Abstract
Background
Lutetium-177 prostate-specific membrane antigen (177Lu-PSMA) radioligand therapy is emerging as a promising treatment for metastatic castration-resistant prostate cancer refractory to established therapies. While there is an increasing body of survival and other data from retrospective analyses and prospective trials, there is no clear understanding of how best to predict therapy response and survival outcomes.
Objective
In this retrospective cohort analysis, we aimed to identify features that are associated with response to radioligand therapy and greater survival based on analysis of real-world data.
Patients and Methods
191 patients aged 70 ± 8 years with metastatic castration-resistant prostate cancer treated with radioligand therapy from November 2015 to February 2019 were included for analysis. Eligible patients had PSMA-expressing metastatic castration-resistant prostate cancer (confirmed by a 68Ga-PSMA-ligand positron emission tomography (PET)/computed tomography (CT) scan), an Eastern Cooperative Oncology Group performance status score ≤ 2 and no significant kidney, liver or bone marrow dysfunction (as characterised by kidney and liver function tests and a full blood count). Patients received one to five cycles of intravenous 177Lu-PSMA-ligand therapy. Endpoints included biochemical [prostate-specific antigen (PSA)] and radiologic (PSMA PET/CT) response, progression-free survival and overall survival, defined according to the Prostate Cancer Working Group 3 guidelines. Survival analysis was conducted by Kaplan–Meier estimation.
Results
Most individuals (89.5%) previously underwent first- and second-line systematic therapy. Of the 191 men treated with 452 cycles with mean injected activity of 6.1 ± 1.0 GBq per cycle, 159 patients were assessed for a biochemical response defined as a PSA decline ≥ 50% from baseline. A ≥ 50% PSA decline was observed in 89 (56%) patients, while any PSA decline occurred in 120 (75%) men. For the entire cohort, median values (interquartile range) of overall survival [n = 191], PSA progression-free survival [n = 132] and PET/CT progression-free survival were 12 (5–18), 4 (3–8) and 6 (3–10) months, respectively. Survival analysis confirmed better outcomes in individuals who had demonstrated therapy response. Predominantly lymph node metastatic disease and chemotherapy-naïve status were significant pre-therapy factors associated with longer survival. Baseline PSA was significantly linked to survival outcomes: lower levels predicted a lower risk of death and disease progression. Treatment-related adverse events included grade 3 or 4 haematological (12%), grade 1 or 2 renal (4.5%), and grade 3 or 4 clinical events (5.7%).
Conclusions
Our findings suggest that 177Lu-PSMA radioligand therapy provides a significant response rate with a low toxicity profile. The evidence promotes greater efficacy of radioligand therapy in predominantly lymph node metastatic castration-resistant prostate cancer, and in individuals with chemotherapy-naïve status and lower levels of baseline PSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with predominantly lymph node metastatic castration-resistant prostate cancer who have not been pre-treated with chemotherapy have higher overall survival compared with patients with bone metastases and visceral disease, and those who have previously received chemotherapy. |
Patients with a ≥ 50% prostate-specific antigen decline had an improved overall survival twice that of patients with no change in prostate-specific antigen after lutetium-177 prostate-specific membrane antigen-targeted therapy. |
1 Introduction
Metastatic castration-resistant prostate cancer (mCRPC) is the second most lethal cancer among men worldwide [1]. In an Australian context, the estimated risk of a male individual being diagnosed with prostate cancer by the age of 85 years is 1 in 6 [2, 3]. Metastatic castration-resistant prostate cancer is characterised by rapid progression towards advanced stages despite treatment with traditional androgen deprivation agents or other systemic therapies [4]. In men who have not responded to curative intent procedures such as radical prostatectomy or primary radiotherapy, systemic therapy is often required beyond the progression of disease [5]. Currently, taxane-based chemotherapy, 223RaCl2 (Xofigo®), immunotherapy and androgen deprivation therapy (ADT) are widely considered life-prolonging treatment modalities for prostate cancer [6].
