guide wire. The feeding tube was passed through the nasal cavity, esophagus and the narrow occlusive segment into the gastroduodenum and jejunum (Fig. 3b). Along the hard guide wire, the self-made feeding tube was pushed slowly into the jejunum, and the seed chain of the feeding tube was placed across the occlusive segment of the esophagus to perform continuous low-dose brachytherapy (Fig. 3d). Fluoroscopy confirmed that the proximal end of the nasogastric feeding tube was located in the jejunum. At 3–5 days after seed chain implantation, the patients received nasal feeding.
Observations and definitions
Baseline demographics such as age, gender, procedure time, comorbidities and tumor site were collected. Technical success was defined as the successful manufacture of the feeding tube and placement of the tube into the tumor-occluded segment. Procedure time was defined as the time required for the preoperative radioactive feeding tube to complete the placement of the seed chain. Local tumor control was evaluated by the response evaluation criteria in solid tumors (RECIST). The local control rate was complete remission and partial remission. Main complications were perioperative death, esophageal perforation, severe infection and massive hemorrhage [5]. The dysphagia score was assessed and analyzed before and after insertion of the feeding tube. Chest CT and esophagography were reexamined every 1–2 months after discharge (Fig. 3d). Neuhaus dysphagia grade, contrast medium patency and tumor local condition were evaluated during follow-up.
Statistical analysis
Continuous variables were shown as means ± standard deviation or median with interquartile ranges (IQR). Kaplan–Meier analysis was used to analyze the survival time. The student’s t test was used for comparing dysphagia score by using Prism 5.0 software (GraphPad Software Inc, San Diego, Calif). Significance was taken at p < 0.05.
Results
A total of 16 patients were enrolled in this study, 11 males and five females, with a mean age of 65.9 ± 8.4. Median course of disease was 8.0 months (IQR 3.5–12.0). Thirteen patients showed esophageal carcinoma, two patients showed carcinoma of the esophagogastric junction, and one patient showed carcinoma of the pharynx. Six patients underwent surgical resection, and eight patients received chemotherapy and/or radiotherapy before radioactive feeding tube insertion (Table 1). Preoperative Karnofsky score was 50.0 (IQR 42.5–57.5); nine patients showed grade IV dysphagia, and seven patients showed grade III dysphagia. Preoperative esophagography showed severe stenosis in four patients and complete obstruction in 12 patients. The median length of the involved esophageal segment was 48.8 mm (IQR 24.8–58.8).
Insertion of radioactive feeding tubes was technically successful in all patients (100%). The median procedure time was 44.0 min (IQR 36.5–56.5). A median of 27.5 seeds (IQR 23.0–30.0) were used per patient. The removal of radioactive feeding tubes was performed in six patients due to dysphagia relief. Tube adjustment or replacement was performed in two patients. The median postoperative Karnofsky score was 65.0 points (IQR 60.0–70.0), and Neuhaus dysphagia score analysis showed grade 0 in six patients, grade I in eight patients, and grade II in two patients. There was a significant improvement compared with preoperative data (p < 0.01). Dysphagia score decreased significantly after radioactive feeding tube insertion (p < 0.0001).
Except for one case with missing follow-up data, 15 patients were successfully followed up for a median period of 12.0 months. Short-term follow-up within 3 months after the procedure showed that all of the contrast media passed smoothly. Complete remission was found in one patient, 13 patients showed partial remission, and two patients died of tumor progression. Local control was 87.5% (14/16), with no severe complications such as bleeding, infection or esophageal fistula (Table 2).
There were no perioperative deaths related to tube insertion or removal. By the end of the follow-up period, six patients were taking food by mouth after pulling out the nasogastric feeding tube due to the improvement of symptoms. During the follow-up period, four patients have survived, one patient died of heart failure at 9.6 months after the procedure, and 10 patients died of tumor progression at 1.1–47.8 months, including the aforementioned two deaths. The survival rates were 60.0%, 46.7% and 12.5% at 6, 12 and 36 months, respectively. The median survival was 12.0 months (Fig. 4).
Discussion
Self-expandable esophageal stent implantation is the main treatment for advanced esophageal cancer complicated by dysphagia [3,4,5,6,7, 13], but esophageal stents can only relieve symptoms and have no therapeutic effect on primary tumors. Complications such as stent restenosis, bleeding, stent migration and a strong sense of foreign matter after implantation are very difficult problems. Although the brachytherapy stent can achieve both symptom relief and a therapeutic effect [14], it is not suitable for some special patients, such as those who tend to stent migration due to the curvature of the cardiac structure or who have severe dyspnea due to tracheal pressure after stent placement [10]. For these patients, enteral nutrition is a common palliative treatment. We propose that it is possible to combine nasal feeding with brachytherapy to play a role of double carving with one stone.
