Abstract
Objective
To evaluate the utility of T1 mapping on gadoxetic acid-enhanced MRI and DWI for staging liver fibrosis and assess the influence of ROI positioning on interobserver variability, T1 relaxation time and ADC value.
Methods
This retrospective study was approved by the institutional review board and included 150 patients (mean age 58 years old; 91 men and 59 women). Liver fibrosis stages (S) were histopathologically determined. T1 relaxation time and ADC value of liver were measured by three distinct ROI protocols (the whole left lobe liver, the whole right lobe liver and the individual ROIs). T1 relaxation time measurements were compared with ADC values according to S scores. Interobserver variability for the T1 relaxation times and ADC values by the three distinct ROI protocols was analyzed by calculating the ICC.
Results
T1 relaxation time measurements by the three distinct ROI protocols on severe fibrosis stage were significantly higher than the relative values on mild fibrosis stage. The mean ADC values on severe fibrosis stage showed no significantly different when measured by means of the whole right lobe liver (p = 0.057) and the individual ROIs (p = 0.10), compared with the relative values on mild fibrosis stage. AUCs of T1 relaxation time and ADC value by the means of the three distinct ROI protocols were 0.614, 0.676, 0.677 and 0.656, 0.585, 0.575 for identification of severe fibrosis stage. The interobserver reproducibility was excellent for measuring the right lobe liver T1 relaxation time and the individual ROIs T1 relaxation time (ICC 0.814, 0.883, respectively).
Conclusions
T1 relaxation time measurements by means of the three distinct ROI protocols on gadoxetic acid-enhanced MR imaging were a potential biomarker in staging of hepatic fibrosis, which were more accuracy than DWI-ADC measurements. The more reproducible results were obtained when measuring T1 relaxation time of the whole right lobe liver and the individual ROIs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic liver diseases represent a major public health problem, accounting for significantly morbidity and mortality worldwide [1]. It can lead to hepatic fibrosis, cirrhosis, end-stage liver disease, and portal hypertension and to the development of hepatocellular carcinoma (HCC) [2, 3]. The diagnosis of liver fibrosis staging remains an important issue in patients with chronic liver disease. The early detection of fibrosis is important for determining disease progression and postponing the evolution of chronic hepatitis into cirrhosis via the implementation of prompt and specific treatment. Fibrosis is a part of the innate wound healing response, which occurs in injured tissues [4]. And within the liver, fibrosis is characterized by the deposition of extracellular matrix. Currently, histopathologic examination of a liver biopsy is still the “gold standard” for liver fibrosis diagnosis and staging [5, 6]. However, liver biopsy is an invasive procedure with rarely but potentially life-threatening complications and prone to sampling errors [7, 8]. The patient, who should be monitored the treatment effect, will have liver biopsy repeatedly, and it will increase the rate on complications (haemorrhage in 0.3 % of patients and mortality in 0.01 %) [9]. Besides, due to sampling errors, liver biopsy is hindered by an approximately 24 % false negative rate for the diagnosis of cirrhosis [10]. Now the noninvasive methodologies for the assessment of fibrosis have developed, such as the indirect markers of liver injury, computed tomography (CT), transient elastography (TE) and magnetic resonance imaging (MRI) [11, 12]. Among them, recent advances in MRI have led to a growing interest in optimizing and applying functional MRI to the assessment of liver disease [13]. Such MRI methods include diffusion-weighted imaging (DWI), perfusion weighted MRI, and MR spectroscopy. Of these methods, DWI particularly can be easily incorporated into a routine MRI protocol. DWI is based on the random Brownian motion of water molecules within the voxel. This motion can be quantified through the apparent diffusion coefficient (ADC) of protons, which depends on the tissue structure. Li H et al. [14] showed that hepatic MR-DWI played a key role in evaluating liver fibrosis following transcatheter arterial chemoembolization (TACE) with low doses of chemotherapy.
