Abstract
Background
Predictive factors that can be routinely used in clinical practice are critically needed for immune checkpoint inhibitor therapy in metastatic renal cell carcinoma (mRCC).
Objective
To comprehensively analyze the predictive impact of peripheral blood markers and C-reactive protein (CRP) in nivolumab therapy for mRCC.
Methods
Fifty-eight patients were retrospectively evaluated. We evaluated neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), absolute eosinophil count (AEC), and absolute monocyte count (AMC) as peripheral blood markers as well as serum CRP levels. The primary endpoints were progression-free survival (PFS) and overall survival (OS) after nivolumab initiation.
Results
Median PFS was significantly shorter in patients with high NLR (≥ 3) versus low NLR (p = 0.0356), high MLR (≥ 0.3) versus low MLR (p = 0.0013), or high PLR (≥ 160) versus low PLR (p = 0.0073), and median OS was significantly shorter in patients with high NLR versus low NLR (p = 0.0025), high MLR versus low MLR (p = 0.0025), high PLR versus low PLR (p = 0.0256), or high CRP (≥ 1.0 mg/dl) versus low CRP (p = 0.0006). Multivariate analyses showed that MLR (HR 2.65, p = 0.0068) was an independent factor for PFS and that NLR (HR 3.34, p = 0.0218), MLR (HR 3.42, p = 0.0381), and CRP (HR 4.98, p = 0.0108) were independent factors for OS.
Conclusions
The systemic inflammatory factors NLR, MLR, and CRP were predictive factors in nivolumab therapy for mRCC. These easily monitored factors can contribute to effective treatment and follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nivolumab, an anti-PD-1 monoclonal antibody, is standard systemic therapy for metastatic renal cell carcinoma (mRCC) [1]. A pivotal phase III trial, “CheckMate025”, demonstrated that nivolumab conferred prolonged overall survival (OS) and a more favorable safety profile than everolimus in second- or third-line therapy after the failure of previous antiangiogenic regimens for advanced clear-cell RCC. In addition, other immune checkpoint inhibitors (ICIs) targeting PD-1 or other molecules, such as PD-L1 or CTLA-4, have been developed and tested in clinical trials as monotherapy or combination therapy [2]. Thus, there is an ongoing paradigm shift in the systemic therapy of mRCC.
However, the rate of patients who cannot obtain any therapeutic benefit from ICIs (i.e., patients with progressive disease as their best overall response) ranged from 20–35%, according to previous trials [3, 4]. Moreover, in the real world, similar or higher rates of such cases (33–47%) were recently reported [5,6,7]. Furthermore, a subset of patients can develop immediate progressive disease, such as hyperprogression, and these patients generally have poor prognosis [8,9,10]. In addition, the cost of ICI therapy has been debated [11]. Therefore, it is important to identify predictive or prognostic factors to provide effective therapy for patients with mRCC.
In mRCC, inflammatory factors such as neutrophil, platelet counts, and serum C-reactive protein (CRP) level and combinational markers consisting of these factors (e.g., neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), modified Glasgow Prognostic Score), are intensively studied predictive or prognostic factors in molecular-targeted therapy [12,13,14,15,16,17,18,19,20]. Moreover, recent studies reported that these factors are associated with survival following ICI therapy in patients with melanoma [21, 22] or non-small-cell lung cancer (NSCLC) [23, 24]. In addition, several studies indicated that other peripheral blood markers, including eosinophil [25,26,27] and monocyte counts [25, 28], are associated with prognosis in ICI therapy. Thus, these factors can be effective in predicting the outcomes of ICI therapy in patients with mRCC. However, there is a limited number of clinical investigations that comprehensively analyze the predictive impact of these factors. Thus, in this retrospective study, we investigated the association between these peripheral blood markers and CRP and prognoses in nivolumab therapy for patients with mRCC.
