Abstract
Purpose
Up to 30% of patients with castration-resistant prostate cancer (CRPC) do not show any response to the first cycle of radioligand therapy (RLT) with [177Lu]Lu-PSMA-617 (Lu-PSMA). We evaluated patient response to the second and third cycles of RLT in patients that underwent at least three cycles. The second aim of this study was to calculate the median overall survival (OS) of responders and non-responders after the first cycle and after all three cycles of RLT.
Methods
CRPC patients were treated with Lu-PSMA, with a median interval of 8 weeks between each cycle. The tumour marker prostate-specific antigen (PSA) was used as the marker for response evaluation.
Results
Fifty-two patients underwent a total of 190 cycles of RLT (3–6 cycles per patient). Of these, 80.8% showed a decline in PSA 2 months after the first cycle, with 44.2% showing a PSA decline of ≥50%. When compared to baseline PSA, 73.1% showed a PSA decline after the third cycle. 50% of patients that did not show any response to the first cycle also did not respond to the second and third cycles. The median OS was 60 weeks in all patients. The median OS was significantly longer for patients that showed any PSA decline after the first cycle compared to patients without PSA decline (68 vs. 33 weeks). There was a significant difference in median OS between responders and non-responders for a change in PSA after the third cycle compared to baseline PSA.
Conclusion
Patients with a positive response to RLT, regardless of the rate of decline, had a significantly longer median OS. Of the patients that did not show any response to the first cycle, 50% responded to the second or third cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate-specific membrane antigen (PSMA) is an attractive target for the diagnosis and therapy of castration-resistant prostate cancer (CRPC) [1,2,3,4,5]. To date, all published studies about radioligand therapy (RLT) with [177Lu]Lu-PSMA-617 (Lu-PSMA) have demonstrated that this therapy is safe, with a low toxicity profile [6,7,8,9,10]. According to the published data, up to 30% of patients do not show any prostate-specific antigen (PSA) decline in response to the first cycle of RLT [6, 7, 10,11,12,13]. However, the response rate of these patients to subsequent therapy cycles has hardly been studied. The first aim of this study was to evaluate the response of patients to the second and third cycles of RLT, especially in those that showed a higher PSA value after the first cycle, in addition to evaluating whether there is a correlation between patient response to the first cycle and third cycle compared to baseline PSA in patients that received at least three consecutive RLT cycles every 2 months. Moreover, the association between a better response and longer overall survival (OS) remains unclear; therefore, the second aim of this study was to calculate OS in terms of patient response to the first cycle and to the first three cycles of RLT.
Patients and methods
Patients
CRPC patients with distant metastases and progressive disease, according to their PSA level, were treated with at least three cycles of Lu-PSMA with a median interval of 8 weeks (range: 6–8 weeks) between each cycle. These patients were followed up for at least 2 months after the last cycle. Some of the data from 26 patients included in this study has been previously reported in our prior publications [6, 7, 9, 14,15,16,17,18,19]; however, the current study included a greater number of patients that all received at least three cycles of PSMA therapy. In addition, we have evaluated patient response patterns after the second cycle of RLT, as well as patient OS, which have not previously been analysed or published elsewhere.
Radioligand therapy
PSMA ligand (PSMA-617) was obtained from ABX GmbH (Radeberg, Germany). The preparation of Lu-PSMA has been explained in detail in a previous publication [7].
The treatment solution was administered by slow intravenous injection within 30–60 s, followed by 1000 ml of NaCl or Ringer’s solution. All patients were discharged 48 h after therapy, in accordance with the rules of the Federal Office for Radiation Protection in Germany (BfS).
Tumour response evaluation
The tumour marker PSA was used as the main marker for response evaluation. Changes in PSA level were classified as either a decrease of ≥50% or as any degree of PSA decline. Any increase in PSA was considered to indicate disease progression.
Statistical analysis
The variables of interest are presented as descriptive statistics. The chi-square test (χ2) was used to compare PSA response between cycles. Survival analysis was performed using the Kaplan–Meier curve method. The log-rank test was carried out with a significance level of p < 0.05. All statistical analyses were performed using a commercially available software package (SPSS 22, IMB, Armonk, NY, USA).