Gadoxetic acid (Primovist®; Bayer-Schering Pharma AG, Berlin, Germany) is a MR imaging contrast medium that is developed for evaluating the hepatobiliary system. Many studies have showed that it is useful for the detection and characterization of focal liver lesions, HCCs particularly [15, 16]. After intravenous injection, gadoxetic acid is gradually taken up by hepatocytes and eventually excreted via the biliary pathway. Furthermore, hepatocyte-phase gadoxetic acid-enhanced MRI can measure hepatocyte function potentially. Previous studies showed that patients with liver dysfunction presented with reduction of liver parenchymal enhancement using gadoxetic acid-enhanced MR imaging on hepatobiliary phase (HBP) by the methods of direct measurement of liver parenchymal signal intensity or calculation of perfusion parameters [17, 18].
An alternative approach to direct measurement of signal intensity and perfusion imaging would be to evaluate T1 relaxation time of liver parenchyma, which was directly correlated with the concentration of gadoxetic acid in theory, more reliable and less subjective. To our knowledge, evaluation of gadoxetic acid-enhanced MR imaging for hepatic fibrosis staging has been reported both in animal models and patients [19, 20]. But, the methods of drawing region of interest (ROI) are inconformity. The progression of fibrosis to cirrhosis has a number of sequelae. Histological staging of fibrosis is a combinatorial assessment of amount of fibrosis and architectural disorganization. It is a continuous process and it maybe distributes unevenly in the whole liver.
Thus, the purpose of our study is to evaluate the potential biomarker for liver fibrosis staging, and compare the results of T1 relaxation time with DWI-ADC value and assess the influence of ROI positioning on interobserver variability, T1 relaxation time and DWI-ADC value on gadoxetic acid-enhanced MR imaging.
Materials and methods
Patients
This retrospective study was approved by Institutional Review Board of our hospital and the requirement for informed consent was waived. Between January 2013 and October 2015, 350 patients who were suspected of having focal hepatic lesions and underwent preoperative gadoxetic acid-enhanced MRI examination were included. Exclusion criteria consist of (a) non-availability of pre-treatment gadoxetic acid-enhanced MR imaging using T1 mapping; (b) difficulty to measure the ADC/T1 mapping values because of motion artifact; (c) diffuse hepatic lesions; (d) previous liver resection. F score was histopathologically determined with hepatectomy conducted to treat hepatic tumors. Finally, 150 patients were evaluated in the study. 91 of the 150 patients were men and 59 were women. Median age was 58 years old (range 25–78). The mean time interval between surgical resection and the MRI examination was 3 days (range 2–7 days).
MR imaging
MR imaging was carried out on a 1.5-T MR system (Magnetom Aera, Siemens Medical Solution, Erlangen, Germany) with phased-array coils. For T1 mapping with Syngo MapIt, a dual flip angle 3D gradient echo sequence with volumetric interpolated breath-hold examination (VIBE) was performed after injection of gadoxetic acid (Primovist®; Bayer-Schering Pharma AG, Berlin, Germany). The parameters were as follows: 4.38/1.93 (repetition time msec/echo time msec); flip angle, 2° and 12°; field of view (FOV), 380–400 × 300–324 mm; matrix, 216 × 288; 200 cm slab thickness resulting in an interpolated 5-mm section thickness. A parallel imaging technique (R = 2) was performed using generalized autocalibrating partially parallel acquisition (GAPPA). In all patients, 0.025 mmol/kg body weight of gadoxetic acid was injected manually at about 1 ml/s, by one investigator through a 20-gange intravenous catheter placed in a cubical or cephalic vein. Immediately afterwards, a 20 ml saline flush was administered at the same injection rate. T1 mapping was obtained at 20 min after gadoxetic acid administration for HBP.
Three scan trace free-breath DW images were obtained prior to gadoxetic acid injection using a single-shot spin-echo echo planar imaging sequence (TR/TE, 3200/56 ms; slice thickness, 5.5 mm; matrix size, 84 × 128; FOV, 380–400 × 300–324 mm) with a b value of 0 and 500 s/mm2. A parallel imaging technique (R = 2) was performed using GAPPA.