2 Patients and Methods
2.1 Study Design
This study was approved by the Internal Ethics Review Board of the Tokyo Women’s Medical University (ID: 5103) and performed in accordance with the principals outlined in the Declaration of Helsinki. Owing to the retrospective observational nature of this study, formal consent was not required.
The inclusion and exclusion criteria for this study are shown in Fig. 1. In our department and its affiliated institution, 76 patients received nivolumab therapy after at least one targeted therapy for mRCC between June 2013 and June 2019. Patients who lacked laboratory data of peripheral blood markers or CRP (n = 11) or lacked other detailed clinical data (n = 3) were excluded. Furthermore, patients whose duration of follow-up was less than 1 month were excluded (n = 4). Finally, the remaining 58 patients were included in this retrospective study. All clinical and laboratory data were obtained from an electronic database and patient medical records.
This study aimed to identify the predictive factors for oncological outcomes in nivolumab therapy. Thus, the primary endpoint was progression-free survival (PFS) and overall survival (OS) after nivolumab initiation. Furthermore, we evaluated objective response rates (ORRs) and clinical benefit (CB) during nivolumab therapy as the secondary endpoint. ORRs were the sum of complete response and partial response rates, and CB was the sum of complete response, partial response, and stable disease rates. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 [29].
2.2 Protocol of Nivolumab Therapy
Nivolumab (3 mg/kg) was intravenously administered every 2 weeks following a protocol used in the CheckMate025 study [3]. Dose modifications were not allowed in any case. Instead, the interval between administrations could be modified according to the patient’s condition or in cases of nivolumab-induced adverse events. In this study, all patients received nivolumab after failure of prior targeted therapy. The sequential targeted therapy regimen adopted in our departments was described in our previous studies [30, 31]. Post-treatment follow-up scans were obtained using computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis regularly at 4- to 12-week intervals, depending on the condition of the patient. Nivolumab was administered until radiographic or clinical disease progression or development of intolerable adverse events was observed.
2.3 Cut-off Values of Peripheral Blood Markers and C-Reactive Protein (CRP)
In this study, we evaluated NLR, monocyte-to-lymphocyte ratio (MLR), PLR, absolute eosinophil count (AEC), and absolute monocyte count (AMC) as the peripheral blood markers. Furthermore, as one of the most intensively studied inflammatory factors, serum CRP levels were evaluated. We set cut-off values of these factors based on those in previous studies. For NLR, the cut-off value was set at 3 based on review or meta-analysis in the setting of systemic therapy including targeted therapy for mRCC [17, 32]; for MLR, the cut-off value was set at 0.3 based on studies in the setting of non-metastatic clear-cell RCC [33, 34]; for PLR, the cut-off value was set at 160 based on one meta-analysis and one study in the setting of nivolumab for NSCLC [35, 36]; for AEC, the cut-off value was set at 100/µl based on one study in the setting of nivolumab for mRCC [6]; for AMC, the cut-off value was set at 650/µl based on one study in the setting of ipilimumab for melanoma [25]; for CRP, the cut-off value was set at 1.0 mg/dl based on studies in the setting of mRCC [37, 38]. We evaluated the data for these factors in all patients within 2 weeks before initiation of nivolumab therapy.
2.4 Statistical Analysis
Categorical variables were analyzed using Fisher’s exact test. PFS was calculated from initiation of nivolumab therapy until disease progression or death, whichever came first. Patients who were alive without disease progression were censored at the time of last follow-up. OS was calculated from initiation of nivolumab therapy until death due to any cause. Patients lost to follow-up were censored at the time of last contact. Survival was calculated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses using the Cox proportional hazards regression models were used to identify risk factors for PFS and OS. Multivariate analyses were conducted using factors whose statistical significance was identified by univariate analyses. Risk was expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses were conducted using JMP software (version 14; SAS Institute Inc., Cary, NC, USA), with p < 0.05 indicating statistical significance.