Results
Fifty-two patients underwent a total of 190 cycles of RLT (3–6 cycles per patient). The median follow-up time after the last cycle was 4.5 months (range 2–12 months). At the time of analysis, 25 patients (48.1%) were still alive. Thirty-nine patients (75%) exhibited good Eastern Cooperative Oncology Group (ECOG) performance status scores (0 or 1). The remaining patients (25%) exhibited an ECOG score of 2. Fourteen patients had a Gleason score < = 7, 35 (67.3%) had a score > 7, and the score was unknown in three patients. All patients underwent at least three cycles of RLT. Nine, eight and three patients received four, five and six cycles of therapy, respectively, of whom eight patients received four cycles and one patient received five consecutive cycles at 8-week intervals immediately after completing the first three cycles. The other patients that received more than three cycles underwent further cycles of RLT as recurrence therapy. The median cumulative administered activity was 18.5 GBq (range 12.8–35.8 GBq). Detailed information regarding patient characteristics, prior therapies and extent of disease are presented in Tables 1 and 2.
Treatment response after the first, second and third cycle
Forty-two patients (80.8%) showed a PSA decline 2 months after the first cycle, 23 (44.2%) of which showed a PSA decline of ≥50%. Compared with the baseline PSA value, 37 patients (71.2%) showed a PSA decline 2 months after the second cycle, with 31 (59.6%) showing a PSA decline >50%, and 38 patients (73.1%) showed a PSA decline 2 months after the third cycle, 31 (59.6%) with a PSA decline ≥50%. Table 3 shows the therapy response after each cycle compared to the previous cycle and to the baseline PSA value in further detail.
Of the patients that did not show any response to the first cycle, 50% (5/10) did not respond to the second and third cycles. Of the other five patients, four responded to the second or third cycle with less than 50% decline in PSA value. One patient showed a 52% increase in PSA after the first cycle, followed by a PSA decline of 42% and 59% after the second and third cycles, respectively (−64% difference between the third cycle and the baseline PSA value).
Treatment response after the third cycle compared to baseline prostate-specific antigen level
Table 4 shows a significant correlation between patient response after the first and third cycle of RLT. Of the patients that showed any PSA decline after the first cycle, 88% also showed any PSA decline 2 months after the third cycle when compared to their baseline PSA value (p < 0.0001), with 71.4% showing a PSA decline of ≥50% (p = 0.001). Of the patients with a PSA response of ≥50% after the first cycle, 91.3% showed a significant response to the first three cycles (PSA decline ≥50%; p < 0.0001).
Ninety percent of patients that did not show any PSA decline after the first cycle also showed no response after the third cycle when compared to their baseline PSA value (Table 4).
Survival analysis
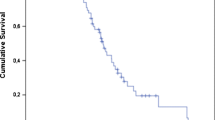
Survival was calculated from the day of the first RLT cycle. The median OS was 60 weeks in all patients irrespective of response (95% CI 44.2- 75.8; Fig. 1).
Regarding the therapeutic response to the first cycle, the median OS was significantly longer for patients that showed any PSA decline compared to those with no PSA decline, with OS of 68 weeks (95% CI 54.3- 81.7) and 33 weeks (95% CI 26.8- 39.2), respectively (p = 0.006; Fig. 2A). However, this difference was not significant when a PSA decline of ≥50% was considered as a response after the first cycle, with OS of 63 weeks (95% CI 56.8- 69.2) and 49 weeks (95% CI 15.5- 82.5), respectively (p = 0.3; Fig. 2B).
Kaplan–Meier survival curves of patients stratified by responders and non-responders. (A) Patient response to the first cycle of radioligand therapy (RLT) considering any prostate-specific antigen (PSA) decline. (B) Patient response to the first cycle of RLT considering a PSA decline of ≥50%. (C) Patient response to the first three cycles of RLT considering any PSA decline. (D) Patient response to the first three cycles considering a PSA decline of ≥50%
There was a significant difference in median OS between responders and non-responders in terms of PSA change in patients after the third cycle compared to their baseline PSA value prior to the first cycle. Considering any PSA decline as a response, the median OS was 70 weeks (95% CI 45.1- 95.0) and 32 weeks (95% CI 27.1- 36.9), respectively (p < 0.0001; Fig. 2C), and the OS was 68 weeks (95% CI 35.8- 100) and 40 weeks (95% CI 27.1- 52.9), respectively, when a PSA decline of ≥50% was considered a response (p = 0.001; Fig. 2D).