Imaging evaluation
Quantitative T1 relaxation time maps and ADC maps were derived automatically on a voxel-by-voxel basis. The MR data sets were transferred to a workstation (Leonardo, Siemens, Erlangen, Germany) to measure the T1 relaxation time and ADC value of the liver parenchyma using operator-defined ROI. The MR images were independently analyzed by two radiologists (Cai-zhong Chen and Li Yang), who performed T1 relaxation times and ADC values. The readers were blinded to each other’ results, the clinical patient data and pathology reports. They manually drew the ROIs on the T1-weighted images and high b value (b = 500 s/mm2) DW images, except for the border of the liver (to avoid partial volume effects), any visible lesions, major hepatic veins and the inferior vena cava, then copied to corresponding T1 maps and ADC maps.
ROI protocols
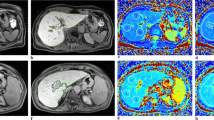
The hepatic parenchyma T1 relaxation time and ADC value were measured according to three distinct ROI protocols: (a) the whole left lobe liver; (b) the whole right lobe liver; (c) four ROIs on the liver randomly and mean T1 relaxation time and ADC value for 4 ROIs being considered as the representative measurement for the liver (individual ROIs)(Fig. 1a–f).
a–f Examples of placement of regions of interest (ROI) in the liver parenchyma on T1 mapping to measure the T1 relaxation time by the whole left lobe liver (a), to measure the T1 relaxation time by the whole right lobe liver (b) and to measure the T1 relaxation time by individual ROIs (c). Examples of placement of regions of interest (ROI) in the liver parenchyma on ADC map to measure the ADC value by the whole left lobe liver (d), to measure the ADC value by the whole right lobe liver (e) and to measure the ADC value by individual ROIs (f)
Histopathology standard
All surgical specimens were evaluated by a senior hepatopathologist with 10 years of experience, having no knowledge of radiologic diagnoses. Fibrosis stages were determined semiquantitatively. Criteria for the fibrosis stages were as follows: F0, no fibrosis; F1, fibrous portal expansion; F2, bridging fibrosis; F3, bridging fibrosis with architectural distortion; and F4, cirrhosis [18].
Statistical analyses
Statistical analyses were performed using MedCalc (MedCalc for Windows, version 11.5.0.0, www.medcalc.be). Interobserver variability for the T1 relaxation times and ADC values of the two readers for the right lobe liver and the left lobe liver and for the each individual ROI method were analyzed according to the method of Bland and Altman and by calculating the intraclass correlation coefficient (ICC) (0.00–0.20 poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good and 0.81–1.00 excellent correlation). T1 relaxation times and ADC values were averaged between the two observers for further analyses. The correlations between MR measurements (T1 relaxation time and ADC value) and F scores were assessed using Spearman’s rank-correlation test. The T1 relaxation time and ADC value, respectively, were compared between F scores (≤2) and F scores (≥3) using a Student’s t test when normally distributed or Mann–Whitney U test when not normally distributed. In addition, receiver operating characteristics (ROC) analyses were constructed to determine the potential diagnostic performance for differentiating F scores (≤2) from F scores (≥3) patients. Corresponding areas under the ROC curve (AUCs) and 95 % confidence intervals (95 % CI) were calculated. Difference in diagnostic performance was analyzed by comparing the ROC curves. Difference in AUCs was analyzed by comparing the ROC curves according to the method of DeLong et al. [19]. Difference with a p value smaller than 0.05 was considered statistically significant.
Results
Baseline patients’ characteristics
A total of 150 patients, who underwent partial hepatectomy, were included finally. And the pathological results were as follows: hepatocellular carcinoma, n = 89; intrahepatic cholangiocarcinoma, n = 13; hemangioma, n = 15; cirrhosis, n = 3; focal nodular hyperplasia, n = 9; colorectal liver metastases, n = 12; inflammatory nodules, n = 2; degenerative nodule, n = 2; angiomyolipomas, n = 3; hepatocellular adenoma, n = 2. Patients were classified as mild fibrosis stage (F score ≤ 2) and patients were classified as severe fibrosis stage (F score ≥ 3).
