Abstract
Background
Association between systemic inflammation and clinical outcome of immune checkpoint inhibitors (ICIs) has received focus. Our objective was to evaluate the utility of the neutrophil-to-lymphocyte ratio (NLR) in metastatic renal cell carcinoma (mRCC) patients treated with nivolumab as well as the prognostic impact of the C-reactive protein (CRP) level.
Materials and methods
Sixty-five mRCC patients treated with nivolumab were enrolled. We retrospectively investigated several factors, including the NLR and the CRP level, for their association with progression-free survival (PFS) and overall survival (OS). In addition, we evaluated their impact on the objective response.
Results
The CRP level was confirmed to be positively correlated with the NLR in a correlation analysis. An NLR ≥ 5 was significantly associated with a worse PFS (hazard ratio [HR]: 4.54, 95% confidence interval [CI] 1.93–10.7; p < 0.001), and an NLR ≥ 5 and a CRP ≥ 2.1 mg/dL were identified as a significant factors predicting worse OS with HRs of 4.88 (95% CI 1.35–17.7; p < 0.016) and 3.89 (95% CI 1.01–15.0; p = 0.049), respectively. In addition, patients with a ≥ 25% decrease in the NLR and CRP level showed a significantly better response to nivolumab than those without a ≥ 25% decrease in the NLR and CRP level, with odds ratios of 9.54 (95% CI 2.09–49.8, p = 0.001) and 4.36 (95% CI 1.03–18.9, p = 0.032), respectively.
Conclusion
Both the NLR and CRP levels were significantly associated with the clinical outcome of nivolumab in mRCC patients. The potential prognostic impact of those markers needs to be further prospectively investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of immune checkpoint inhibitors (ICIs) has dramatically changed the treatment strategy of metastatic renal cell carcinoma (mRCC). Since a randomized clinical trial demonstrated a survival advantage of nivolumab, an anti-programmed death-1 (PD-1) monoclonal antibody, in the treatment of patients with mRCC [1], ICIs have been a mainstay treatment for advanced RCC [2]. In addition, therapeutic options have further expanded to include the combination of ICIs, such as nivolumab and ipilimumab, an anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody [3]. Most recently, several clinical trials have shown a survival benefit with the combined use of an ICI plus a multitargeted receptor tyrosine kinase inhibitor (TKI) [4, 5]. Thus, there will be more opportunities to use ICIs in the near future.
As the ideal treatment strategy for mRCC has become complicated in the ICI era, biomarkers predicting the clinical outcome of ICI treatment are more crucial than ever for the selection of appropriate treatment agents. Anti-programmed death-ligand 1 (PD-L1) has been expected to be a candidate predictive biomarker of nivolumab. However, while the previous reports have shown that the PD-L1 expression of tumor tissue may be associated with the clinical outcome of anti-PD-1 antibody treatment in several cancers [6,7,8], the survival benefit observed in a randomized clinical trial of mRCC patients treated with nivolumab was not associated with the PD-L1 status [1]. This contradiction may be attributed to several causes, such as variety in the PD-L1 expression pattern among cancer types, intratumoral heterogeneity and differences in the definition of PD-L1 positivity [8,9,10].
Recently, cancer-related systemic inflammation has been shown to be a major determinant of the disease progression and survival in most cancers [11, 12]. The neutrophil-to-lymphocyte ratio (NLR) has received focus as a biomarker for predicting the efficacy of nivolumab in several cancers, including mRCC. Several previous reports showed that a high NLR was significantly associated with worse clinical outcomes [13,14,15,16]. In addition, the level C-reactive protein (CRP), a common inflammatory marker, has also been studied as a prognostic biomarker of nivolumab in several cancers, such as lung cancer and melanoma [17,18,19], but its utility in mRCC patients treated with nivolumab has never been demonstrated.
The objectives of our study were to validate the utility of the NLR for predicting the clinical outcomes of our mRCC patients treated with nivolumab and to evaluate the impact of the CRP level on those patients.
Materials and methods
Patients
A total of 65 patients with metastatic or unresectable RCC who had been treated with nivolumab after 1 or more TKI regimens at Kobe University Hospital in Japan between December 2016 and February 2019 were included in this study. Informed consent to participate in the present study was obtained from all patients, and the study design was approved by the Research Ethics Committee of our institution (No. B190059), which was conducted in accordance with the Declaration of Helsinki.