3 Results
3.1 Patient Characteristics
Patient characteristics are shown in Table 1. Briefly, 45 patients (77.6%) were male and 34 patients (58.6%) were more than 65 years old. Forty-five patients (77.6%) were diagnosed with a clear-cell histotype. Based on the Internal Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk at nivolumab initiation [39], four (6.90%), 32 (55.2%), and 22 (37.9%) patients were categorized as favorable, intermediate, and poor risk, respectively. Nivolumab was administered in the third- or later-line in 24 patients (41.4%), and most of the previous targeted therapies were tyrosine kinase inhibitors, both in the first- (57/58, 98.3%) and second-line therapies (20/22, 90.9%). High NLR (≥ 3), high MLR (≥ 0.3), high PLR (≥ 160), low AEC (< 100/µl), high AMC (≥ 650/µl), and high CRP (≥ 1 mg/dl) were observed in 34 (58.6%), 37 (63.8%), 38 (65.5%), 23 (39.7%), nine (15.5%), and 34 (58.6%) patients, respectively.
3.2 Survival According to Peripheral Blood Markers and CRP
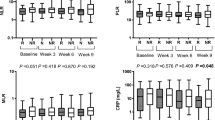
During the follow-up period, 44 (75.9%) and 21 (36.2%) patients experienced disease progression and died due to any cause, respectively (Table 1). Median PFS was significantly shorter in patients with high NLR, MLR, or PLR than in those with low NLR, MLR, or PLR (NLR 4.24 (95% CI 2.33–5.92)] vs. 8.38 (4.04–13.4) months, p = 0.0356; MLR 3.62 (2.27–5.19) vs. 10.1 (5.95–58.9) months, p = 0.0013; PLR: 3.76 (2.66–5.49) vs. 10.5 (5.92 not reached (NR)) months, p = 0.0073) (Fig. 2). The other factors were not significantly associated with median PFS (AEC 6.30 (3.58–10.1) vs. 4.34 (2.70–7.89) months, p = 0.731; AMC 6.97 (0.46–13.1) vs. 5.19 (3.62–8.05) months, p = 0.735; CRP 5.19 (2.33–8.38) vs. 5.95 (3.91–13.4) months, p = 0.184). Median OS was significantly shorter in patients with high NLR, MLR, PLR, or CRP than in those with low NLR, MLR, PLR, or CRP (NLR 22.0 (7.36–26.0) vs. NR (21.4–NR) months, p = 0.0025; MLR 15.4 (7.36–NR) vs. NR (21.4–NR) months, p = 0.0025; PLR 22.0 (9.30–NR) vs. NR (21.4–NR) months, p = 0.0256; CRP 21.4 (8.02–NR) vs. NR (NR–NR) months, p = 0.0006) (Fig. 3). The other factors were not significantly associated with median OS (AEC 23.3 (21.4–NR) vs. NR (8.02–NR) months, p = 0.685; AMC 22.0 (0.72–NR) vs. NR (21.4–NR) months, p = 0.0538).
