Abstract
Automated breast ultrasound (ABUS) is a recently introduced ultrasonography technique, developed with the purpose to standardize breast ultrasonography and overcome some limitations of handheld ultrasound (HHUS), such as operator dependence and the considerable amount of medical time necessary to perform and interpret HHUS. This new ultrasonography technique separates the moment of image acquisition (that may be performed also by a technician) from that of its interpretation, increasing reproducibility, reducing operator-dependence and physician time. Moreover, multiplanar reconstructions, especially the coronal view, introduce new diagnostic information. ABUS, with those advantages, has the potential to be used as an adjunctive tool to screening mammography, especially in the dense breast, where mammography has a relatively low sensitivity. Women’s awareness of risks related to breast density is a hot topic, especially in the USA where legislative breast density notification laws increase the demand for supplemental ultrasound screening. Therefore, ABUS might have the potential to respond to this need. The purpose of this article is to present a summary of current state-of-the-art of ABUS technology and applications, with an emphasis on breast cancer screening. This article discusses also how to overcome some ABUS limitations, in order to be familiar with the new technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignant disease of the female population [1] and mammographic screening is an effective proven method for reducing mortality, through early diagnosis [2]. Despite this, in dense breasts, mammographic sensitivity can be very low, even less than 50% [3]. For this reason and because of breast density has been established as an independent risk factor for breast cancer, the need for an additional tool is gaining ever greater interest [4]. Handheld ultrasound (HHUS) is a valid supplemental screening tool being widely available and cheap. Several studies evaluated HHUS as a screening tool in women with dense breast [5,6,7,8,9], the most significant is the large multi-institutional trial published by Berg et al. [5], reporting a supplemental yield of 4.2 per 1000 women screened. This study, however, revealed some “weak points” of HHUS screening, such as the high number of false positives (FP) and the considerable effort in terms of physician time for exam execution and interpretation [5]. Due to these limitations, technological research tried for years to propose ultrasonography machines able to standardize and simplify US examination. Automated breast ultrasound (ABUS), also known as automated volumetric scanner (ABVS), depending on the vendor, was introduced with the purpose to overcome the operator-dependence of HHUS, increasing the reproducibility of the examination. ABUS is a technological advance, which provides ultrasonography 3D representation of the breast tissue, with the advantage of multiplanar reformations and the capability to review images retrospectively once the examination has been acquired [4, 10]. The concept of automated breast ultrasound dates back to the 70s [11]; however, old generation scanners had transducers with relatively low frequency (4–7 MHz) and image quality was not sufficiently good. Interest in ABUS research further increased with the development of modern scanners, provided with high-frequency transducers [12]. Therefore, several studies were performed mostly for the evaluation of ABUS as a supplemental screening tool [13,14,15] and in recent years some studies also considered its use in the clinical setting [12, 16,17,18,19].
In the present review, we performed a computerized search by using the PubMed database (www.ncbi.nlm.nih.gov/pubmed/), including articles listed up to 30 April 2017. The following search terms were used: automated breast ultrasound/ultrasonography, automated breast volume scanner/scanners/scanning and automated whole breast ultrasound/ultrasonography. Only articles in English were included. Titles and abstracts of search results were examined. When considered suitable, the full text was reviewed. The references of retrieved studies were examined to identify additional papers.
Articles regarding the use of ABUS in the screening or clinical setting were included. Excluded were studies (1) on non-human subjects, (2) that evaluated newly developed prototype devices (e.g. fusion devices) or (3) testing computerized applications for ABUS (e.g. image segmentation, density analysis or Computer Aided Detection).
Equipment and technique
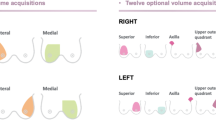
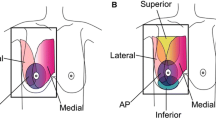
Nowadays, several types of automated breast ultrasound systems are available, including two main categories: prone- and supine-scanners [4].
The first device, a supine-type scanner, was a hybrid system (SonoCine, Reno) provided with a conventional ultrasound transducer mounted on a computer-guided articulating arm [20]. This system received FDA clearance as an adjunct to screening mammography in 2008 [21]. The transducer moves automatically over the breast, in a way similar to that of HHUS, acquiring transverse images in cranio-caudal linear overlapping rows. A hydrogel nipple pad is applied to allow proper contact with the skin and a trained operator is responsible for appropriate vertical orientation of the probe and adequate contact pressure with the skin. Overall examination time is about 20–30 min (10–20 min for scanning, 5–10 min for preparing the patient) [13]. Following 2D axial scan’s acquisition, the automated whole breast ultrasound software creates a cine loop, containing 2000–5000 images each [13]. Initially, such systems did not allow 3D reconstructions of the row data [4]; however, new 3-D whole breast multiplanar reconstruction software has recently been announced.