Docetaxel has shown proven efficacy in a chemotherapy-naïve setting [7], and the second-generation chemotherapeutic cabazitaxel is effective in patients who have received prior docetaxel [8], while enzalutamide, abiraterone, apalutamide and darolutamide extend overall and radiologic progression-free survival (PFS) in patients with advanced prostate cancer [9]. However, current chemotherapy and hormone therapies are often associated with side effects and drug resistance [10]. The alpha emitter 223RaCl2 has been reported to provide an overall survival (OS) benefit of 3.6 months but its therapeutic benefit is limited to the setting of skeletal metastases only [11]. Immunotherapy has demonstrated a survival benefit of a few months but with a lesser impact on progression, and with persistence of toxicity [12]. Recent insights into the mechanism of action of sipuleucel-T indicate a potentially greater benefit in the earlier stages of prostate cancer [13]. As our understanding of immunogenic mechanisms in mCRPC increases, the way is paved for new targets and therapies [14]. For instance, Gao and co-workers recently noted the role of the V-type immunoglobulin domain-containing suppressor of T-cell activation in the immune-regulatory mechanism in prostate cancer [15]. Although immune checkpoint monotherapy has not shown great therapeutic benefit in prostate cancer [16], anticancer effects could be potentiated by appropriate combination therapy.
There is currently no curative treatment for advanced prostate cancer and no global consensus on treatment, though contemporary targeted radiopharmaceutical therapeutic agents are providing promising results and potentially improved prognosis for this disease [5, 17]. Radioligand therapy (RLT) with 177Lu-labelled prostate-specific membrane antigen (PSMA) [177Lu-PSMA] is a therapeutic option for patients with confirmed PSMA expression by baseline PSMA-directed imaging and progressive mCRPC progressive disease after exhaustion of approved therapies [18]. Although 177Lu-PSMA RLT has not yet been approved by the US Food and Drug Administration or the European Medicines Agency, several studies have reported prolonged survival in certain patient populations [4, 19,20,21,22,23]. Currently used PSMA-targeting ligands are small-molecule inhibitors that bind with high affinity to PSMA. When bound via a chelator to Lu-177, the resulting complex emits beta-particle radiation that is delivered effectively and with reasonable specificity to tumour cells, with potentially minimal harm to distant healthy cells. Prostate-specific membrane antigen is a type II membrane protein that is expressed on cells of the prostate and has enzymatic activity as a glutamate-preferring carboxypeptidase. Prostate-specific membrane antigen protein is overexpressed 100- to 1000-fold on prostate cancer cells, with even greater expression generally correlated with metastatic and/or more aggressive cancer [24, 25]. We conducted a retrospective longitudinal cohort study of the safety and efficacy of 177Lu-PSMA RLT in a large cohort of patients with patients with mCRPC and determined the advantages and disadvantages of 177Lu-PSMA RLT based on real-world data.
2 Materials and Methods
2.1 Patients
A cohort of 191 consecutive patients with mCRPC with progressive PSMA-ligand avid disease, who underwent 177Lu-PSMA RLT at different sites across Australia under the management of GenesisCare Theranostics between November 2015 and February 2019, were evaluated in this retrospective study. The cut-off time point for assessments was set as 31 May, 2019.
The study inclusion criteria were PSMA-ligand avid mCRPC, Eastern Cooperative Oncology Group performance status score ≤ 2. All patients at the stage of referral to the initiation of therapy did not have acute or chronic kidney dysfunction (estimated glomerular filtration rate < 30 mL/min/1.73 m2), liver injury (bilirubin >1.5 × upper limit of normal [ULN], or if >1.5 × ULN, normal conjugated bilirubin; aspartate aminotransferase or alanine aminotransferase < 2 × ULN or < 5 × ULN in the presence of liver metastases) or signs of significantly impaired bone marrow function (haemoglobin < 80 g/L, platelet count < 75 × 109/L, neutrophil count < 1.0 × 109/L, lymphocyte count < 0.5 × 109/L). Prior treatments included radical prostatectomy, extended surgery (including pelvic lymph node dissection), external beam radiation therapy, standard ADT, second-generation anti-androgens (e.g., abiraterone and enzalutamide) or taxane-based chemotherapy (e.g., docetaxel and cabazitaxel).
Progressive disease was defined by progression on positron emission tomography/computed tomography (PET/CT) or bone scintigraphy, or new pain in an area of a radiologically confirmed lesion. The study was designed and conducted in accordance with the World Medical Association Declaration of Helsinki, Good Clinical Practice principles and ethical standards of the relevant institutional research committee. Patients provided written informed consent for use of their data for research purposes.
2.2 Patient Assessment
We evaluated metastatic distribution and grouped the cohort by the following approach. Patients who had lymph node metastases (LNM) and no more than three sites of bone lesion and no more than one site of visceral metastases were considered to have predominantly LNM. Patients who had extensive bone metastatic disease (more than three sites) regardless of the extension of lymph node or visceral metastases were considered to have predominantly bone metastases. Patients who had visceral metastases and no bone or lymph metastatic disease were considered to have predominantly visceral metastases. For a small number of patients, pre-RLT images were not available in full, but general interpretations (as per the radiologist report) were at hand.
