Abstract
Purpose
Automated three-dimensional (3D) breast ultrasound (US) systems are meant to overcome the shortcomings of hand-held ultrasound (HHUS). The aim of this study is to analyze and compare clinical performance of an automated 3D-US system by comparing it with HHUS, mammography and the clinical gold standard (defined as the combination of HHUS, mammography and—if indicated—histology).
Methods
Nine hundred and eighty three patients (=1,966 breasts) were enrolled in this monocentric, explorative and prospective cohort study. All examinations were analyzed blinded to the patients´ history and to the results of the routine imaging. The agreement of automated 3D-US with HHUS, mammography and the gold standard was assessed with kappa statistics. Sensitivity, specificity and positive and negative predictive value were calculated to assess the test performance.
Results
Blinded to the results of the gold standard the agreement between automated 3D-US and HHUS or mammography was fair, given by a Kappa coefficient of 0.31 (95 % CI [0.26;0.36], p < 0.0001) and 0.25 (95 % CI [0.2;0.3], p < 0.0001), respectively. Our results showed a high negative predictive value (NPV) of 98 %, a high specificity of 85 % and a sensitivity of 74 % based on the cases with US-guided biopsy. Including the cases where the lesion was seen in a second-look automated 3D-US the sensitivity improved to 84 % (NPV = 99 %, specificity = 85 %).
Conclusion
The results of this study let us suggest, that automated 3D-US might be a helpful new tool in breast imaging, especially in screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since its introduction in 1951 [1] hand-held breast ultrasound (HHUS) has become an important instrument in complementary breast imaging and is accepted in assessing suspicious lesions of the breast [2–5]. Concerning breast cancer screening of asymptomatic women, especially in women with dense breast tissue sensitivity of breast imaging may be increased by a HHUS in addition to mammography [6–11].
The challenge is to obtain reproducible and standardized data with an ultrasound examination. Until today hand-held breast ultrasound (HHUS) is highly dependent on the examiner´s experience. There have been multiple attempts to solve these problems with automated breast ultrasound systems during the last decades [12–22].
The new generation of automated breast ultrasound systems combines automation and three-dimensional (3D) scanning [23–33]. The SomoV™ (U-Systems, Inc., Sunnyvale, CA, USA) system allows an automated recording of the whole breast 3D volume by a technician. The acquired 3D volume data can be evaluated time- and location-independent by different physicians.
The aim of this study was to evaluate and compare clinical performance of the automated SomoV™ system by analyzing 1,966 cases in comparison to the standard breast imaging methods and histology if available.
Materials and methods
Study design and procedures
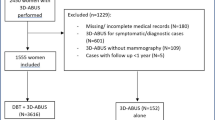
Nine hundred and eighty three patients were included in this monocentric, explorative, prospective, institutional review board approved cohort study. The indication for examination had a wide range including routine check-up, follow-up, preoperative staging of breast cancer, evaluation of palpable lumps and work-up of abnormalities found in HHUS or mammography. The examination with SomoV™ was integrated in the routine of the breast clinic. Mammography, HHUS and SomoV™ were interpreted and classified according to the current American college of radiologists breast imaging reporting and data system (ACR BI-RADS®) [34]. The decision for further histological work-up (biopsy) was based on the conventional methods for breast imaging (mammography and/or ultrasound BI-RADS® category 4 or 5), not on the results of SomoV™. Thereby the standard methods (HHUS, mammography and—if indicated—histology) served as gold standard.
SomoV™ examination
Automated ultrasound of the breast was performed by a technician. Both breasts (thoracic wall after mastectomy respectively) were scanned with SomoV™. Nine hundred and eighty three patients and therefore 1,966 cases (=breasts) were included.
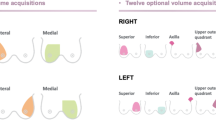
The SomoV™ system consists of a scanning unit and the diagnostic workstation. The scanning unit contains a 10 MHz high-frequency linear transducer. This transducer is able to capture a volume of 17 × 14.5 × 5 cm3 in a single scan. In the course of a single scan which takes about 60 s the SomoV™ generates 340 two-dimensional slices. The number of required scans to image the whole breast was determined by the patient´s breast size and ranged from one to five scans per breast (Fig. 1a, b).
The acquired volume data were automatically sent from the SomoV™ scanning unit to the diagnostic workstation to process the 3D volume dataset in various multi-planar reconstructions and orientations. For the purpose of this study transverse, coronal and sagittal views were available. Technical details of the SomoV™ method and handling have already been described previously (see Fig. 2, SomoV™ diagnostic workstation) [28, 31, 35].