Effect of ROI methods
The mean values of T1 relaxation time and DWI-ADC were displayed in Table 1 for the mild fibrosis stage and severe fibrosis stage of each respective ROI protocol. The mean T1 relaxation time values on severe fibrosis stage were significantly higher when measured by means of the whole left lobe liver (p = 0.035), the whole right lobe liver (p = 0.002) and the individual ROIs (p = 0.0006), compared with the relative values on mild fibrosis stage. The mean ADC values on severe fibrosis stage showed no significantly different when measured by means of the whole right lobe liver (p = 0.057) and the individual ROIs (p = 0.10), compared with the relative values on mild fibrosis stage. Only by means of the whole left lobe liver, the mean value on severe fibrosis stage showed significantly lower than the value on mild fibrosis stage (p = 0.003).
Diagnostic performance for assessment of severe fibrosis stage
The ROC curves performed to compare the diagnostic performance of T1 relaxation time and ADC value by means of the three distinct ROI protocols for assessment of severe fibrosis stage were provided in Table 2. AUCs of T1 relaxation time value for identification of severe fibrosis stage were 0.614, 0.676 and 0.677, respectively. AUCs of ADC value for identification of severe fibrosis stage were 0.656, 0.585 and 0.575, respectively. The comparison of ROC analyses on severe fibrosis stage using T1 relaxation time and ADC value by the three distinct ROI protocols were showed in Table 3. The values of T1 relaxation time by means of three distinct ROI protocols (AUC 0.614, 0.676 and 0.677, respectively) were better than that of ADC by means of measuring the right lobe liver and the individual ROIs (AUC 0.585, 0.575, respectively) for identification of severe fibrosis stage. However, there was no significant difference among the each measuring methods on T1 relaxation time and ADC value.
Interobserver variability
ICCs between the two readers were provided in Table 4 for the three distinct ROI protocols. The interobserver reproducibility was excellent for the measuring the right lobe liver T1 relaxation time and the individual ROIs T1 relaxation time (ICC 0.814, 0.883, respectively), and good for measuring the left lobe liver T1 relaxation time (ICC 0.768). For the measuring the ADC values by the three distinct ROI protocols, the ICCs ranged from 0.247 to 0.640.
Discussion
Our study results mainly revealed that T1 relaxation time measurement by the means of the three distinct ROI protocols on gadoxetic acid-enhanced MR imaging showed significantly different between mild fibrosis stage with severe fibrosis stage (p = 0.035, p = 0.002 and p = 0.0006, respectively) and had good accuracy for identification of severe fibrosis stage with AUCs of 0.614, 0.676 and 0.677. However, by means of measuring the whole right lobe liver and the individual ROIs, the mean ADC values on mild fibrosis stage showed no significantly different with the relative values on severe fibrosis stage. The results were in keeping with the previous study. Watanabe et al. [21] indicated that the contrast enhancement index with gadoxetic acid-enhanced MR imaging was an efficient biomarker in the staging of liver fibrosis and was more accurate than ADC values. It is clinically useful for the hepatologists to determine the stage of fibrosis to manage patients with chronic liver disease properly. The risks of various complications would increase dramatically if patients reached the stage of cirrhosis. Although, there are numerous ways of diagnosing and quantifying liver fibrosis with imaging tools, such as ultrasound methods. The gold standard on non-invasive methods for liver fibrosis detection is still not unanimous. However, a recently developed hepatobiliary contrast agent, gadoxetic acid, has been widely used to determine early-stage carcinogenesis of liver nodules; consequently, it has been used to identify early-stage liver fibrosis [22]. Therefore, we believed that T1 relaxation times could be differential from mild and severe fibrosis stage, which was very useful for the patients both on treatment in time and follow-up effectively. T1 relaxation time is an absolute value, which appears more reliable and less subjective than the direct hepatic signal intensity [23]. Syngo MapIt enables fast MRI sequencing using 3D-VIBE and has a high resolution [24]. Therefore, we include this sequence in routine liver MR imaging in our department. The signal intensity on liver parenchyma would change during the stage of liver fibrosis with the hepatic architectural disruption. ADC value is significantly influenced by technical parameters, such as the b value. Thus, results of ADC values are the lack of a well cut-off value and it is difficult to be used in clinical practice. Also, in our study, ADC values could not differentiate mild fibrosis stage from severe fibrosis stage and were relatively low for identification of severe fibrosis stage.