Analysis of therapy outcomes included evaluation of observational and follow-up periods. The observational period was defined as the time between the commencement of RLT and the cut-off date, while the follow-up period was specified as the time between the last cycle of RLT and the cut-off date. Adjuvant systemic therapy applied between cycles or new systemic therapy commencing after failure of 177Lu-PSMA RLT was also recorded.
177Lu-PSMA-ligand therapy was provided to patients in strict compliance with the Australian Therapeutic Goods Administration Special Access Scheme for compassionate use. Different (but structurally similar) PSMA ligands (either ‘617’ or ‘I&T’) were used at different sites early in the series, with all patients receiving PSMA-I&T from 2017 onwards. No significant differences in biodistribution, safety or effect of these ligands have been reported in the literature [26]. All patients were clinically monitored during radiopharmaceutical administration.
2.2.1 Imaging Response
All individuals underwent the following investigations before the start of therapy, between cycles and at follow-up with clinically reasoned intervals: 68Ga-PSMA-11 PET/CT (to identify PSMA-ligand avid disease and assess therapeutic efficacy), 18F-fluorodeoxyglucose PET/CT (where indicated, e.g., minimal PSMA-ligand avidity and suspected neuroendocrine differentiation), contrast-enhanced CT of the chest, abdomen, or pelvis, and bone scintigraphy (where indicated).
2.2.2 Toxicity
Within 2 weeks before and 4 weeks after each treatment, a full blood count, electrolytes, renal and liver function tests, lactate dehydrogenase, serum PSA and testosterone were measured. Common Terminology Criteria for Adverse Events (version 5.0, 2017) was used as a guide to grade post-therapy adverse events. Incidence of kidney damage (acute kidney injury, acute kidney disease or chronic kidney disease) was assessed in compliance with Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. Patients with suspicious obstructive nephropathy were assessed by 99mTc-mercaptoacetyltriglycine (MAG3) renal scintigraphy to accurately evaluate kidney function.
2.2.3 Biochemical Response
The primary endpoint was PSA response rate defined as the proportion of patients with a PSA decline of ≥ 50% from baseline with confirmation 3–4 weeks apart according to the Prostate Cancer Working Group 3 guidelines [27]. The additional primary endpoint was radiographic response (based on 68Ga-PSMA-ligand PET/CT). Secondary endpoints were OS and biochemical and metabolic PFS defined as the time from the treatment start until tumour progression or death. Biochemical disease progression was recognised in compliance with the Prostate Cancer Working Group 3: (a) if the first PSA increase was ≥ 25% and ≥ 2 ng/mL above the nadir (in the case of a previous PSA decline from baseline), and which is confirmed by a second value ≥ 3 weeks later or (b) PSA ≥ 25% and a ≥ 2-ng/mL increase from baseline beyond 12 weeks if no PSA decline had been observed. Metabolic disease progression was recognised if any new metastatic lesion was detected by PET/CT in comparison with the previous scan. All secondary endpoints were measured in months from the date of the first treatment. The measure of ‘any PSA decline’ was utilised as an additional biochemical endpoint and defined as any PSA decline from baseline with confirmation 3–4 weeks apart after 12-weeks post-therapy.
2.3 Statistical Analysis
Statistical analysis was performed using the Microsoft Office® package and freely available online statistics applications (http://vassarstats.net/odds2x2.html; https://www.medcalc.org/calc; https://www.quantitativeskills.com/sisa/index.htm). For descriptive purposes, mean ± standard deviation (SD) or median with interquartile range (IQR) were reported. For the assessment of statistical significance in the analysis of contingency tables, the two-tailed p value was estimated in the Fisher’s exact test. The Student’s t test or Mann–Whitney U test was used to compare any two groups in dependence on normality of distribution of variables. Categorical data were compared by the chi-square test. Survival analysis was performed using the Kaplan–Meier estimator, and the comparison of survival curves was implemented by the log-rank test. Statistical significance (compatibility) was accepted if the p value was < 0.05.