Some researchers have begun to try to bind radioactive seeds to plastic tubes or drainage tubes to make seed chains and use them in the human cavity for short-range radiotherapy. Insertion of seed chains has been successfully applied to bile duct obstruction [15, 16]; portal vein cancer thrombus [17], and vena cava obstruction caused by tumor invasion [18], and has achieved a good clinical effect. Therefore, we speculated that the seed chain could be bound a nasal feeding tube for patients with advanced esophageal cancer who could not or refused to receive esophageal stents in order to achieve the dual objective of intraluminal brachytherapy for esophageal cancer and enteral nutrition. In addition, there is little space-occupying effect on the nasal feeding tube, and there is no need to worry about the occurrence of dyspnea caused by airway compression after esophageal stent placement, the massive bleeding caused by friction between the stent and the esophageal wall, or stent migration. If there are complications associated with brachytherapy, the nasal feeding tube can be removed at any time to stop radiotherapy. The radioactive feeding tube can be adjusted or replaced easily if migration happens.
Postoperative CT showed that the local wall was reduced in varying degrees, but the local wall was still thickened. Gastroscopic biopsy showed that the tumor remained, and the following reasons were considered: in the early stage of implantation, the effect of brachytherapy was good because the seeds were close to the lesion, but with tumor shrinkage, the lumen enlarged and the tumor and the seeds were not close to each other, which might affect the curative effect. Due to the decrease in the radiation dose of the seeds, the dose of irradiation to the local tumor was uneven. A three-row seed chain with an angle of 120° or a four-row seed chain with an angle of 90° may show a better therapeutic effect, but these seed chains require further clinical research.
Owing to the extra complexity of delivery compared with esophageal stents, brachytherapy may be insufficient to increase patient acceptance [19]. Sinha et al. compared brachytherapy to esophageal stent placement for relief of dysphagia and found that patients who received esophageal stents showed better improvements in dysphagia scores initially [20]. Their complications rate of stent placement was 33%, mainly due to late hemorrhage (13%). In our study, no perioperative death or severe complications such as bleeding, infection or esophageal fistula occurred during tube insertion or removal. Zhu et al. [14] found that patients who received esophageal stents loaded with radioactive iodine (125I) seeds had prolonged overall survival compared to those who received conventional covered stents. Median overall survival was 177 days and 147 days in the irradiation group and conventional stent group, respectively. In our study, the median survival was 12.0 months.
Our study has several shortcomings. It is a retrospective analysis of treatment for esophageal tumors with radioactive feeding tube in a single center and in a small series of patients; the study is lacking comparison with other palliative treatments. Experience with intranasal feeding tube placement for malignant esophageal occlusive lesions is still a preliminary. Whether long-term patients really benefit from survival, how many rows of seeds should be used for better efficacy, how to choose seed activity, and when to stop intracavitary radiotherapy remain to be solved, which requires more comparative randomized studies.
In conclusion, the preparation of a radioactive feeding tube is simple and easy. The insertion of this kind of tube achieves parenteral nutrition and brachytherapy simultaneously and is safe and effective in dysphagia palliation in patients with malignant esophageal stricture. The radiological-radiotherapeutic procedure could be an alternative tool in the case of refusing other treatments by the patients.