Modern automated breast scanners were subsequently developed (Somo-V, GE Healtcare; ACUSON S2000, Siemens Medical Solutions); both received FDA clearance in 2012 [21] and operate differently from previous hybrid machines. Those systems are provided with special high-frequency and large footprint transducers (15 × 17 cm), similar in size to a mammography compression paddle and mounted on a mechanical arm. One of those modern systems is provided with a curved transducer designed to follow the contour of the breast [4]. The acquisition starts after placing the probe over the breast with only mild compression. A replaceable membrane covers the transducer surface to allow adequate contact with the skin of the entire area. In this system, the patient lies in the supine position with the arm of the side under examination over the head. Conventionally, three 1 min scans are sufficient for scanning the entire breast, excluding the axilla. However, in case of larger breast, more than one scan may be required to cover the entire field of interest. The average total time to complete the examination is approximately 15 min. Trained technicians are able to perform examination. Of note, learning to perform ABUS, with correct positioning and adequate compression power, requires time; therefore, training is a relevant part for image quality [22]. Compression errors can create artifacts that interfere with the interpretation (Fig. 1). After image acquisition, row data are stored on the systems’ hard disk and then transferred to a dedicated workstation, where images, displayed both in the native axial plane of acquisition and reformatted coronal and sagittal planes, can be reviewed for further interpretation and analysis. The coronal plane, also known as the “surgical view” (in which the breast is positioned in the same way that it is oriented on the surgical table), introduces new diagnostic information, i.e. the retraction phenomenon [23]. The main limitations of ABUS systems are exclusion of axillary regions from the field of view and the absence of tools to assess vascularity and tissue elasticity [4].
Artifacts: nipple shadowing (arrow) and dense breast parenchyma artifacts (asterisk) displayed on automated breast ultrasonography using Siemens ABVS (a axial view, b coronal view, c sagittal view). Re-scanning the same patient and increasing scanning pressure, these artifacts were eliminated (d axial view, e coronal view, f sagittal view) allowing to obtain an optimal visualization of the entire breast parenchyma
Prone-type scanners are still under development; however, one of these systems received FDA clearance in 2014 [21]. A circumferential transducer performs the scan with the patient lying prone on the table with the breast suspended in a warm-water bath beneath an opening in the table top [22, 24]. The transducer has a ring configuration and collects data by rotating 360°. After acquisition, data are processed and reconstructed to allow volumetric rendering [22, 24].
ABUS interpretation
Automated breast ultrasound technology allows the radiologist to interpret ultrasonography images in a separate time after acquisition. Different interpretation times have been reported, ranging from 5 to 10 min [14, 23, 25,26,27,28,29], probably according to differences in readers’ experience and complexity of each case.
Regarding the reading technique there is no uniform protocol: some authors used a reading protocol starting from transverse plane [26], while other authors preferred starting from coronal plane [28, 30, 31].
Clinical applications
Screening
The application of automated breast ultrasound was initially focused on the screening setting and first works aimed to test the new technique in dense breast. Interest has continued to increase in past years due to a greater awareness for the problem of breast density, especially in the USA where legislative breast density notification laws increase the demand for supplemental US screening [22, 26].
First screening works were performed using a hybrid system [13, 25]. Kelly et al. [13], in a multicenter study, compared mammography alone versus automated whole breast ultrasound (AWBU) plus mammography in 4419 women with dense breasts and/or at elevated risk of breast cancer. The above mentioned authors found an improvement in cancer detection of 3.6 per 1000 women screened with the addition of AWBU [13]. Sensitivity increased from 40% for mammography alone to 81% for the combined modalities, while AWBU alone reached a sensitivity of 67% [13]. The detection of invasive ductal cancers and smaller lesions (≤10 mm) was significantly higher for AWBU alone than mammography (sensitivities for cancers ≤10 mm of 81 and 33%, respectively) [13]. Of note, recalls increased from 4.2% for mammography alone to 9.6% adding AWBU [13]. Specificity based on recalls was 89.9% for AWBU, 95.15% for mammography and 98.7% for the combined modalities [13]. Previous authors published another study evaluating radiologists’ performance in breast cancer detection using mammography alone versus mammography plus AWBU in women with dense breast [25]. By adding AWBU to mammography, the sensitivity of breast cancer detection increased from 50 to 81% and all readers in this study significantly improved the identification of asymptomatic cancers [25]. Indeed, the cancer detection for true positive cases showed an increase of 63%, with only a 4% decrease in correct identification of the true negative cases [25]. By adding AWBU the detection of cancers ≤1 cm increased to 65%, while with mammography alone the detection of those cancers was 26% [25]. Of note, mean interpretation time per AWBU was 7 min 58 s [25], shorter than the time reported in the ACRIN 6666 trial regarding HHUS screening (19 min) [5].