3 Results
3.1 Patient Cohort Characteristics
In this retrospective cohort study, 191 patients with mCRPC underwent 452 cycles of 177Lu-PSMA RLT (one to five cycles). The mean injected Lu-177 activity was 6.1 ± 1.0 GBq per cycle, and the mean cumulative injected activity was 13.3 (IQR 10.3–19.7) GBq. The mean age at start of treatment was 70 ± 8 (minimum/maximum range from 50 to 92) years. Thirty-four patients (18%) underwent one cycle, 87 (46%) two cycles, 40 (21%) three cycles, 26 (13%) four cycles and 4 (2%) underwent five cycles. Of the 191 patients, 76 (40%) were receiving ongoing follow-up, 66 (35%) were deceased and 42 (22%) were lost to follow-up. In six (3%) cases, the treatment was ceased because of adverse events (two cases with grade 3 bone pain and grade 3 anaemia), progression as a result of concurrent disease (one case of myasthenia gravis, one case of discovered hypocellular bone marrow) and personal reasons. Progression in two cases was considered unrelated to RLT but a significant risk factor for continuation of RLT.
The details of cohort baseline characteristics and demographics are represented in Table 1. The median time interval between the primary diagnosis of prostate cancer and the initiation of 177Lu-PSMA RLT was 6 (IQR 3–10) years. Gleason score history was available for 122 (64%) patients with a mode of 9. Based on 68Ga-PSMA PET/CT imaging reports, 136 patients (71%) had predominantly bone metastases, 37 (20%) had LNM and 8 (4%) had visceral metastases. Full pre-therapy imaging reports were not available for ten (5%) individuals. The group with predominantly visceral metastases was not included in the analysis because of the low number of patients. The influence of the features related to metastatic distribution on endpoints and outcomes was assessed only between the bone metastases and LNM groups. The majority of patients (87%) received prior ADT, while prior chemotherapy was trialled in 115 (60%) individuals. 72 patients (38%) remained chemotherapy-naïve before 177Lu-PSMA RLT. A small number (1.6%) of men received immunotherapy prior to RLT after failure of ADT and chemotherapy. Of note, only 20 (10.5%) men did not undergo ADT, chemotherapy or another systemic treatment before 177Lu-PSMA RLT (Table 1). Non-systemic treatments mostly included radical prostatectomy, extended surgery and external-beam radiation therapy, along with, less frequently, brachytherapy.
3.2 Analysis of Therapy Outcomes
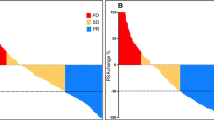
Both biochemical (PSA) and radiographic (post-therapy 68Ga-PSMA PET/CT data) responses were assessed. The PSA response rate (≥ 50% PSA decline from baseline) was 56% (89 patients) while the radiographic response rate was 49.6% (63 patients), where the total number of patients for which data were available was 159 and 127, respectively. Any PSA decline was observed in 120 individuals (75%, n = 159) (Fig. 1) while 39 had PSA elevation after therapy initiation. For the entire cohort, median OS, PSA PFS and 68Ga-PSMA PET/CT PFS were 12 (IQR 5–18, n = 191), 4 (IQR 3–8, n = 158) and 6 (IQR 3–10, n = 124) months, respectively. Of note, five patients (3.6%, n = 137) had not experienced disease progression at the assessment cut-off time point. The proportion of patients who had not experienced disease progression at the 6- and 12-month time points after RLT start were 52% (n = 138) and 35% (n = 81), respectively.
A comparison between the group of patients who achieved a PSA response and the group who did not respond biochemically demonstrated that the mortality rate was higher in the non-response group (51%, n = 70) than the response group (15%, n = 89, p < 0.001) across the whole observational period (Table 2). The rate of disease progression at 6- and 12-month time points was, perhaps predictably, significantly higher in individuals who did not respond to therapy as defined by PSA response. The response group had a longer period of follow-up, OS, PSA PFS and PET/CT PFS compared with the non-response group. Baseline PSA and Gleason score were similar between the two groups. Notably, the time since the diagnosis of prostate cancer in the ‘response’ group was longer, although age at the start of therapy and age at diagnosis did not significantly differ between groups. A trial of adjuvant therapy in some patients did not have an influence on RLT response rate. Both cumulative and average (per cycle) 177Lu activity administered were higher in the ‘response’ group. Interestingly, the comparison highlighted the occasional discordance between PSA and 68Ga-PSMA-PET/CT response: 9% of those who did not have PSA response had a 68Ga-PSMA PET/CT-confirmed response.