Progression-free survival according to peripheral blood markers and CRP. Higher NLR (≥ 3), higher MLR (≥ 0.3), and higher PLR (≥ 160) were significantly associated with shorter median PFS (NLR 4.24 vs. 8.38 months, p = 0.0356; MLR: 3.62 vs. 10.1 months, p = 0.0013; PLR 3.76 vs. 10.5 months, p = 0.0073). Other factors, namely AEC, AMC, or CRP, were not associated with PFS (AEC: 6.30 vs. 4.34, p = 0.731; AMC: 6.97 vs. 5.19, p = 0.735; CRP 5.19 vs. 5.95, p = 0.184). CRP C-reactive protein, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, PFS progression-free survival, AEC absolute eosinophil count, AMC absolute monocyte count
Overall survival according to peripheral blood markers and CRP. Higher NLR (≥ 3), higher MLR (≥ 0.3), higher PLR (≥ 160), and higher CRP (≥ 1 mg/dl) were significantly associated with shorter median OS (NLR: 22.0 months vs. NR, p = 0.0025; MLR: 15.4 vs. NR, p = 0.0025; PLR: 22.0 months, vs. NR, p = 0.0256; CRP: 21.4 months vs. NR, p = 0.0006). Other factors, namely AEC or AMC, were not associated with OS (AEC: 23.3 vs. NR, p = 0.685; AMC: 22.0 vs. NR, p = 0.0538). CRP C-reactive protein, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, OS overall survival, AEC absolute eosinophil count, AMC absolute monocyte count
3.3 Factors for Survival
Univariate analysis showed that histopathology, IMDC risk, NLR, MLR, and PLR were significant factors for PFS (all, p < 0.05) (Table 2). Univariate analysis also showed that IMDC risk, Karnofsky Performance Status score, liver metastasis status, NLR, MLR, PLR, and CRP were significant factors for OS (all, p < 0.05). Multivariate analysis for PFS showed that MLR (HR 2.65 (95% CI 1.30–5.86), p = 0.0068) was a sole independent factor (Table 3). Multivariate analysis for OS showed that NLR (HR 3.34 (1.18–11.9), p = 0.0218), MLR (HR 3.42 (1.06–15.3), p = 0.0381), and CRP (HR 4.98 (1.39–31.9), p = 0.0108) were independent factors.
3.4 Objective Response Rates and Clinical Benefit According to Peripheral Blood Markers and CRP
We also evaluated an association between ORRs and CB and peripheral blood markers and CRP. As shown in Table 4, MLR was significantly associated with ORRs (18.9% vs. 52.4%, p = 0.0166) and CB (48.6% vs. 85.7%, p = 0.0057), and NLR was significantly associated with CB (47.1% vs. 83.4%, p = 0.0064).
4 Discussion
This retrospective study showed that NLR, MLR, and PLR were associated with PFS and that NLR, MLR, PLR, and CRP were associated with OS. Multivariate analyses further showed that MLR was the sole independent factor for PFS and that NLR, MLR, and CRP were independent factors for OS. In addition, MLR was associated with ORRs and CB, and NLR was associated with CB during nivolumab therapy. To the best of our knowledge, this study is the first comprehensive investigation of the predictive impact of peripheral blood markers and CRP in nivolumab therapy for patients with mRCC.
Among these factors, NLR is one of the most intensively studied inflammatory factors and has recently been reported to be associated with survival in mRCC patients treated with nivolumab [6, 40] and in patients with melanoma [21, 22] and NSCLC [23] treated with ICIs. Elevated NLR reflects high activity of the immune system. Chronic inflammation favors tumor development by preventing or suppressing the antitumor activity of the immune system, resulting in tumor growth [41, 42].
PLR has been also indicated as a predictive factor in several cancers including RCC. Platelets also play an active role in inflammation by releasing VEGF, which mediates the migration and extravasation of leukocytes, and PDGF, a chemokine that recruits neutrophils and monocytes [43]. The association between PLR and prognoses has already been reported during ICI therapy in NSCLC [36, 44]. Furthermore, in markers consisting of monocytes including AMC and MLR, AMC was reported to be associated with prognoses in ICI therapy for melanoma [25] and NSCLC [28]. Monocytes are progenitors of monocyte-derived macrophages. Recent studies revealed the mechanistically specific properties of monocytes—inflammatory monocytes are required for the efficacy of transferred activated cytotoxic T-cells but can exert potent tissue-damaging effects. On the other hand, other monocytes can provoke resistance to chemotherapy and aid tumor growth [45]. Interestingly, from our data, oncological outcomes were associated with MLR rather than AMC. Possibly, this superior predictive performance of MLR may be caused by inclusion of lymphocyte counts in MLR, which also have the potential to predict prognoses.