In the past years other important studies have been published using modern automated ultrasound systems [14, 15, 32]. Wilczek et al. [14], in a single-center study, evaluated 1668 asymptomatic women, with heterogeneously/extremely dense breast parenchyma. The combination of digital mammography plus ABUS determined an increase in cancer detection of 2.4 per 1000 women screened [14]. The increase in sensitivity was 36.4% for combined modalities vs. mammography alone at study entry, while, including interval cancers, sensitivity increased by 25% [14]. Recall rates increased by 9.0 per 1000 (13.8 per 1000 for mammography alone and 22.8 for the combined imaging approach) and specificity decreased by 0.7% when ABUS was added to mammography [14]. The Somoinsight study [15], a multicenter prospective trial including 15,318 asymptomatic women with dense breast, compared mammography versus mammography plus ABUS. Combined imaging approach led to an increase in cancer detection rate of 1.9 per 1000 women with an increase in sensitivity of 26.7% [15]. Cancers detected only with ABUS were significantly more likely to be invasive in comparison to those detected by screening mammography alone (93.3 vs. 62.2%, p = 0.001) [15]. Moreover, ABUS alone detected cancers which presented with lower stage at diagnosis, suggesting positive prognostic implications [15]. In this study, recall rates increased from 150.2 per 1000 women with mammography alone to 284.9 per 1000 women by adding ABUS and the specificity decreased by 13.4% for the combined modalities (85.4% for mammography alone vs. 72% for mammography + ABUS) [15]. Giger et al. [32], in a multi-reader study on asymptomatic women with BI-RADS C or D breast density, showed an improvement in detection of both mammography-negative and mammography-positive breast cancers with the use of ABUS. The improvement in sensitivity was 23.9%, for mammography-negative breast cancers (p = 0.004) and 5.9% for mammography-positive breast cancers (p = 0.234) [32]. Specificity decreased non-significantly (from 78.1% for mammography alone to 76.2% for the combined modalities) due to a robust training program [32].
Table 1 presents the results of above presented screening studies. From all ABUS screening studies emerged an improvement in cancer detection rate, ranging from 1.9 to 3.6 per 1000 by adding ABUS to screening mammography [13,14,15]. These results are similar to those reported in the studies that used HHUS as an adjunct to screening mammography, in women with dense breast (from 1.9 to 5.3 additional cancer per 1000 women) [5, 7, 33,34,35]. On the other hand, an increase of recall rates was reported [13,14,15], a problem which emerged also from studies considering screening with HHUS [36, 37]. However, recall rate is expected to decrease progressively over the time as readers’ experience increases [38]. In a retrospective study, Arleo et al. [38] evaluating ABUS as a screening tool in women with dense breasts, reported an overall recall rate of 19% during the 3-month study time period. Of note, recall rate trended down from 24.7% in the first study’s month to 12.6% in the third study’s month showing the clinical implication that ABUS has a learning curve [38]. Therefore, such a problem could be overcome improving readers’ experience and by providing radiologists’ training programs to help minimize false positives [32, 38].
Clinical setting
Automated breast ultrasound has been and still is actively studied for breast examination in different clinical scenarios. Most of the studies compared ABUS with HHUS in terms of detection [23, 28, 31, 39,40,41,42] and characterization [12, 43,44,45,46,47,48] of breast lesions, while others evaluated the diagnostic performance of ABUS [23, 29, 30, 39, 41, 43,44,45,46, 49, 50]. Some publications focused on the inter-observer agreement of ABUS [12, 16, 26, 30, 39, 41, 43, 51] and others evaluated ABUS in the pre-operative setting [17,18,19, 52], in the assessment of response to neoadjuvant chemotherapy [53] and as a second look procedure [10, 54, 55]. Furthermore, imaging-histologic correlation between ABUS semeiotics and molecular subtypes of breast cancer has been investigated [56].
Detection
Detection of breast lesions was reported to be similar between the two US modalities for most of the authors [23, 39,40,41], even if some studies reported higher detection rates for ABUS than HHUS (Table 2) [31, 42]. Chang et al. [28] retrospectively evaluated the detection performance of benign and malignant masses. Overall sensitivity and specificity of three readers were, respectively, 71.9 and 79.5%, and the sensitivity was higher for detection of malignant masses (87.5–95.8%) vs. benign masses (56.3–66.7%) [28]. Mass size, shape and surrounding tissue changes were the variables affecting detectability at ABUS [28]. Shin et al. [12] also demonstrated the relation between size and lesion detectability: the lesion detection rate increased along with the increase of lesion size.