3.3 Survival Analysis
Kaplan–Meier survival analyses were performed to assess the impact of any PSA decline and a ≥ 50% PSA decline on OS and PSA and 68Ga-PSMA-PET/CT PFS after RLT. Among 159 patients, 120 demonstrated a statistically significant survival outcome if they had any PSA decline in contrast to no PSA decline (n = 39), log-rank comparison of survival curves noting a p value of 0.0037 and a hazard ratio (HR) for death of 0.33 with a 95% confidence interval (CI) of 0.15–0.69 (Fig. 2a). The OS in patients with a PSA decline of ≥ 50% was 86% higher than in patients where PSA declined by less than 50%, HR for death 0.1 (95% CI 0.07–0.26) with a p value < 0.0001 (Fig. 2b). Similarly, a statistically significant increase in PFS was confirmed in patients with a > 50% decline in PSA (p < 0.0001) and 68Ga-PET/CT objective response (p < 0.0001) (Fig. 2c, d). Thus, we conclude that in this cohort of patients, the risks of disease progression and death were significantly lower in individuals who had a PSA response after treatment (Table 3).
Overall survival of patients with a any prostate-specific antigen (PSA) decline vs. no decline (p = 0.0037) and b a ≥ 50% PSA decline vs ≤ 50% (p < 0.0001). Progression-free survival in patients c with a% PSA decline greater or smaller than 50, hazard ratio 0.36 (95% confidence interval 0.25–0.53) and d as assessed by 68Ga- prostate-specific membrane antigen-positron emission tomography/computed tomography (PET/CT), hazard ratio 0.34 (95% confidence interval 0.22–0.53)
Notably, baseline PSA was a strong predictor of survival outcomes. Serum PSA baseline levels below the cohort median (< 70.4) were significantly associated with a lower chance of death (log rank comparison with p < 0.0001) (Fig. 3a) and disease progression defined by both biochemical and radiographic measures (Table 3). Moreover, analysis of baseline PSA sorted by quartiles demonstrated that the risk of death gradually decreases across baseline PSA decline from 0.25 to 0.09 (Table 4). The risk of death increased almost 12 times with an increase in PSA baseline level, from below 11.7 ng/mL (Q1) to a baseline PSA > 262 ng/mL (Q4) [(95% CI 5.74–23.6] (Fig. 3b).
Overall survival in patients a with baseline prostate-specific antigen (PSA) levels greater or less than 70.4 ng/mL, hazard ratio (HR) 0.25, 95% confidence interval (CI) 0.15–0.41, b by PSA level (ng/mL) in quartiles, where Q1 < 11.7, Q2 (11.7–70.4), Q3 (70.4–262) and Q4 >262, c with lymph node metastases vs bone metastases, median overall survival 40 months (95% CI 33.2–40.7) vs 20 months (95% CI 20.5–26.8) and d with or without prior chemotherapy HR 0.29 (95% CI 0.18–0.48). Estimated median overall survival in the group receiving prior chemotherapy is 14 months (95% CI 18.8–25.8), while median overall survival was not reached at the cut-off date for chemotherapy-naïve patients
Patients with predominantly LNM had a better OS compared with men with predominantly bone metastases: HR for death was 0.19 (95% CI 0.11–0.34; p = 0.0001) (Fig. 3c). This observation was confirmed by a decline in PSA and 68Ga-PSMA-PET/CT verified disease progression with more than half as many patients with LNM noting a decrease in PSA [HR 0.60 (95% CI 0.41–0.88; p = 0.0237)] and 68Ga-PSMA-PET/CT verified disease progression 0.49 (0.32–0.74; p = 0.0063) [Table 3]. The survival outcome was also improved in patients who were chemotherapy-naïve compared to patients receiving prior chemotherapy, with chemotherapy-naïve patients almost 70% less likely to die than those who received prior chemotherapy (p < 0.0001) (Fig. 3d). Furthermore, the risk of disease progression was 0.58 (95% CI 0.42–0.79; p = 0.0011) and 0.51 (95% CI 0.36–0.73; p = 0.0005) as assessed by PSA- and 68Ga-PSMA-PET/CT-based estimations, respectively (Table 3). The small number of patients with visceral-only disease precluded any meaningful analyses; however, seven of the eight patients had a biochemical response (≥ 50% PSA decline) after RLT with a mean OS of 26.20 months (95% CI 19.60–33.0).