A recent study showed that these systemic inflammatory factors including NLR, MLR, and PLR had potential for outcome prediction during ICI therapy for advanced cancers whose major components of histology were melanoma and gastrointestinal and lung/head and neck cancers [46]. However, another study investigated the predictive performance of PLR in nivolumab therapy for mRCC and concluded that PLR was not an independent factor for OS based on multivariate analysis [47].
Decreased eosinophil count is also reported to be associated with poor prognosis in ICI therapy for patients with melanoma [25,26,27]. Eosinophils have a tumor surveillance function, and they have been reported to be effector cells for tumor rejection [48, 49]. Recently, Zahoor et al. indicated a significant association between low AEC and poor prognosis in nivolumab therapy for mRCC [6], which is inconsistent with our findings. One explanation for this discrepancy may be the differences in patient cohorts.
Finally, CRP is also a well-studied effective predictive factor representing inflammation in targeted therapy for mRCC [13, 14, 18], but its potential for prediction remains unknown in ICI therapy. Thus, to the best of our knowledge, this is the first report to indicate the significant association between CRP and prognoses in nivolumab therapy for mRCC. In other cancers, the predictive impact of CRP has been reported in ICI therapy [24, 27]. Moreover, in gastric cancer, a significant correlation between the development of ICI-induced hyperprogression and elevated CRP was reported [50]. Another study suggested that IL-6, which induces CRP production from the liver, and CRP were predictive factors in melanoma patients treated with ICIs [51]. Their in vitro data also suggested that CRP can affect T-cell signaling and activation. Thus, these data may support our findings in terms of the close association between high inflammatory status and poor prognosis and lower tumor response.
Taken together, our analyses indicate that among multiple factors, systemic inflammation has the potential to predict oncological outcomes. These identified factors can contribute to improve the predictive performance of existing prognostic models. For example, a previous study reported that addition of NLR to the IMDC risk model instead of neutrophil count significantly improved the predictive performance for OS in targeted therapy [12]. Thus, our identified factors may be considered for inclusion in future clinical research or trials to build effective predictive or prognostic models in ICIs for mRCC.
This study has several limitations. First, this study was conducted retrospectively using a small sample size. Thus, any findings could be affected by the unavoidable selection biases. Second, peripheral blood markers might be affected by previous targeted therapies (i.e., neutropenia or thrombocytopenia), which can affect the results of our analyses. Third, in this study, nivolumab was administered as a second- or later-line therapy after the failure of previous targeted therapies regardless of the IMDC risk classification in all patients, although this regimen has been not strongly recommended under the current guideline [1]. Fourth, the relatively short duration of follow-up and the small number of patients who died can affect the analyses, especially in OS.
5 Conclusions
This retrospective study showed that systemic inflammation factors including NLR, MLR, and CRP were significant predictive factors in nivolumab therapy for patients with mRCC. Because these factors can be easily evaluated and monitored in routine clinical practice, usage of these factors can contribute to effective treatment and follow-up. However, further prospective large-scale studies are needed to confirm our findings.
References
Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Montes SF, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2019.02.011.
George S, Rini BI, Hammers HJ. Emerging role of combination immunotherapy in the first-line treatment of advanced renal cell carcinoma: a review. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.4604.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
De Giorgi U, Carteni G, Giannarelli D, Basso U, Galli L, Cortesi E, et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int. 2018. https://doi.org/10.1111/bju.14461.
Zahoor H, Barata PC, Jia X, Martin A, Allman KD, Wood LS, et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J Immunother Cancer. 2018;6(1):107. https://doi.org/10.1186/s40425-018-0425-8.
Ishihara H, Takagi T, Kondo T, Tachibana H, Fukuda H, Yoshida K, et al. Correlation between the magnitude of best tumor response and patient survival in nivolumab therapy for metastatic renal cell carcinoma. Med Oncol (Northwood, London, England). 2019;36(4):35. https://doi.org/10.1007/s12032-019-1261-5.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8. https://doi.org/10.1158/1078-0432.ccr-16-1741.