Zhang et al. [42] and Xiao et al. [31] demonstrated that ABUS detected significantly higher number of breast lesions than HHUS. Furthermore, in the retrospective study by Xiao et al. [31], evaluating 300 patients with 417 breast lesions, ABUS was found to detect all malignant lesions (32/32) while HHUS identified 31/32 lesions (p = 0.158).
Characterization
Many studies focused on the comparison between ABUS and HHUS in the characterization of breast lesions according to BI-RADS lexicon [12, 43,44,45,46,47].
Agreement between ABUS and HHUS in final BI-RADS category assessment ranged from fair to almost perfect [12, 43,44,45,46,47]. Golatta et al. [43] found fair agreement between ABUS and HHUS in the comparison of BI-RADS scores (k = 0.34), but dichotomizing BI-RADS categories into suspicious (BI-RADS 4-5) vs. not suspicious (BI-RADS 1-2) agreement was substantial (k = 0.68). The same authors, 2 years later, performed another study on 983 patients showing fair agreement (k = 0.31) between ABUS and HHUS in assigning BI-RADS scores (dichotomized as “benign” for BI-RADS 1-2 and “unclear/suspicious” for BI-RADS 0, 4 and 5) [44]. Those results were caused probably by imbalanced blinding of readers (HHUS readers had clinical information and availability of mammography while ABUS readers did not) [44]. On the other hand, Shin et al. [12] found substantial agreement (k = 0.64) for final BI-RADS assessment. In addition, good results were shown in the comparison of the two US modalities for the evaluation of clock-face position, distance from the nipple and largest diameter with intra-class correlation coefficients (ICC) of 0.75, 0.89 and 0.94, respectively [12]. Also Kim et al. [46], analyzing 106 solid breast masses (52 cancers, 54 benign lesions), found substantial agreement in BI-RADS final assessment (k = 0.773 ± 0.104).
Regarding comparison between ABUS and HHUS for BI-RADS descriptors, fair to substantial agreement was shown [46]. Kotsianos-Hermle et al. [45] reported good correlation for the criterion “margin” (ρ = 0.88). In the study by Kim et al. [46] “posterior echo feature” presented the worst agreement (k = 0.371 ± 0.225), while “orientation” the best (k = 0.608 ± 0.210).
Automated breast ultrasound was also shown to detect calcifications better than HHUS [31] (Fig. 2) and to identify a new semeiotic feature, the retraction phenomenon (Fig. 3) [48]. Retraction phenomenon is a peculiarity of coronal plane, unique of ABUS, with high diagnostic accuracy for breast malignancy [31, 39, 40, 48]. Zheng et al. [48] found that retraction phenomenon and microlobulated margins were the best predictors for malignant masses, while Lin et al. [40] reported 100% specificity and 80% sensitivity for retraction phenomenon in detecting breast cancer with high accuracy to determine malignant and benign lesions (91.4%).
A case of 48-year-old woman with invasive ductal carcinoma (IDC) of the right breast, diagnosed on US-guided core needle biopsy. Tomosynthesis (a MLO view) showed an irregular opacity with spiculated/lobulated margins and fine pleomorphic calcifications. Automated breast ultrasonography, performed using Siemens ABVS, detected a heterogeneous hypoechoic mass, with lobulated margins containing calcifications, which are well seen as hyperechoic spots (b axial view, c coronal view, d sagittal view)
A case of 55-year-old woman with invasive ductal carcinoma (IDC) of the left breast diagnosed on US-guided CNB. Automated breast ultrasonography detected a small (8 mm) hypoechoic mass presenting the retraction phenomenon, which is a useful feature visible on coronal plane (a, b, c three contiguous coronal images, d axial view). Tomosynthesis (e MLO view, f CC view) showed a small spiculated opacity
Diagnostic accuracy
Diagnostic accuracy in differentiation between malignant and benign lesions has been widely analyzed and most studies (Table 3) demonstrated high values for sensitivity and specificity, as reported in a recent meta-analysis (pooled values of 92.0 and 84.9%, respectively) [50].
Golatta et al. [44] compared ABUS, HHUS and mammography with a clinical gold standard, defined as the combination of mammography, HHUS and histology (if indicated), on a population of 983 patients. They reported values of negative predictive value (NPV) of 98%, specificity of 85% and sensitivity of 74% for ABUS [44]. In this study 31 of 119 malignant lesions were rated as BI-RADS 1-2 by ABUS; of those, 12 lesions were seen after re-reading ABUS examinations and 8 lesions were primarily seen with MRI or mammography, therefore found on second look [44]. Remaining 11 of 119 lesions were definitively not seen by ABUS because of their location (behind the nipple or too peripheral), difficulties to perform examination (presence of ulcerated cancer) or in image interpretation (due to the lack of clinical information, e.g. previous surgical treatments) [44]. Imbalanced blinding caused those results, since radiologists evaluating HHUS were aware of patients’ history and mammography while ABUS readers were not [44]. Relevant problems concerning the image quality emerged from the study, such as the presence of artifacts and lacking data due to inadequate contact between the transducer and the skin [44].