3.4 Treatment-Related Adverse Events
A total of 175 patients were evaluated for adverse events (Table 5). Severe haematological events (grade 3 or 4 single event or combination) were observed in 21 patients (12%); these included anaemia (n = 9), thrombocytopenia (n = 6), lymphopenia (n = 10) and neutropenia (n = 1). Renal adverse events were noted in eight patients, three cases of acute kidney injury and five cases developed chronic kidney disease (grade 1 or 2). In nine patients, there were grade 3 or 4 clinical adverse events (as individual cases), which included tiredness (n = 2), bone pain (n = 3), nausea and vomiting (n = 1), proctitis (n = 1), generalised seizures (n = 1) and dehydration (n = 1). Clinical and haematological adverse events required modifications to the disease management plan, including treatment cessation in two patients (one case of anaemia and one case of severe bone pain). The renal adverse events did not influence the RLT regime.
4 Discussion
Our study is one of the largest reported single-institution (GenesisCare) retrospective studies, evaluating the efficacy and safety of 177Lu-PSMA (either 177Lu-PSMA-617 or 177Lu-PSMA-I&T) RLT in a cohort of 191 patients with mCRPC treated at different sites under the management of GenesisCare Theranostics. In contrast with other studies, a large subset of the patients (10.5%) had not been heavily pre-treated by salvage therapies. The efficacy of 177Lu-PSMA RLT was demonstrated either by PSA decline or 68Ga-PSMA PET/CT response. It is well documented that a significant decline in PSA is observed in patients with mCRPC treated with 177Lu-PSMA RLT [4, 19, 20, 22, 23, 28,29,30]. A recent meta-analysis pooled data from 17 studies with a total of 744 patients showing the proportion of patients with any PSA decline and a ≥ 50% PSA decline of 75% (493/671) [95% CI 70–79] and 46% (307/681) [95% CI 40–53], respectively [31]. In addition, a meta-analysis performed by Calopedos et al. aiming to assess biochemical response of 177Lu-PSMA by any PSA decline and a ≥ 50% PSA decline from baseline noted that the pooled proportion of patients with any PSA decline was 68% (95% CI 61–74) with minor heterogeneity between results (I2 statistic 39.1%, p = 0.11). The pooled proportion of patients with a ≥ 50% PSA decline was 37% (95% CI 22–52) with substantial heterogeneity between results (I2 statistic 91%, p < 0.001). In comparison, we reported a 75% (120/159) decline in PSA with 56% (39/159) of patients showing a ≥ 50% PSA decline, comparable to that reported from the pooled meta-analysis data.
A German multicentre retrospective study reported a ≥ 50% PSA decline in 45% of individuals while any PSA decline occurred in 60%, slightly lower than our report [29]. However, 45% of patients showed an objective response as determined by imaging that compares very favourably with the 49.6% (63/127) we observed. The researchers showed that prior chemotherapy and bone or LNM did not significantly influence response rates after 177Lu-PSMA RLT, while the absence of visceral metastases and the number of therapy cycles were relevant independent predictors of biochemical response. Several groups specifically reported that visceral metastases had a negative impact on OS after 177Lu-PSMA RLT [19, 32,33,34]. A small subset of our patients had visceral-only disease and, surprisingly in this subset, seven of the eight patients reported a ≥ 50% PSA decline with OS of 26.20 months (95% CI 19.60–33.0). In addition, our data suggest better outcomes in patients with predominantly LNM and a chemotherapy-naïve status.
This finding was also noted in a study by von Eyben et al., which evaluated outcomes following 177Lu-PSMA RLT in patients with mCRPC with predominantly LNM [6]. The study involved 35 patients from different centres worldwide. The authors noted better survival in this group of individuals. Additionally, docetaxel-naïve status was a suggested beneficial factor for outcomes. Moreover, patients with LNM without bone lesions had a higher response rate than patients with as few as one or two bone metastases. A separate group published their results on the effect of 177Lu-PSMA RLT in 167 patients with mCRPC who were chemotherapy naïve or pre-treated with taxane-based chemotherapy [35]. Pre-treated individuals had poorer performance status, a higher prevalence of bone metastases and higher PSA levels compared to naïve patients. The median OS was 10.7 vs 27.1 months (p < 0.001) while median radiographic PFS was 6.0 months and 8.8 months (p = 0.003) for pre-treated and chemotherapy-naïve patients, respectively. The PSA response rate was 40% in pre-treated individuals vs 57% in naïve individuals (p = 0.054). The results of these two studies are consistent with our data and emphasise that predominantly lymph node mCRPC and a chemotherapy-naïve status appear to be predictors of beneficial therapy outcomes.