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–50. https://doi.org/10.1158/1078-0432.ccr-16-3133.
Ishihara H, Kondo T, Takagi T, Tachibana H, Fukuda H, Yoshida K, et al. Immediate progressive disease in patients with metastatic renal cell carcinoma treated with nivolumab: a multi-institution retrospective study. Target Oncol. 2018;13(5):611–9. https://doi.org/10.1007/s11523-018-0591-0.
Verma V, Sprave T, Haque W, Simone CB 2nd, Chang JY, Welsh JW, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):128. https://doi.org/10.1186/s40425-018-0442-7.
Tanaka N, Mizuno R, Yasumizu Y, Ito K, Shirotake S, Masunaga A, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma treated with first-line and subsequent second-line targeted therapy: a proposal of the modified-IMDC risk model. Urol Oncol. 2017;35(2):39.e19–28. https://doi.org/10.1016/j.urolonc.2016.10.001.
Teishima J, Kobatake K, Kitano H, Nagamatsu H, Sadahide K, Hieda K, et al. The impact of change in serum C-reactive protein level on the prediction of effects of molecular targeted therapy in patients with metastatic renal cell carcinoma. BJU Int. 2016;117(6b):E67–74. https://doi.org/10.1111/bju.13260.
Beuselinck B, Vano YA, Oudard S, Wolter P, De Smet R, Depoorter L, et al. Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int. 2014;114(1):81–9. https://doi.org/10.1111/bju.12494.
Santoni M, De Giorgi U, Iacovelli R, Conti A, Burattini L, Rossi L, et al. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013;109(7):1755–9. https://doi.org/10.1038/bjc.2013.522.
Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer (Oxford, England 1990). 2012;48(2):202–8. https://doi.org/10.1016/j.ejca.2011.09.001.
Na N, Yao J, Cheng C, Huang Z, Hong L, Li H, et al. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget. 2016;7(28):44039–46. https://doi.org/10.18632/oncotarget.9836.
Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J, et al. Effect of systemic inflammation on survival in patients with metastatic renal cell carcinoma receiving second-line molecular-targeted therapy. Clin Genitourin Cancer. 2017;15(4):495–501. https://doi.org/10.1016/j.clgc.2017.01.018.
Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia and the modified glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Target Oncol. 2016;11(5):605–17. https://doi.org/10.1007/s11523-016-0430-0.
Nunno VD, Mollica V, Gatto L, Santoni M, Cosmai L, Porta C, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. Immunotherapy. 2019;11(7):631–43. https://doi.org/10.2217/imt-2018-0175.
Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. https://doi.org/10.1186/s40425-018-0383-1.
Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn J Clin Oncol. 2019. https://doi.org/10.1093/jjco/hyy201.
Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2017;106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013.
Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8(61):103117–28. https://doi.org/10.18632/oncotarget.21602.
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–18. https://doi.org/10.1158/1078-0432.ccr-15-2412.
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–96. https://doi.org/10.1158/1078-0432.ccr-16-0127.
Heppt MV, Heinzerling L, Kahler KC, Forschner A, Kirchberger MC, Loquai C, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer (Oxford, England: 1990). 2017;82:56–65. https://doi.org/10.1016/j.ejca.2017.05.038.
Soyano AE, Dholaria B, Marin-Acevedo JA, Diehl N, Hodge D, Luo Y, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6(1):129. https://doi.org/10.1186/s40425-018-0447-2.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990). 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J, et al. Time to progression after first-line tyrosine kinase inhibitor predicts survival in patients with metastatic renal cell carcinoma receiving second-line molecular-targeted therapy. Urol Oncol. 2017;35(9):542. https://doi.org/10.1016/j.urolonc.2017.05.014.