Until the end stage, the progression of fibrosis to cirrhosis has a number of sequelae. First it will distort hepatic architecture and vasculature, second it will have a deleterious effect on hepatic function and the third it will increase the propensity for neoplastic transformation. It is a continuous process. Therefore, histological staging of fibrosis maybe distributes unevenly in the whole liver. In our study we showed that T1 relaxation time measurements obtained by the three distinct ROI protocols could all differentiate mild fibrosis stage from severe mild fibrosis stage. But the AUCs of T1 relaxation time values obtained from the whole left lobe liver was relatively low for identification of severe fibrosis stage. We recommended that measuring T1 relaxation time used the ROI protocols of the whole right lobe liver and the individual ROIs.
Another interesting finding was that measuring T1 relaxation time in patients by means of the three distinct ROI protocols was more reproducible. T1 relaxation time measurements obtained from the whole right lobe liver and the individual ROIs were more reproducible than those obtained from the whole left lobe liver. They resulted in excellent interobserver reproducibility (ICC 0.814, 0.883). The main reason may be that the left lobe liver is near to the heart. The signal intensity is affected by the cardiac impulse easily. Besides, sometimes it is affected by the aorta beating artifacts. We also found that ADC values obtained by the means of the three distinct ROI protocols were less reproducible. ADC measurements by means of the three distinct ROI protocols resulted in lower interobserver agreement (ICC 0.247–0.640), compared with T1 relaxation time measurements (ICC 0.768–0.883). The reasons are as follows: ① ghosting artifacts; ② low spatial resolution using respiratory-triggered diffusion-weighted imaging.
There were some limitations in our study. Firstly, because of its retrospective nature and the relatively small patients, our study could not avoid sampling bias. Secondly, in our study we only used b value of 0 and 500 s/mm2 to calculate ADC. It might affect the accuracy of ADC values. Another new technique called intravoxel incoherent motion MR imaging (IVIM) has been used, which is a measurement of numerous points of ADC at different b values [25].
In conclusion, T1 relaxation time measurements by means of the three distinct ROI protocols on gadoxetic acid-enhanced MR imaging were a potential biomarker in staging of hepatic fibrosis, which were more accuracy than DWI-ADC measurement. The more reproducible results were obtained when measuring T1 relaxation time of the whole right lobe liver and the individual ROIs.