In another meta-analysis, Kim and Kim evaluated the therapeutic responses and OS after the first cycle of RLT based on data from ten studies with 455 patients [22]. The pooled rate of any PSA and a ≥ 50% PSA decline was 68% (95% CI 64–72) and 35% (95% CI 30–39), respectively; these rates are lower than what we report in the present study. The pooled HRs for death in patients with any PSA decline was 0.29 (95% CI 0.21–0.40) with a p-value < 0.00001, while individuals with a ≥ 50% PSA decline had an HR of 0.82 (95% CI 0.54–1.25) with no statistical significance (p = 0.39). Interestingly, our study noted an HR of 0.33 (95% CI 0.15–0.69, p < 0.05) in patients with any PSA decline, similar to that reported by Kim and Kim; however, we noted a further decrease in HR to 0.14 (95% CI 0.07–0.24, p < 0.0001) in patients with a ≥ 50% PSA decline. The researchers concluded that any PSA decline can be anticipated in approximately two thirds of patients, and a ≥ 50% PSA decline can be anticipated in one third of patients in response to the first cycle of RLT.
von Eyben et al. evaluated whether 177Lu-PSMA RLT and third-line treatment (including abiraterone, enzalutamide and cabazitaxel) have similar effectiveness and toxicity [36], and included 12 studies with 669 patients in the evaluation. Overall, 51% (95% CI 43–60) of the patients had a ≥ 50% PSA decline after RLT with a median OS of 14 months. This is similar to our own findings. After RLT, adverse effects were mostly transient and no toxicity-related therapy discontinuation cases were found. In conclusion, the researchers highlighted that 177Lu-PSMA RLT appeared to be more beneficial in safety and efficacy than third-line therapies with cabazitazel or androgen receptor antagonists.
The recent publication from Yordanova et al. reported the value of various biochemical markers as potential predictors of survival after 177Lu-PSMA RLT [37]. In this retrospective analysis of 137 patients, similar to our own, the researchers found that the baseline PSA (first quartile cut-off) was significantly correlated with survival. A PSA level below 47 ng/mL at baseline was associated with longer OS than patients with higher PSA levels: 83 weeks (95% CI 43.4–122.6) vs 47 weeks (95% CI 35.8–58.2) with p = 0.007 and an HR of 1.97 (95% CI 1.19–3.27). Moreover, the authors highlighted that the significance of any measured PSA changes (whether decreased or increased) was higher than the value classified according to the Prostate Cancer Working Group 3. In turn, our results demonstrated that baseline PSA was a strong predictor of survival outcome. The levels below the cohort median (< 70.4 ng/mL) and within the first quartile (< 11.7 ng/mL) were significantly associated with a lower chance of death and disease progression. The highest risk of death was noted in the fourth quartile (PSA >262 ng/mL) with almost a 12-fold increase in the risk of death compared with the first quartile.
Apart from retrospective studies and a meta-analysis, a recent prospective phase II trial involving 30 men with mCRPC also reported favourable effects of 177Lu-PSMA RLT with low toxicity [23]. The trial was extended to include 20 new patients with longer term outcomes [20]. The cohort was characterised by extensive prior treatment including prior docetaxel (84%), cabazitaxel (48%), and abiraterone and/or enzalutamide (92%). The observed biochemical response rate was 64% (95% CI 50–77) while 56% achieved an objective response by RECIST 1.1. Interestingly, Violet et al. reported that a PSA response at 12 weeks was predictive of survival with an optimal cut-off defined at 34%. Median OS was 13.3 months (95% CI 10.5–18.7) with a significantly longer survival of 18.4 months (95% CI 13.8–23.8, n = 32) in patients achieving a PSA response compared to 8.7 months (95% CI 6.5–13.4) if a PSA decline was < 50% [20]. These values are consistent with our results. In our study, patients with a biochemical response had an OS of 18 months (IQR 12–22, n = 89) and those who did not respond to RLT had an OS of 9 months (IQR 6–15, n = 70). Median PSA PFS was 6.9 months (95% CI 6.0–8.7). Prostate-specific antigen PFS was also significantly longer in patients with a PSA decline of ≥ 50% (8.2 months) [95% CI 6.9–10.3] compared to 4.2 months (95% CI 3.9–7.1) for those with a decline of PSA of < 50% [20]. The reported adverse events were also relevant to our results. The haematological adverse events contained grade 3 or 4 thrombocytopenia (10%), anaemia (10%), neutropenia (6%), and lymphopenia (32%), and the rates were similar to our data except for the incidence of neutropenia and lymphopenia. Grade 1 or 2 renal adverse effects occurred in 10% of patients, which was higher than that in the present cohort; however, this can be explained by a difference in the assessment of kidney injury [38].