Most of published studies did not find significant differences between HHUS and ABUS in diagnostic performance (Table 3) [23, 39, 41, 45, 49]. However, Choi et al. [29] evaluated a large population of asymptomatic women who were subdivided into two groups (1866 patients for ABUS and 3700 patients for HHUS) and showed that diagnostic accuracy and specificity were significantly higher for ABUS than HHUS (respectively, diagnostic accuracy 97.7 vs. 96.5% and specificity 97.8 vs. 96.7%). On the other hand, Kim et al. [46] found a significantly higher accuracy, in terms of areas under the ROC curve, for ABUS than for HHUS when considering the mean values by multi-reader analysis (respectively, 0.963 vs. 0.991); differences between ABUS and HHUS did not reach statistical significance when considering each reader.
Examining FP and false negatives (FN) Wang et al. [23] showed that most common pathology types for FP (19.5% with ABUS and 17.5% with HHUS) were adenosis, intraductal papilloma, fibroadenoma and mastitis. FN (4.7% with ABUS and 9.4% with HHUS) were lesions not detected because of small size or not suspicious ultrasonography features, such as smooth and circumscribed margins (at histology: phyllodes tumor, medullary carcinoma and invasive solid papillary carcinoma) [23]. One of the FN of HHUS (misinterpreted as adenosis) was a 6.5 cm invasive ductal carcinoma, presenting as an extensive hypoechoic area but correctly detected by ABUS, thanks to its wide scanning area [23].
Inter-observer reliability
Inter-observer reliability has a fundamental role in determining the diagnostic accuracy of ABUS with a significant impact on its clinical practice [50]. Agreement between readers has been tested for description of BI-RADS features, BI-RADS final category assessment, location and size of lesions. Key results from main studies are summarized in Table 4.
Data regarding the agreement in assigning BI-RADS scores were heterogeneous, ranging from slight to substantial [12, 26, 30, 39, 41, 43, 51]. Agreement proved to increase by dichotomizing BI-RADS scores into benign and suspicious groups [30, 43] and also by adding mammography to ABUS [26]. Skaane et al. [26] in their retrospective study comparing reader performance (5 radiologists) of ABUS vs. ABUS plus mammography found a considerable inter-observer variability in giving BI-RADS assessment scores. Reader agreement improved for mammography plus ABUS (mean k ranged from 0.14 to 0.44) compared to ABUS alone (mean k ranged from 0.07 to 0.34) [26].
Few studies considered BI-RADS feature assessment showing fair to substantial agreement for the main descriptors [12, 41, 51], while agreement in size, location and distance from the nipple varied from moderate to excellent [12, 16, 41], whereas only “lesion depth from the skin” showed low reliability (ICC = 0.342) [16]. Chang et al. [16] analyzing 24 patients with ABUS two times before biopsy or surgery (within a mean interval of 1.3 days) found that the different values of depth were probably related to variations in positioning and scanning pressure of the probe. Compression may also affect lesion characteristics; in particular, some benign lesions are more deformable than the stiffer solid masses and could flatten [16]. Therefore, margins and shape of benign lesions could change by increasing compressive pressure [16].
Other applications of ABUS
In the preoperative setting, one of the preliminary studies for ABUS, which was performed in a population of 40 patients including both in situ and invasive cancers, showed promising results in the extent of cancer assessment [19]. Li et al. [18], analyzing 33 patients with histologically proven DCIS, found that ABUS assessed the extent of disease better than HHUS (compared to histopathology) and that mean lesion size assessed by ABUS did not differ significantly from that determined by histopathology. Moreover, Huang et al. [52] focusing on the pre-operative assessment of DCIS, found that ABUS is superior to HHUS in the detection of malignancies and it is also more accurate in the tumor largest diameter assessment.
Considering 3D evaluation of 51 invasive ductal carcinomas, Xu et al. [17] assessed largest tumor diameter, tumor volume and tumor surface area on HHUS and ABUS and compared these with tumor size and volume on pathology specimen after surgical excision (gold standard). All ABUS measurements showed stronger correlation coefficient than those of HHUS [17]. Furthermore, volumetric measurements determined by ABUS had significantly higher accuracy than those determined by HHUS [17].