Treatments and procedures
Nivolumab (3 mg/kg or 240 mg/body) was administered every 2 or 3 weeks until the occurrence of disease progression, unacceptable adverse events, withdrawal, or death. We collected the following data from the medical records of patients: patient demographics, histology, Karnofsky performance status (KPS), blood test results, and adverse events (AEs). Patients were classified into three risk categories (favorable-, intermediate-, and poor-risk groups) according to the International metastatic renal cell carcinoma Database Consortium (IMDC) classification [20]. The elevation of lactate dehydrogenase (LDH) was defined as a value of > 222 U/L, which is considered to be the upper limit of normal in our hospital. The treatment response to nivolumab was evaluated by computed tomography (CT) or bone scintigraphy at least once every 12 weeks and classified according to the response evaluation criteria in solid tumours (RECIST) 1.1. The objective response rate (ORR) was defined as the percentage of patients with confirmed complete or partial responses among all treated patients.
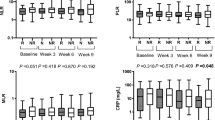
The NLR was derived from the absolute neutrophil and absolute lymphocyte counts of a full blood count. In present study, we used an NLR of 5 as the threshold value, as its clinical utility in predicting patient outcomes in a variety of cancers was examined in a previous systematic review [21]. In addition, the changes in the NLR and CRP level were compared between baseline and four weeks after the induction of nivolumab.
Statistical analyses
We assessed the objective response, progression-free survival (PFS) and overall survival (OS) of nivolumab treatment. Fisher’s exact test was performed to evaluate the association of the NLR and CRP values with an objective response. The PFS and OS were estimated using the Kaplan–Meier method, and we assessed several potential factors for predicting the PFS and OS with nivolumab using the Cox proportional hazards model.
In addition, we evaluated the Pearson product-moment correlation coefficient to analyze the correlation between the NLR and CRP level. The optimal threshold of the CRP level for predicting an NLR ≥ 5 was determined as the value maximizing the sum of the sensitivity and specificity in the receiver operating characteristic (ROC) analysis.
For all statistical analyses, we employed EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [22], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). It is a modified version of R commander designed to add statistical functions frequently used in biostatistics. Each test was 2-sided, and a value of p < 0.05 was considered significant.
Results
Patients’ characteristics
The clinical characteristics of the 65 patients are shown in Table 1. The median age was 68 years (range 44–87 years), and most patients were male (n = 47; 72.3%), had a KPS ≥ 80% (n = 47; 72.3%), had undergone nephrectomy (n = 56; 86.2%), and had been diagnosed with clear cell carcinoma (n = 47; 72.3%). According to the IMDC classification, three patients had a favorable risk, 34 (52.3%) had an intermediate risk and 28 (43.1%) had a poor risk. Thirty-six (55.4%) patients had received only one previous TKI, and the remaining 29 (44.6%) patients had received ≥ 2 such therapies. The median values of the NLR, platelet count and LDH and CRP levels were 3.1 (range 1.1–10.0), 24.4 (×104/µL, range 7.9–47.6), 199 (IU/L, range 104–2104) and 0.48 (mg/dL, 0.0–20.0), respectively. Immune-related adverse events (irAEs) of any grade occurred in 40 (62.5%) patients.
Treatment response and survival outcomes
As of database lock, the response assessment to nivolumab was available in 64 of 65 patients. Of these, 3 (4.7%) patients achieved a complete response, and 12 (18.7%) achieved a partial response; the ORR was 23.4% (Table 2). The median PFS of nivolumab was 7.2 months (95% confidence interval [CI] 2.6–9.7), and the median OS was not reached during the median 9.5 months of observation in this study (Fig. 1a, b).
Impact of the NLR and CRP on clinical outcomes
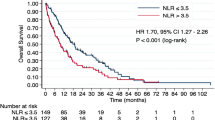
As shown in Fig. 2, both the PFS and OS of patients with NLR ≥ 5 was significantly shorter in comparison to those with NLR < 5. The median PFS of patients with NLR < 5 and NLR ≥ 5 was 7.9 months and 1.1 months (Fig. 2a, p < 0.001), and the median OS was not reached and 6.0 months (Fig. 2b, p < 0.001), respectively.
Next, our analysis of the correlation between the NLR and CRP level revealed a moderate correlation between the two variables (Fig. 3a, correlation coefficient: 0.568), and the optimal cut-off value of the CRP level for predicting an NLR ≥ 5, as estimated by an ROC analysis, was 2.14 mg/dL (Fig. 3b, sensitivity: 0.714, specificity: 0.843).