Discussion
Apart from approved therapies for metastatic prostate cancer (abiraterone, enzalutamide, docetaxel, cabazitaxel and 223Ra) [20,21,22,23,24,25], according to retrospective studies, RLT with Lu-PSMA has been shown to be effective for inducing PSA decline in almost 70% of patients, with a favourable toxicity profile and mild side effects [6, 7, 11, 12, 26, 27]. In a recently published study by our research group, we evaluated the impact of different pre-therapeutic parameters on therapy response, considering changes in PSA after the first cycle of RLT [14]. In this previous study, the multivariate analysis showed that patients with a high platelet count or a regular need for analgesics had a significantly worse response after the first RLT cycle, considering any PSA decline after 2 months. When a PSA decline >50% was considered, patients with a regular need for analgesics showed a worse response; however, other pre-therapeutic parameters including prior therapies and the amount of PSMA uptake in 68Ga-PSMA PET imaging, measured by SUV max, had no impact on patient response [14].
Kratochwil et al. [10] reported that 90% of patients that showed a PSA decline after the first cycle, who subsequently received a total of three treatment cycles, presented a continued decrease in PSA when compared to their baseline PSA value. This is consistent with the results of the current study, in which 88.1% of patients (37/42) that showed a positive response to the first cycle also showed a positive response after finishing the third cycle.
Of the patients that did not show any response to the first cycle, 50% (5/10) also did not respond to the second or third cycles; however, 50% responded to the second or third cycle. This means that 9.6% of the patients in this cohort did not respond to any of the cycles. 10% of the patients (1/10) that showed no PSA decline after the first cycle went on to show a PSA decline >50% 2 months after the third cycle when compared to their baseline PSA value. These results demonstrate the importance of performing the second and third cycles in patients who did not show a response to the first cycle, firstly because we found that 50% of these patients respond to the second or the third cycle, and secondly because there is no available data regarding the OS in non-responding patients compared to a best supportive care group.
In oncology, a good response, characterised by size reduction or regression of metabolism, does not always translate to a longer OS. Rahbar et al. [11] compared 28 patients that were treated with a total of 50 cycles of RLT (1–2 cycles per patient) with a historical patient cohort that were treated with the best supportive care prior to the availability of Lu-PSMA. The estimated median survival in their study was 29.4 weeks, which was significantly longer than survival in the historical best supportive care group of 19.7 weeks (p = 0.031). Rahbar et al. did not compare the median OS between responders and non-responders, and the majority of patients in this study underwent further cycles of Lu-PSMA therapy after the data had been published. The preliminary results of Rahbar et al. were promising, as they demonstrated the efficacy of RLT with Lu-PSMA [11].
In the current study, the median OS was 60 weeks in all patients, and there were significant differences for any PSA decline between responders and non-responders after the first and third cycles, and in PSA decline ≥50% after the third cycle in comparison to the baseline PSA value prior to the first cycle. The median OS was not significantly longer in patients who showed a PSA decline ≥ 50% after the first cycle compared to whom with no PSA decline or a decline less than 50% (63 vs. 49 weeks; p = 0.3). Patients with a positive response had a significantly longer OS; however, this does not mean that patients with a poor response did not benefit from PSMA therapy in terms of prolonged OS when compared with patients in the same situation that did not receive PSMA therapy.
The current study included patients that underwent at least three cycles of Lu-PSMA therapy; this specific inclusion criteria may introduce a bias regarding evaluation of the median OS, as we excluded all patients that received less than three cycles, and those that did not undergo any further therapies after the first or second cycle due to cancer progression, a change in therapy plan, or an excellent response to the first cycles. Nevertheless, we believe that the results of the current study can assist in planning RLT therapies, especially during consultations, as patients can be given useful information about the treatment response. In patients with continually increasing PSA levels after the first and second cycles, it may be better to discuss the indication for performing a third cycle with a multidisciplinary tumour board, in addition to the patient and his family.
The results of this study, like other retrospective studies, should be confirmed by prospective randomised studies.