Automated breast ultrasound has been also evaluated in the assessment of response to neoadjuvant chemotherapy [53]. In a study by Wang et al. [53] ABUS was performed before treatment and after two cycles of chemotherapy in a population of 290 patients. The product change of two largest perpendicular diameters (PC) in axial and coronal planes and the longest diameter change in axial and coronal planes were the four prediction methods examined in the study [53]. The overall performance of ABUS in predicting complete response after four cycles of chemotherapy was high (area under the receiver operating characteristic curve [AUC]: 0.83–0.85) and all four prediction methods revealed high sensitivities (85.7–88.1%) while specificity was high only for PC (81.5–85.1%) [53]. Lower values of AUC were observed when mid-treatment ABUS was used to predict poor pathological outcomes [53].
Automated breast ultrasound showed promising results as a second look (SL) tool after breast MRI [10, 54, 55] (Fig. 4). Chae et al. [54] found 80 additional suspicious lesions on 58 preoperative breast MRI. ABUS-SL detected 70/80 lesions while HHUS-SL detected 65/80 lesions. Only 4% of HHUS detected lesions were not detected on ABUS while 10% of ABUS detected lesions were not detected on HHUS [54]. Girometti et al. [10] compared HHUS with ABUS in the second-look scenario evaluating a population of 131 patients that had undergone breast MRI for different reasons beyond breast cancer staging (e.g. high risk of breast cancer, B3 lesions, evaluation of response to neoadjuvant chemotherapy etc.). They found comparable values of both techniques in terms of detection rates for MRI-detected lesions (69.3 vs. 71.5%, for ABUS and HHUS, respectively), with an almost perfect agreement in assessing them as significant or not (k = 0.94) [10]. Kim et al. [55], on a population of 40 patients undergoing preoperative breast MRI with 76 additional MRI findings, found significantly higher values of detection rate for ABUS than HHUS (94.7 vs. 86.8%, respectively; p < 0.05).
A case of 70-year-old woman with multifocal and multicentric invasive lobular carcinoma (ILC) of the left breast. Automated breast ultrasonography (a coronal view, b axial view, c sagittal view) detected two hypoechoic lesions with retraction phenomenon on the upper quadrants of the left breast, well represented on coronal reconstruction (index lesions: thin arrow; multifocality: arrowhead). Another small hypoechoic area (circle on coronal, axial and sagittal views) was detected on automated ultrasonography second-look examination performed after staging breast MRI, due to the presence of suspicious mass like enhancement on the inferior-outer quadrant of the same breast (d large arrow). Coronal view well depicts all three neoplastic lesions in the same image
When evaluating the correlation between ABUS imaging features and molecular subtypes of breast cancer, Zheng et al. [56] demonstrated that the luminal-A, luminal-B, HER2 and triple-negative subtypes have specific predictive factors (luminal-A: retraction phenomenon, post-acoustic shadowing, echogenic halo, absence of calcifications; luminal-B: presence of calcifications, absence of retraction phenomenon; HER2: presence of calcifications, absence of retraction phenomenon, non-mass lesions, absence of echogenic halo, post-acoustic enhancement; triple negative: absence of retraction phenomenon, post-acoustic enhancement, absence of echogenic halo, absence of calcifications, regular shape). Retraction phenomenon showed a strong correlation with molecular subtypes resulting in the strongest independent predictor for the luminal-A subtype when present and for the triple negative subtype when absent [56].
Conclusion
Automated breast ultrasound is an emergent ultrasonography technique, developed to support screening mammography, especially in dense breast, where mammography’s sensitivity is relatively low. ABUS has the advantage to be more reproducible and less operator-dependent than HHUS. In addition, multiplanar reconstructions, a peculiarity of ABUS, are very useful for image interpretation. However, ABUS presents some limitations because of the lack of tools to assess vascularity and tissue elasticity; another possible limitation is the exclusion of axillary regions from the field of view. Furthermore, an increase of false positives has been reported when using ABUS. Increasing readers’ experience and improving the scanning technique could overcome this problem. ABUS applications are currently under research, both in the screening setting and in the clinical setting. With the increase of screening demand, ABUS has the potential to be the method of choice as an adjunctive tool to screening mammography in women with dense breast tissue.