5 Conclusions
In this single-centre retrospective analysis of patients with mCRPC, we revealed a significant response rate (56%) and a low toxicity profile associated with 177Lu-PSMA RLT treatment. Patients demonstrating PSA response had longer overall survival, as well as biochemical and radiographic PFS. In addition, a lower level of baseline PSA was a strong predictor of improved survival, while greater efficacy was seen in patients with predominantly lymph node mCRPC and individuals with a chemotherapy-naïve status. Our real-world data analysis suggests 177Lu-PSMA RLT may be considered not only as a last-line treatment but as a beneficial option in combination with other systemic treatments in mCRPC.
References
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89.
Cancer Council. Prostate cancer. 2020. https://www.cancer.org.au/about-cancer/types-of-cancer/prostate-cancer/. Accessed 24 Mar 2020.
Australian Institute of Health and Welfare. Cancer mortality. 2020. https://ncci.canceraustralia.gov.au/outcomes/cancer-mortality/cancer-mortality. Accessed 24 Mar 2020.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. (177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91.
Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52–60.
von Eyben FE, Singh A, Zhang J, Nipsch K, Meyrick D, Lenzo N, et al. (177)Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget. 2019;10:2451–61.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, et al. Sequencing of Sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. 2017;23:2451.
Madan RA, Antonarakis ES, Drake CG, Fong L, Yu EY, McNeel DG, et al. Putting the pieces together: completing the mechanism of action jigsaw for Sipuleucel-T. J Natl Cancer Inst. 2020;112:562–73.
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23:551–5.
Goswami S, Aparicio A, Subudhi SK. Immune checkpoint therapies in prostate cancer. Cancer J. 2016;22:117–20.
Jones W, Griffiths K, Barata PC, Paller CJ. PSMA theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers. 2020;12:1367.
Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58:1196–200.
Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54.
Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang S-P, Kong G, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of (177)Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:857–65.
Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8:3581–90.
Kim YJ, Kim Y-I. Therapeutic responses and survival effects of 177Lu-PSMA-617 radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin Nucl Med. 2018;43:728–34.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40.
Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl. 10):S13–8.
Lütje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SAMW, Rosenbaum-Krumme S, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–401.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Calopedos RJS, Chalasani V, Asher R, Emmett L, Woo HH. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:352–60.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–70.
Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with (177)Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–6.
Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving (225)Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–9.
Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45:19–31.
Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of (177)Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–62.
von Eyben FE, Roviello G, Kiljunen T, Uprimny C, Virgolini I, Kairemo K, et al. Third-line treatment and (177)Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45:496–508.
Yordanova A, Linden P, Hauser S, Feldmann G, Brossart P, Fimmers R, et al. The value of tumor markers in men with metastatic prostate cancer undergoing [(177) Lu]Lu-PSMA therapy. Prostate. 2020;80:17–27.
Gallyamov M, Meyrick D, Barley J, Lenzo N. Renal outcomes of radioligand therapy: experience of 177lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin Kidney J. 2019;13(6):1049–55.
Acknowledgements
The authors thank Drs. Guiseppe Cardaci, David Macfarlane and Catherine Lucas for their care of patients included in this retrospective study. The permission granted by participants to use data for research and analysis purposes is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of Interest
Danielle Meyrick, Marat Gallyamoa, Shanthi Sabarimurugan, Nadia Falzone and Nat Lenzo have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The study was conducted in accordance with institutional ethical compliance for off-trial palliative treatment of patients with metastatic castration-resistant prostate cancer on a compassionate basis. From a regulatory perspective, this is provided under Category A of the Therapeutic Goods Administration Special Access Scheme.
Consent to Participate
Written informed consent for publication was obtained from all patients.
Consent for Publication
Not applicable.
Availability of Data and Material
Data and material are available after request to the corresponding author.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Marat Gallyamov, Shanthi Sabarimurugan and Danielle Meyrick. The first draft of the manuscript was written by Marat Gallyamov and Danielle Meyrick, final editing performed by Nadia Falzone, Danielle Meyrick and Nat Lenzo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Meyrick, D., Gallyamov, M., Sabarimurugan, S. et al. Real-World Data Analysis of Efficacy and Survival After Lutetium-177 Labelled PSMA Ligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Targ Oncol 16, 369–380 (2021). https://doi.org/10.1007/s11523-021-00801-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00801-w