References
Sundelof M, Ye W, Dickman PW, Lagergren J (2002) Improved survival in both histologic types of oesophageal cancer in Sweden. Int J Cancer 99:751–754. https://doi.org/10.1002/ijc.10420
Kubba AK, Krasner N (2000) An update in the palliative management of malignant dysphagia. Eur J Surg Oncol 26(2):116–129. https://doi.org/10.1053/ejso.1999.0754
Bethge N, Sommer A, von Kleist D, Vakil N (1996) A prospective trial of self-expanding metal stents in the palliation of malignant esophageal obstruction after failure of primary curative therapy. Gastrointest Endosc 44(3):283–286. https://doi.org/10.1016/S0016-5107(96)70165-7
Baron TH (2001) Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344(22):1681–1687. https://doi.org/10.1056/NEJM200105313442206
Conio M, Repici A, Battaglia G et al (2007) A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol 102(12):2667–2677. https://doi.org/10.1111/j.1572-0241.2007.01565.x
Bona D, Sarli D, Saino G, Quarenghi M, Bonavina L (2007) Successful conservative management of benign gastro-bronchial fistula after intrathoracic esophagogastrostomy. Ann Thorac Surg 84(3):1036–1038. https://doi.org/10.1016/j.athoracsur.2007.04.043
Wlodarczyk JR, Kuzdzal J (2018) Stenting in palliation of unresectable esophageal cancer. World J Surg. https://doi.org/10.1007/s00268-018-4722-7
Riccioni ME, Shah SK, Tringali A, Ciletti S, Mutignani M, Perri V, Zuccala G, Coppola R, Costamagna G (2002) Endoscopic palliation of unresectable malignant oesophageal strictures with self-expanding metal stents: comparing Ultraflex and Esophacoil stents. Dig Liver Dis 34(5):356–363. https://doi.org/10.1016/S1590-8658(02)80130-X
Nathwani RA, Kowalski T (2007) Endoscopic stenting of esophageal cancer: the clinical impact. Curr Opin Gastroenterol 23(5):535–538. https://doi.org/10.1097/MOG.0b013e3282a56968
Tian D, Wen H, Fu M (2016) Comparative study of self-expanding metal stent and intraluminal radioactive stent for inoperable esophageal squamous cell carcinoma. World J Surg Oncol 14(1):18. https://doi.org/10.1186/s12957-016-0768-x
Zhongmin W, Xunbo H, Jun C, Gang H, Kemin C, Yu L, Fenju L (2012) Intraluminal radioactive stent compared with covered stent alone for the treatment of malignant esophageal stricture. Cardiovasc Interv Radiol 35(2):351–358. https://doi.org/10.1007/s00270-011-0146-6
Guo JH, Teng GJ, Zhu GY, He SC, Fang W, Deng G, Li GZ (2008) Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology 247(2):574–581. https://doi.org/10.1148/radiol.2472070999
Roy-Choudhury SH, Nicholson AA, Wedgwood KR, Mannion RA, Sedman PC, Royston CM, Breen DJ (2001) Symptomatic malignant gastroesophageal anastomotic leak: management with covered metallic esophageal stents. AJR Am J Roentgenol 176(1):161–165. https://doi.org/10.2214/ajr.176.1.1760161
Zhu HD, Guo JH, Mao AW et al (2014) Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol 15(6):612–619. https://doi.org/10.1016/S1470-2045(14)70131-7
Ma J, Luo J, Gu J, Liu Q, Liu L, Zhang W, Zhang Z, Yan Z (2018) Malignant obstructive jaundice treated with intraluminal placement of Iodine-125 seed strands and metal stents: an analysis of long-term outcomes and prognostic features. Brachytherapy 17(4):689–695. https://doi.org/10.1016/j.brachy.2018.04.001
Zhang W, Yang ZQ, Shi HB, Liu S, Zhou WZ, Zhao LB (2015) Placement of 125I seed strands and stents for a type IV Klatskin tumor. World J Gastroenterol 21(1):373–376. https://doi.org/10.3748/wjg.v21.i1.373
Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP (2016) Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int 10(1):185–195. https://doi.org/10.1007/s12072-015-9663-8
Yang QH, Zhang W, Liu QX, Liu LX, Wu LL, Wang JH, Yan ZP, Luo JJ (2016) TACE combined with implantation of irradiation stent versus TACE combine with bare stent for HCC complicated by IVCTT. Cardiovasc Interv Radiol 39(9):1280–1288. https://doi.org/10.1007/s00270-016-1372-8
Sinha S, Varagunam M, Park MH, Maynard ND, Trudgill N, Crosby T, Cromwell DA (2019) Brachytherapy in the palliation of oesophageal cancer: effective but impractical? Clin Oncol (R Coll Radiol) 31(7):e87–e93. https://doi.org/10.1016/j.clon.2019.03.045
Homs MY, Steyerberg EW, Eijkenboom WM et al (2004) Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 364(9444):1497–1504. https://doi.org/10.1016/S0140-6736(04)17272-3
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81501569). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Ethical approval
This study was approved by the institutional review board of Zhengzhou University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bi, Y., Zhu, X., Yu, Z. et al. Radioactive feeding tube in the palliation of esophageal malignant obstruction. Radiol med 125, 544–550 (2020). https://doi.org/10.1007/s11547-020-01151-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01151-9