Ishihara H, Takagi T, Kondo T, Tachibana H, Yoshida K, Omae K, et al. Efficacy and safety of third-line molecular-targeted therapy in metastatic renal cell carcinoma resistant to first-line vascular endothelial growth factor receptor tyrosine kinase inhibitor and second-line therapy. Int J Clin Oncol. 2018;23(3):559–67. https://doi.org/10.1007/s10147-018-1241-3.
Boissier R, Campagna J, Branger N, Karsenty G, Lechevallier E. The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: a review. Urol Oncol. 2017;35(4):135–41. https://doi.org/10.1016/j.urolonc.2017.01.016.
Chen Z, Shao Y, Yao H, Zhuang Q, Wang K, Xing Z, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget. 2017;8(29):48291–302. https://doi.org/10.18632/oncotarget.15162.
Chen Z, Wang K, Lu H, Xue D, Fan M, Zhuang Q, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2019;11:909–19. https://doi.org/10.2147/cmar.s186976.
Wang X, Su S, Guo Y. The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(48):84506–14. https://doi.org/10.18632/oncotarget.21108.
Russo A, Franchina T, Ricciardi GRR, Battaglia A, Scimone A, Berenato R, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J Cell Physiol. 2018;233(10):6337–43. https://doi.org/10.1002/jcp.26609.
Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109(2):205–12. https://doi.org/10.1002/cncr.22400.
Naito S, Kinoshita H, Kondo T, Shinohara N, Kasahara T, Saito K, et al. Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: a multicenter study of 556 patients. Urology. 2013;82(4):846–51. https://doi.org/10.1016/j.urology.2013.06.035.
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300. https://doi.org/10.1016/s1470-2045(14)71222-7.
Bilen MA, Dutcher GMA, Liu Y, Ravindranathan D, Kissick HT, Carthon BC, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin Genitourin Cancer. 2018;16(3):e563–75. https://doi.org/10.1016/j.clgc.2017.12.015.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. https://doi.org/10.1038/nature01322.
Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40. https://doi.org/10.1016/j.semcancer.2011.12.005.
Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. Journal Interferon Cytokine Res. 2002;22(9):913–22. https://doi.org/10.1089/10799900260286623.
Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam, Netherlands). 2017;111:176–81. https://doi.org/10.1016/j.lungcan.2017.07.024.
Murray PJ. Immune regulation by monocytes. Semin Immunol. 2018;35:12–8. https://doi.org/10.1016/j.smim.2017.12.005.
Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–34. https://doi.org/10.1002/cncr.31778.
De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.ccr-18-3661.
Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol (Baltimore, Md: 1950). 2007;178(7):4222–9.
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–17. https://doi.org/10.1038/ni.3159.
Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019. https://doi.org/10.1007/s10120-018-00922-8.
Weber JS, Tang H, Hippeli L, Qian M, Wind-Rotolo M, Larkin JMG, et al. Serum IL-6 and CRP as prognostic factors in melanoma patients receiving single agent and combination checkpoint inhibition. J Clin Oncol. 2019;37(15_suppl):100. https://doi.org/10.1200/jco.2019.37.15_suppl.100.
Acknowledgements
The authors thank Ms. Nobuko Hata (Department of Urology, Tokyo Women’s Medical University and Department of Urology) for secretarial work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tsunenori Kondo received honoraria from Ono Pharmaceutical. Hiroki Ishihara, Hidekazu Tachibana, Toshio Takagi, Hironori Fukuda, Kazuhiko Yoshida, Junpei Iizuka, Hirohito Kobayashi, Masayoshi Okumi, Hideki Ishida, and Kazunari Tanabe have no conflicts of interest to declare.
Funding
This study did not receive any funding.
Rights and permissions
About this article
Cite this article
Ishihara, H., Tachibana, H., Takagi, T. et al. Predictive Impact of Peripheral Blood Markers and C-Reactive Protein in Nivolumab Therapy for Metastatic Renal Cell Carcinoma. Targ Oncol 14, 453–463 (2019). https://doi.org/10.1007/s11523-019-00660-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00660-6