All scans were interpreted by a physician specialized on breast diagnostics. SomoV™ interpretation took place independent of the patient´s work-up; the reviewer was blinded to the findings on the corresponding mammograms, HHUS and to all clinical information including the medical history of the patient.
Conventional breast imaging
Mammography and HHUS were evaluated in one session by the same breast diagnostics specialist knowing the patients´ medical history and clinical findings. Bilateral HHUS of the whole breast was performed by a physician specialized in breast imaging by using the Acuson Antares ultrasound system (Siemens Medical Solutions, Mountain View, CA, USA) equipped with a linear-array transducer with a bandwidth of 7.5–13.5 MHz.
Digital mammography was conducted by medical technical assistants using the Mammomat Novation DR (Siemens Medical Solutions, Mountain View, CA, USA). Usually the examination consisted of the cranio caudal (CC) and the medio latero oblique (MLO) projections. Whenever necessary based on the decision of a physician, additional positions as the medio lateral (ML) projection, spotfilms or magnification views were performed.
All mammography images were soft copy double-read by experienced physicians in breast diagnostics.
Data evaluation and statistical analysis
As an explorative study, all statistical analyses are descriptive. Reported p-values have no confirmatory character. Statistical analyses were performed with SPSS Statistics software Version 21.0.
At first, the study cohort is described by calculating absolute and relative frequencies for categorical variables and means and standard deviations for metric outcomes.
The main objective of this study was to evaluate the diagnostic potential of SomoV™ to truly differentiate cancer and normal/benign breast tissue without the knowledge of other clinical information. Therefore, we compared the BI-RADS® scores given for the SomoV™ examinations with those for HHUS, mammography and the gold standard. In order to do this, the BI-RADS® score results were dichotomized as follow:
For SomoV™, the BI-RADS® 1 and 2 were summarized as benign, the BI-RADS® scores 0, 4 and 5 were combined and rated as unclear/suspicious. BI-RADS® score 3 was not used to characterize findings with SomoV™ in this study simulating a screening situation. BI-RADS® 0 was given in all cases with unclear findings as for example possible scar tissue or artifacts.
For HHUS and mammography the BI-RADS® 1, 2 and 3 were summarized as benign, the BI-RADS® scores 4 and 5 were combined and rated as unclear/suspicious/malignant.
Kappa statistics were applied to measure agreement between the different diagnostic methods [36]. Moreover the corresponding 95 % confidence intervals are provided.
We also calculated sensitivity, specificity and positive and negative predictive values.
Results
Study population
In total, 983 patients were examined with SomoV™ which leads to 1,966 cases (=breasts/thoracic walls) to be evaluated. The mean age was 55.7 years (range 19–92 years). A number of 348 patients (35 %) came for further evaluation of lesions detected in an outpatient clinic, 283 patients (29 %) came for a routine checkup, 274 patients (28 %) attended their follow-up visit after breast cancer and 78 (8 %) came for re-evaluation of lesions that were under observation (further details see Table 1). Table 2 shows the given BI-RADS® scores for SomoV™, HHUS and mammography.
Agreement of SomoV™ interpretation with HHUS and mammography
Table 3 shows the absolute numbers of agreement and disagreement for SomoV™ and HHUS yielding a kappa coefficient of 0.31 (95 % CI [0.26;0.36], p < 0.0001). In 1,638 cases (83 %) both methods came to the same interpretation. Mammography and SomoV™ agreed slightly worse (kappa coefficient 0.25 (95 % CI [0.2;0.3], p < 0.0001) (see Table 4).
Performance of SomoV™ compared with the gold standard
As some lesions were explicitly not seen in HHUS e.g. microcalcification (even not in a second-look US after MRI) the lesions were consequently biopsied under mammographical guidance (53 lesions). These cases are not expected to be seen in SomoV™ and therefore have been excluded from the further analysis.
The absolute numbers of agreement which result in a kappa coefficient of 0.3 and a total agreement rate of 84 % are shown in Table 5. In this group 119 breast cancers were detected. Sensitivity, specificity, positive and negative predictive values (PPV and NVP) are given as 74, 85, 24 and 98 %, respectively.