Conclusion
Patients with a positive response to RLT using Lu-PSMA (responders), regardless of the rate of decline, had a significantly longer median OS compared to non-responders. Of the patients that did not show any response to the first cycle, 50% responded to the second or third cycles. Therefore, we should not give up, if the patients show no response after the first cycle.
References
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The Theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: Biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:1697–705. doi:10.2967/jnumed.115.161299.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95. doi:10.1007/s00259-012-2298-2.
Rai BP, Baum RP, Patel A, Hughes R, Alonzi R, Lane T, et al. The role of positron emission tomography with (68)gallium (Ga)-labeled prostate-specific membrane antigen (PSMA) in the Management of Patients with Organ-Confined and Locally Advanced Prostate Cancer Prior to radical treatment and after radical prostatectomy. Urology. 2016;95:11–5. doi:10.1016/j.urology.2015.12.048.
Ahmadzadehfar H, Azgomi K, Hauser S, Wei X, Yordanova A, Gaertner F, et al. 68Ga-PSMA-11 PET as a gate-keeper for the treatment of metastatic prostate cancer with radium-223: proof of concept. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016; doi:10.2967/jnumed.116.178533.
Braat A, Ahmadzadehfar H. Lutetium-177 labelled PSMA ligands for the treatment of metastatic castrate-resistant prostate cancer. Tijdschr Nucl Geneesk. 2016;38:1627–34.
Ahmadzadehfar H, Eppard E, Kurpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88. doi:10.18632/oncotarget.7245.
Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. doi:10.1186/s13550-015-0114-2.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 Radioligand therapy in advanced prostate cancer patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58:85–90. doi:10.2967/jnumed.116.183194.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1334–8. doi:10.2967/jnumed.116.173757.
Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1170–6. doi:10.2967/jnumed.115.171397.
Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–8. doi:10.1097/RLU.0000000000001240.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen Radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1006–13. doi:10.2967/jnumed.115.168443.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92. doi:10.1007/s00259-014-2713-y.
Ferdinandus J, Eppard E, Gaertner FC, Kurpig S, Fimmers R, Yordanova A, et al. Predictors of response to Radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58:312–9. doi:10.2967/jnumed.116.178228.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016; doi:10.2967/jnumed.116.183194.
Schlenkhoff CD, Gaertner F, Essler M, Schmidt M, Ahmadzadehfar H. Positive influence of 177Lu PSMA-617 therapy on bone marrow depression caused by metastatic prostate cancer. Clin Nucl Med. 2016;41:478–80. doi:10.1097/RLU.0000000000001195.
Schlenkhoff CD, Knupfer E, Essler M, Ahmadzadehfar H. Metastatic prostate cancer with restored Hormone-response after Radioligand therapy with 177Lu-PSMA-617. Clin Nucl Med. 2016;41:572–3. doi:10.1097/RLU.0000000000001200.
Wei X, Schlenkhoff C, Sopora C, Essler M, Ahmadzadehfar H. Successful treatment of hepatic metastases of Hormone refractory prostate cancer using Radioligand therapy with 177Lu-PSMA-617. Clin Nucl Med. 2016;41:894–5. doi:10.1097/RLU.0000000000001358.
Ahmadzadehfar H, Zimbelmann S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi:10.1056/NEJMoa1207506.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology. 2015;16:152–60. doi:10.1016/S1470-2045(14)71205-7.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi:10.1056/NEJMoa1213755.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi:10.1016/S0140-6736(10)61389-X.
Kantoff PW, Higano CS, Small EJ, Whitmore JB, Frohlich MW, Schellhammer PF. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104:1107–1109; author reply 9-12. doi:10.1093/jnci/djs279.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi:10.1056/NEJMoa041318.
Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016; doi:10.2967/jnumed.115.171397.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016; doi:10.2967/jnumed.116.173757.
Acknowledgements
We are grateful to the nursing staff of the treatment ward in our department. We give special thanks to our study nurse, Mrs. Ulrike Kuhn-Seifer (Department of Nuclear Medicine Bonn).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or non-financial competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ahmadzadehfar, H., Wegen, S., Yordanova, A. et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging 44, 1448–1454 (2017). https://doi.org/10.1007/s00259-017-3716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3716-2