References
Castera L (2011) Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol 25:291–303
Hu Y, Gong HY, Lin HJ (2015) Real-time tissue elastography for assessment of liver stiffness in adults without known liver disease. J Ultrasound Med Sep 18. pii: 14.10001. (Epub ahead of print)
Nakayama H, Takayama T (2015) Management before hepatectomy for hepatocellular carcinoma with cirrhosis. World J Hepatol 7:2292–2302
Robert S, Indra NG (2014) Non-invasive monitoring of liver fibrosis. Br Med Bull 112:97–106
Bedossa P, Carrat F (2009) Liver biopsy: the best, not the gold standard. J Hepatol 50:1–3
Castera L (2008) Assessing liver fibrosis. Gastroenterol Hepatol 2:541–552
Rockey DC, Caldwell SH, Goodman ZD et al (2009) Liver biopsy. Hepatology 49:1017–1044
Ichikawa S, Motosugi U, Morisaka H et al (2015) Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging 33:26–30
Castera L, Negre I, Samii K, Buffet C (2001) Patient-administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: a randomized placebo-controlled trial. Am J Gastroenterol 96:1553–1557
Aube C (2015) Imaging modalities for the diagnosis of hepatic fibrosis and cirrhosis. Clin Res Hepatol Gastroenterol 39:38–44
Lucidarme D, Foucher J, Le Bail B et al (2009) Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology 49:1083–1089
Castéra L, Vergniol J, Foucher J et al (2005) Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128:343–350
Bohte AE, de Niet A, Jansen L et al (2014) Non-invasive evaluation of liver fibrosis: a comparison of ultrasound-based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur Radiol 24:638–648
Tokgoz O, Unal I, Turgut GG et al (2014) The value of liver and spleen ADC measurements in the diagnosis and follow up of hepatic fibrosis in chronic liver disease. Acta Clin Belg 69:426–432
Rihyeon K, Jeong ML, Cheong S et al (2015) Differentiation of intrahepatic mass-forming cholangiocarcimona from hepatocellular carcinoma on gadoxetic acid-enhanced liver MR imaging. Eur Radiol. doi:10.1007/s00330-015-4005-8
Haimerl M, Wachtler M, Platzek I et al (2013) Added value of Gd-EOB-DTPA-enhanced Hepatobiliary phase MR imaging in evaluation of focal solid hepatic lesions. BMC Med Imaging 13:41
Saito K, Ledsam J, Sourbron S et al (2014) Measuring hepatic functional reserve using low temporal resolution Gd-EOB-DTPA dynamic contrast-enhanced MRI: a preliminary study comparing galactosyl human serum albumin scintigraphy with indocyanine green retention. Eur Radiol 24:112–119
Jang YJ, Cho SH, Bae JH et al (2013) Noninvasive assessment of hepatic fibrosis using gadoxetate-disodium-enhanced 3T MRI. Ann Hepatol 12:926–934
Ding Y, Rao SX, Chen CZ et al (2015) Liver fibrosis staging using T1 mapping on Gadoxetic acid-enhanced MR imaging compared with DW imaging. Clin Radiol 70:1096–1103
Tsuda N, Okada M, Murakami T (2010) New proposal for the staging of nonalcoholic steatohepatitis: evaluation of liver fibrosis on Gd-EOB-DTPA-enhanced MRI. Eur J Radiol 73:137–142
Watanabe H, Kanematsu M, Goshima S et al (2011) Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging—preliminary observations. Radiology 259:142–150
Lagadec M, Doblas S, Giraudeau C et al (2015) Advanced fibrosis: correlation between pharmacokinetic parameters at dynamic gadoxetate-enhanced MR imaging and hepatocyte organic anion transporter expression in rat liver. Radiology 274:379–386
Bae KE, Kim SY, Lee SS et al (2012) Assessment of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig Dis 30:617–622
Yoshimura N, Saito K, Saguchi T et al (2013) Distinguishing hepatic hemangiomas from metastatic tumors using T1 mapping on gadoxetic-acid-enhanced MRI. Magn Reson Imaging 31:23–27
Marzi S, Stefanetti L, Seperati F et al (2015) Relationship between diffusion parameters derived from intravoxel incoherent motion MRI and perfusion measured by dynamic contrast-enhanced MRI of soft tissue tumors. NMR Biomed. doi:10.1002/nbm.3446
Acknowledgments
This study was funded by the Youth Program on Shanghai Municipal Commission of Health and Family Planning (Grant No. 20154Y0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no any conflict of interest information for all authors.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional. This retrospective study was approved by Institutional Review Board of our hospital and the requirement for informed consent was waived.
Rights and permissions
About this article
Cite this article
Ding, Y., Rao, S., Yang, L. et al. Comparison of the effect of region-of-interest methods using gadoxetic acid-enhanced MR imaging with diffusion-weighted imaging on staging hepatic fibrosis. Radiol med 121, 821–827 (2016). https://doi.org/10.1007/s11547-016-0669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0669-7