SomoV™ detected breast cancer correctly in 88 cases (74 % sensitivity). Thirty one breast cancer cases were not detected with SomoV™. After reevaluating these cases another 12 were seen when rereading the original SomoV™ scans for evaluation (84 % sensitivity, 85 % specificity, 27 % PPV and 99 % NVP). A further eight cases were seen primarily with MRI or mammography and the US-guided biopsy was done after a second-look HHUS. Within the remaining 11 cases not seen by SomoV™, the cancer was located behind the nipple or very peripheral in five cases so that acquisition technique came to its limits. In two cases an ulcerated carcinoma made the examination difficult and in one case a recurrent focus near the chest wall after mastectomy and reconstruction was difficult to interpret without the clinical information. In a clinical situation when informed about the patients´ history and combining that knowledge with the clinical findings and breast imaging only 11 cancers would not have been found. Respectively 108 of 119 breast cancers would have been detected resulting in a sensitivity of 91 %.
Discussion
SomoV™ was at a disadvantage in comparison to HHUS and mammography due to the blinding since the examiners of HHUS and mammography had knowledge about the clinical situation (scars, lumps, history of the patient, etc.). In addition, the results of mammography were available during HHUS examination and interpretation. In contrast the examiners evaluating SomoV™ data were given no further information about the patients` medical history or results from any other examination. We were aware that this would lead to a diagnostic imbalance between the different methods and a disadvantage for SomoV™, but we aimed to analyze the diagnostic test performance in a situation similar to screening. By including diagnostic cases we intended to increase the number of potential findings in the SomoV™ data.
Nevertheless the study revealed kappa coefficients that indicate fair agreement (k = 0.25–0.31) with HHUS and mammography. Otherwise total agreement rates for dichotomized (benign vs. malignant) were even above 80 %.
The specificity rates are acceptable, which means that a negative result with SomoV™ is very likely to be truly negative. The sensitivity ranged from 74 to 91 % depending on the fact if second-look US and clinical information were included or not.
Challenges of the scanning technique
Some problems concerning the quality of the acquired data arise from the scanning unit´s construction. As soon as a breast contour is modified (for example after surgery) the contact between transducer and tissue can be inadequate and a lack of data and artifacts can be the consequence.
Strength of the study
The strength of this study is the large series of cases. Contrary to other studies, where nearly every examined breast showed a finding, the examiners in our study had a more heterogeneous group and were blinded to all clinical information. This made evaluation more difficult. Wenkel et al., for example, examined only suspicious breasts with SomoV™ so that there was a finding in nearly every data set [31]. Kotsianos-Hermle et al. included only suspicious findings in their study [37]. In the study of Prosch et al. the examiners did not know the results from HHUS and mammography but they were informed about the patients´ clinical history so that they knew about scars, etc. [28].
Outline: implementation of SomoV™ as a screening tool
It is very important for the application of a screening method that nearly every patient with a lesion is detected. From the 144 carcinomas confirmed by biopsy, 92 were detected with SomoV™. From the remaining 52 cases 21 cancers could not be seen with HHUS, neither with SomoV™. Excluding all cases where the failure was definitely not due to the SomoV™ technique (see results), only 19 cancers would not have been detected with SomoV™. Moreover the number of undetected lesions would decrease with the examiners gaining experience and a setting where the examiners are given additional information concerning the patients´ history. Therefore Somo V™ could be a valuable screening tool for either young women in addition to the clinical examination or women between 50 and 70 in combination with mammography. The problem of false positive results is that they cause unnecessary anxiety for patients on the one hand and further diagnostic procedures, as a second-look HHUS and in some cases core cut biopsies on the other hand. This means an emotional drain for the concerned patients. The false positive rate in our study would probably decrease with growing experience and when medical history and the clinical information can be taken into account. The BI-RADS® score 0 was given exceptionally frequently for SomoV™ data. It is likely that being given more information about the patients´ history and clinical findings and having more experience with SomoV™ examination, the examiners could decide more often whether a lesion is suspicious or not and the number of BI-RADS® score 0 would decrease, which would lead to decreasing false positive rates.
Conclusion
The results of this explorative study let us suggest, that automated 3D ultrasound might be a helpful new tool in breast imaging, especially in screening.