References
Brem RF, Lenihan MJ, Lieberman J, Torrente J (2015) Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol 204:234–240
Tabár L, Fagerberg CJ, Gad A et al (1985) Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1:829–832
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175
Kaplan SS (2014) Automated whole breast ultrasound. Radiol Clin N Am 52:539–546
Berg WA, Blume JD, Cormack JB et al (2008) Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299:2151–2163
Kolb TM, Lichy J, Newhouse JH (1998) Occult cancer in women with dense breasts: detection with screening US–diagnostic yield and tumor characteristics. Radiology 207:191–199
Kaplan SS (2001) Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology 221:641–649
Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dünser M (2000) Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR 21:325–336
Gordon PB, Goldenberg SL (1995) Malignant breast masses detected only by ultrasound. A retrospective review. Cancer 76:626–630
Girometti R, Zanotel M, Londero V, Bazzocchi M, Zuiani C (2017) Comparison between automated breast volume scanner (ABVS) versus hand-held ultrasound as a second look procedure after magnetic resonance imaging. Eur Radiol 27:3767–3775
Jackson VP, Kelly-Fry E, Rothschild PA, Holden RW, Clark SA (1986) Automated breast sonography using a 7.5-MHz PVDF transducer: preliminary clinical evaluation. Work in progress. Radiology 159:679–684
Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH (2011) Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am J Roentgenol 197:747–754
Kelly KM, Dean J, Comulada WS, Lee SJ (2010) Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol 20:734–742
Wilczek B, Wilczek HE, Rasouliyan L, Leifland K (2016) Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol 85:1554–1563
Brem RF, Tabár L, Duffy SW et al (2015) Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology 274:663–673
Chang JM, Cha JH, Park JS, Kim SJ, Moon WK (2015) Automated breast ultrasound system (ABUS): reproducibility of mass localization, size measurement, and characterization on serial examinations. Acta Radiol 56:1163–1170
Xu C, Wei S, Xie Y, Guan X, Yang B (2016) Three-dimensional assessment of automated breast volume scanner compared with handheld ultrasound in pre-operative breast invasive ductal carcinomas: a pilot study of 51 cases. Ultrasound Med Biol 42:2089–2096
Li N, Jiang YX, Zhu QL et al (2013) Accuracy of an automated breast volume ultrasound system for assessment of the pre-operative extent of pure ductal carcinoma in situ: comparison with a conventional handheld ultrasound examination. Ultrasound Med Biol 39:2255–2263
Tozaki M, Fukuma E (2010) Accuracy of determining preoperative cancer extent measured by automated breast ultrasonography. Jpn J Radiol 28:771–773
Shin HJ, Kim HH, Cha JH (2015) Current status of automated breast ultrasonography. Ultrasonography 34:165–172
Gazhonova V (2017) 3D automated breast volume sonography. Springer, Cham
Durand MA, Hooley RJ (2017) Implementation of whole-breast screening ultrasonography. Radiol Clin N Am 55:527–539
Wang HY, Jiang YX, Zhu QL et al (2012) Differentiation of benign and malignant breast lesions: a comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur J Radiol 81:3190–3200
OʼFlynn EAM, Fromageau J, Ledger AE et al (2017) Ultrasound tomography evaluation of breast density: a comparison with noncontrast magnetic resonance imaging. Investig Radiol 52:343–348
Kelly KM, Dean J, Lee SJ, Comulada WS (2010) Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol 20:2557–2564
Skaane P, Gullien R, Eben EB, Sandhaug M, Schulz-Wendtland R, Stoeblen F (2015) Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: a reader performance study. Acta Radiol 56:404–412
Chang JM, Moon WK, Cho N et al (2011) Breast cancers initially detected by hand-held ultrasound: detection performance of radiologists using automated breast ultrasound data. Acta Radiol 52:8–14
Chang JM, Moon WK, Cho N, Park JS, Kim SJ (2011) Radiologists’ performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur J Radiol 78:99–103
Choi WJ, Cha JH, Kim HH et al (2014) Comparison of automated breast volume scanning and hand-held ultrasound in the detection of breast cancer: an analysis of 5,566 patients evaluations. Asian Pac J Cancer Prev 15:9101–9105
Wojcinski S, Gyapong S, Farrokh A, Soergel P, Hillemanns P, Degenhardt F (2013) Diagnostic performance and inter-observer concordance in lesion detection with the automated breast volume scanner (ABVS). BMC Med Imaging 13:36. doi:10.1186/1471-2342-13-36
Xiao YM, Chen ZH, Zhou QC, Wang Z (2015) The efficacy of automated breast volume scanning over conventional ultrasonography among patients with breast lesions. Int J Gynaecol Obstet 131:293–296
Giger ML, Inciardi MF, Edwards A et al (2016) Automated breast ultrasound in breast cancer screening of women with dense breasts: reader study of mammography-negative and mammography-positive cancers. AJR Am J Roentgenol 206:1341–1350