References
Wild JJ, Neal D (1951) Use of high-frequency ultrasonic waves for detecting changes of texture in living tissues. Lancet 1(6656):655–657
Kreienberg R (2012) Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Leitlinienprogramm Onkologie der AWMF, Deutschen Krebsgesellschaft e.V. und Deutschen Krebshilfe e.V. http://www.awmf.org/leitlinien/detail/ll/032-045OL.html
Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, Brenner RJ, Bassett L, Berg W, Feig S, Hendrick E, Mendelson E, D’Orsi C, Sickles E, Burhenne LW (2010) Breast cancer screening with imaging: recommendations from the society of breast imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol 7(1):18–27. doi:10.1016/j.jacr.2009.09.022
Newell MS, Birdwell RL, D’Orsi CJ, Bassett LW, Mahoney MC, Bailey L, Berg WA, Harvey JA, Herman CR, Kaplan SS, Liberman L, Mendelson EB, Parikh JR, Rabinovitch R, Rosen EL, Sutherland ML (2010) ACR appropriateness criteria(R) on nonpalpable mammographic findings (excluding calcifications). J Am Coll Radiol 7(12):920–930. doi:10.1016/j.jacr.2010.07.006
Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L (2008) European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition–summary document. Ann Oncol 19(4):614–622. doi:10.1093/annonc/mdm481
Albert US, Altland H, Duda V, Engel J, Geraedts M, Heywang-Kobrunner S, Holzel D, Kalbheim E, Koller M, Konig K, Kreienberg R, Kuhn T, Lebeau A, Nass-Griegoleit I, Schlake W, Schmutzler R, Schreer I, Schulte H, Schulz-Wendtland R, Wagner U, Kopp I (2009) 2008 Update of the guideline: early detection of breast cancer in Germany. J Cancer Res Clin Oncol 135(3):339–354. doi:10.1007/s00432-008-0450-y
Bae MS, Moon WK, Chang JM, Koo HR, Kim WH, Cho N, Yi A, Yun BL, Lee SH, Kim MY, Ryu EB, Seo M (2014) Breast cancer detected with screening US: reasons for nondetection at mammography. Radiology 270(2):369–377. doi:10.1148/radiol.13130724
Zonderland HM, Coerkamp EG, Hermans J, van de Vijver MJ, van Voorthuisen AE (1999) Diagnosis of breast cancer: contribution of US as an adjunct to mammography. Radiology 213(2):413–422
Corsetti V, Ferrari A, Ghirardi M, Bergonzini R, Bellarosa S, Angelini O, Bani C, Ciatto S (2006) Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med 111(3):440–448. doi:10.1007/s11547-006-0040-5
Crystal P, Strano SD, Shcharynski S, Koretz MJ (2003) Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol 181(1):177–182
Leconte I, Feger C, Galant C, Berliere M, Berg BV, D’Hoore W, Maldague B (2003) Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol 180(6):1675–1679
Dick DE, Elliott RD, Metz RL, Rojohn DS (1979) A new automated, high resolution ultrasound breast scanner. Ultrason Imaging 1(4):368–377
Egan RL, Egan KL (1984) Detection of breast carcinoma: comparison of automated water-path whole-breast sonography, mammography, and physical examination. AJR Am J Roentgenol 143(3):493–497
Egan RL, Egan KL (1984) Automated water-path full-breast sonography: correlation with histology of 176 solid lesions. AJR Am J Roentgenol 143(3):499–507
Hollenhorst M, Hansen C, Huttebrauker N, Schasse A, Heuser L, Ermert H, Schulte-Altedorneburg G (2010) Ultrasound computed tomography in breast imaging: first clinical results of a custom-made scanner. Ultraschall Med 31(6):604–609. doi:10.1055/s-0029-1245506
Jackson VP, Kelly-Fry E, Rothschild PA, Holden RW, Clark SA (1986) Automated breast sonography using a 7.5-MHz PVDF transducer: preliminary clinical evaluation. Work in progress. Radiology 159(3):679–684
Kimme-Smith C, Bassett LW, Gold RH (1988) High frequency breast ultrasound. Hand-held versus automated units; examination for palpable mass versus screening. J Ultrasound Med 7(2):77–81
Maturo VG, Zusmer NR, Gilson AJ, Smoak WM, Janowitz WR, Bear BE, Goddard J, Dick DE (1980) Ultrasound of the whole breast utilizing a dedicated automated breast scanner. Radiology 137(2):457–463
Richter K, Heywang-Kobrunner SH, Winzer KJ, Schmitt KJ, Prihoda H, Frohberg HD, Guski H, Gregor P, Blohmer JU, Fobbe F, Doinghaus K, Lohr G, Hamm B (1997) Detection of malignant and benign breast lesions with an automated US system: results in 120 cases. Radiology 205(3):823–830