Leconte I, Feger C, Galant C et al (2003) Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol 180:1675–1679
Berg WA, Zhang Z, Lehrer D et al (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307:1394–1404
Schaefer FK, Waldmann A, Katalinic A et al (2010) Influence of additional breast ultrasound on cancer detection in a cohort study for quality assurance in breast diagnosis—analysis of 102,577 diagnostic procedures. Eur Radiol 20:1085–1092
Berg WA, Bandos AI, Mendelson EB, Lehrer D, Jong RA, Pisano ED (2016) Ultrasound as the primary screening test for breast cancer: analysis from ACRIN 6666. J Natl Cancer Inst 108:djv367
Ohuchi N, Suzuki A, Sobue T et al (2016) Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 387:341–348
Arleo EK, Saleh M, Ionescu D, Drotman M, Min RJ, Hentel K (2014) Recall rate of screening ultrasound with automated breast volumetric scanning (ABVS) in women with dense breasts: a first quarter experience. Clin Imaging 38:439–444
Wang ZL, Xu JH, Li JL, Huang Y, Tang J (2012) Comparison of automated breast volume scanning to hand-held ultrasound and mammography. Radiol Med 117:1287–1293
Lin X, Wang J, Han F, Fu J, Li A (2012) Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol 81:873–878
Kim SH, Kang BJ, Choi BG et al (2013) Radiologists’ performance for detecting lesions and the interobserver variability of automated whole breast ultrasound. Korean J Radiol 14:154–163
Zhang Q, Hu B, Hu B, Li WB (2012) Detection of breast lesions using an automated breast volume scanner system. J Int Med Res 40:300–306
Golatta M, Franz D, Harcos A et al (2013) Interobserver reliability of automated breast volume scanner (ABVS) interpretation and agreement of ABVS findings with hand held breast ultrasound (HHUS), mammography and pathology results. Eur J Radiol 82:e332–e336
Golatta M, Baggs C, Schweitzer-Martin M et al (2015) Evaluation of an automated breast 3D-ultrasound system by comparing it with hand-held ultrasound (HHUS) and mammography. Arch Gynecol Obstet 291:889–895
Kotsianos-Hermle D, Hiltawsky KM, Wirth S, Fischer T, Friese K, Reiser M (2009) Analysis of 107 breast lesions with automated 3D ultrasound and comparison with mammography and manual ultrasound. Eur J Radiol 71:109–115
Kim H, Cha JH, Oh HY, Kim HH, Shin HJ, Chae EY (2014) Comparison of conventional and automated breast volume ultrasound in the description and characterization of solid breast masses based on BI-RADS features. Breast Cancer 21:423–428
Wenkel E, Heckmann M, Heinrich M et al (2008) Automated breast ultrasound: lesion detection and BI-RADS classification—a pilot study. Rofo 180:804–808
Zheng FY, Yan LX, Huang BJ et al (2015) Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur J Radiol 84:2123–2129
Schmachtenberg C, Fischer T, Hamm B, Bick U (2017) Diagnostic performance of automated breast volume scanning (ABVS) compared to handheld ultrasonography with breast MRI as the gold standard. Acad Radiol 24:954–961
Meng Z, Chen C, Zhu Y et al (2015) Diagnostic performance of the automated breast volume scanner: a systematic review of inter-rater reliability/agreement and meta-analysis of diagnostic accuracy for differentiating benign and malignant breast lesions. Eur Radiol 25:3638–3647
Zhang J, Lai XJ, Zhu QL et al (2012) Interobserver agreement for sonograms of breast lesions obtained by an automated breast volume scanner. Eur J Radiol 81:2179–2183
Huang A, Zhu L, Tan Y et al (2016) Evaluation of automated breast volume scanner for breast conservation surgery in ductal carcinoma in situ. Oncol Lett 12:2481–2486
Wang X, Huo L, He Y et al (2016) Early prediction of pathological outcomes to neoadjuvant chemotherapy in breast cancer patients using automated breast ultrasound. Chin J Cancer Res 28:478–485
Chae EY, Shin HJ, Kim HJ et al (2013) Diagnostic performance of automated breast ultrasound as a replacement for a hand-held second-look ultrasound for breast lesions detected initially on magnetic resonance imaging. Ultrasound Med Biol 39:2246–2254
Kim Y, Kang BJ, Kim SH, Lee EJ (2016) Prospective study comparing two second-look ultrasound techniques: handheld ultrasound and an automated breast volume scanner. J Ultrasound Med 35:2103–2112
Zheng FY, Lu Q, Huang BJ et al (2017) Imaging features of automated breast volume scanner: correlation with molecular subtypes of breast cancer. Eur J Radiol 86:267–275
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
The images of automated breast volumetric scanner (ABVS) and MRI shown in the figures were retrospectively selected among examinations previously performed according to an Ethical Committee approved trial investigating the role for ABVS in clinical practice, with informed consent obtained from all individual participants.
Funding
No funding was received.
Rights and permissions
About this article
Cite this article
Zanotel, M., Bednarova, I., Londero, V. et al. Automated breast ultrasound: basic principles and emerging clinical applications. Radiol med 123, 1–12 (2018). https://doi.org/10.1007/s11547-017-0805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0805-z