Shipley JA, Duck FA, Goddard DA, Hillman MR, Halliwell M, Jones MG, Thomas BT (2005) Automated quantitative volumetric breast ultrasound data-acquisition system. Ultrasound Med Biol 31(7):905–917. doi:10.1016/j.ultrasmedbio.2005.03.007
Sinha SP, Goodsitt MM, Roubidoux MA, Booi RC, LeCarpentier GL, Lashbrook CR, Thomenius KE, Chalek CL, Carson PL (2007) Automated ultrasound scanning on a dual-modality breast imaging system: coverage and motion issues and solutions. J Ultrasound Med 26(5):645–655
Vilaro MM, Kurtz AB, Needleman L, Fleischer AC, Mitchell DG, Rosenberg A, Miller C, Rifkin MD, Pennell R, Baltarowich O et al (1989) Hand-held and automated sonomammography. Clinical role relative to X-ray mammography. J Ultrasound Med 8(2):95–100
Giuliano V, Giuliano C (2012) Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin Imaging. doi:10.1016/j.clinimag.2012.09.018
Golatta M, Franz D, Harcos A, Junkermann H, Rauch G, Scharf A, Schuetz F, Sohn C, Heil J (2013) Interobserver reliability of automated breast volume scanner (ABVS) interpretation and agreement of ABVS findings with hand held breast ultrasound (HHUS), mammography and pathology results. Eur J Radiol 82(8):e332–e336. doi:10.1016/j.ejrad.2013.03.005
Kelly KM, Richwald GA (2011) Automated whole-breast ultrasound: advancing the performance of breast cancer screening. Semin Ultrasound CT MR 32(4):273–280. doi:10.1053/j.sult.2011.02.004
Lin X, Wang J, Han F, Fu J, Li A (2012) Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol 81(5):873–878. doi:10.1016/j.ejrad.2011.02.038
Padilla F, Roubidoux MA, Paramagul C, Sinha SP, Goodsitt MM, Le Carpentier GL, Chan HP, Hadjiiski LM, Fowlkes JB, Joe AD, Klein KA, Nees AV, Noroozian M, Patterson SK, Pinsky RW, Hooi FM, Carson PL (2013) Breast mass characterization using 3-dimensional automated ultrasound as an adjunct to digital breast tomosynthesis: a pilot study. J Ultrasound Med 32(1):93–104
Prosch H, Halbwachs C, Strobl C, Reisner LM, Hondl M, Weber M, Mostbeck GH (2011) Automated breast ultrasound vs. handheld ultrasound: BI-RADS classification, duration of the examination and patient comfort. Ultraschall Med 32(5):504–510. doi:10.1055/s-0031-1273414
Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH (2011) Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am J Roentgenol 197(3):747–754. doi:10.2214/AJR.10.5841
Wang ZL, Xw JH, Li JL, Huang Y, Tang J (2012) Comparison of automated breast volume scanning to hand-held ultrasound and mammography. Radiol Med 117(8):1287–1293. doi:10.1007/s11547-012-0836-4
Wenkel E, Heckmann M, Heinrich M, Schwab SA, Uder M, Schulz-Wendtland R, Bautz WA, Janka R (2008) Automated breast ultrasound: lesion detection and BI-RADS classification -a pilot study. Roefo 180(9):804–808. doi:10.1055/s-2008-1027563
Wojcinski S, Farrokh A, Hille U, Wiskirchen J, Gyapong S, Soliman AA, Degenhardt F, Hillemanns P (2011) The automated breast volume scanner (ABVS): initial experiences in lesion detection compared with conventional handheld B-mode ultrasound: a pilot study of 50 cases. Int J Womens Health 3:337–346. doi:10.2147/IJWH.S23918
Zhang Q, Hu B, Li WB (2012) Detection of breast lesions using an automated breast volume scanner system. J Int Med Res 40(1):300–306
Mendelson E, Baum J, Berg W (2003) Breast imaging reporting and data system: ACR BI-RADS—breast imaging atlas. In: BI-RADS: Ultrasound Reston, American College of Radiology, VA
Chang JM, Moon WK, Cho N, Park JS, Kim SJ (2011) Radiologists’ performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur J Radiol 78(1):99–103. doi:10.1016/j.ejrad.2011.01.074
Landis J, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Kotsianos-Hermle D, Hiltawsky KM, Wirth S, Fischer T, Friese K, Reiser M (2009) Analysis of 107 breast lesions with automated 3D ultrasound and comparison with mammography and manual ultrasound. Eur J Radiol 71(1):109–115. doi:10.1016/j.ejrad.2008.04.001
Ethical standards
The vote of an independent ethics committee has been received.
Conflict of interest
There is no actual or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golatta, M., Baggs, C., Schweitzer-Martin, M. et al. Evaluation of an automated breast 3D-ultrasound system by comparing it with hand-held ultrasound (HHUS) and mammography. Arch Gynecol Obstet 291, 889–895 (2015). https://doi.org/10.1007/s00404-014-3509-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3509-9