The PFS and OS curves based on the CRP level stratified at 2.1 mg/dL are shown in Fig. 4a, b. The PFS of patients with a CRP level ≥ 2.1 mg/dL was significantly shorter than that of patients with a CRP level of < 2.1 mg/dL (Fig. 4a, median PFS: 2.1 months and 7.9 months, respectively; p = 0.004). Similarly, the OS in patients with a CRP level of ≥ 2.1 mg/dL was significantly shorter than that in patients with a CRP level of < 2.1 mg/dL (Fig. 4b, median OS: 10.3 months and not reached, respectively; p < 0.001).
We then evaluated the association between the clinical characteristics and PFS of patients treated with nivolumab. As shown Table 3, a poor risk on IMDC classification, NLR ≥ 5, and elevated LDH were identified as factors significantly associated with poor PFS, with hazard ratios (HRs) of 3.08 (95% CI 1.40–6.81; p = 0.005), 4.54 (95% CI 1.93–10.7; p < 0.001) and 3.02 (95% CI 1.43–6.40; p = 0.004), respectively. However, the CRP level was not associated with PFS.
In the analysis of the relationship between the clinical characteristics and OS of nivolumab-treated patients (Table 4), we observed that a history of nephrectomy, the NLR and the CRP level significantly contributed to the predicted OS. While NLR ≥ 5 (HR: 4.88, 95% CI 1.35–17.7; p < 0.001) and CRP ≥ 2.1 mg/dL (HR: 3.89, 95% CI 1.01–15.0; p = 0.049) were significantly associated with poor OS, a poor risk on IMDC classification was not.
According to previous reports [23, 24], we focused on the change in the NLR and CRP but not their actual values for the analysis of factors associated with an objective response. There was also a moderate correlation between the change in the NLR and that in the CRP level (Fig. 3c, correlation coefficient: 0.599). As shown Fig. 5, patients with a ≥ 25% decrease in their NLR and CRP level showed a significantly better response to nivolumab than those without a ≥ 25% decrease in these values, with odds ratios of 9.54 (95% CI 2.09–49.8, p = 0.001) and 4.36 (95% CI 1.03–18.9, p = 0.032), respectively. The actual NLR and CRP values had no impact on the objective response (data not shown).
Discussion
In this retrospective analysis, we showed that the NLR and CRP level were significantly associated with clinical outcomes in mRCC patients treated with nivolumab. To our knowledge, this was the first report of the utility of the CRP level as a prognostic biomarker of nivolumab in patients with mRCC.
Systemic inflammation is recognized as a key determinant of the outcome in patients with cancer [21]. Due to their widespread availability in clinical practice, subtypes of white blood cells, such as neutrophils and lymphocytes, have been used parameters of the cancer-related inflammatory response. Neutrophilia has been shown to be associated with a poor survival in several cancer types [25,26,27]. Jeyakumar et al. reported that tumor-associated neutrophils may enhance angiogenesis, tumor growth and progression to a metastatic phenotype [28]. An elevated neutrophil count is included as a risk factor in the IMDC classification (anaemia, thrombocytosis, neutrophilia, hypercalcaemia, KPS < 80%, and < 1 year from diagnosis to treatment) which is often used to predict the clinical outcomes in patients with mRCC [20].
On the other hand, lymphocytes have been shown to be effective suppressors of cancer progression and to reflect host immunity [29]. Cytotoxic T lymphocytes (CTL) are known to induce apoptosis of cancer cells by Fas signaling: an interaction between CD95L molecules on the CTL and CD95 molecules on target tumor cells [30]. The association between lymphocytes and cancer outcomes has also been investigated. Fogar et al. reported that lymphopenia was associated with negative outcomes in patients with pancreatic cancer [31]. In addition, previous reports have shown that tumor-infiltrating lymphocytes were associated with a reduced tumor recurrence and favorable prognosis [32,33,34].
However, Basem et al. suggested the superiority of the NLR to leukocyte counts (e.g., neutrophils and lymphocytes), because the NLR is stable compared to absolute counts, which can be influenced by various physiological, pathological and physical factors. In addition, the NLR can represent both inflammatory and immune pathways [35]. We, therefore, used the NLR for our assessments in the present study. Our study showed that while a high NLR was associated with a poor OS, a poor risk according to the IMDC classification, which was established in the TKI era, was not associated with a poor OS. The NLR may be suitable for inclusion in a new classification system in the ICI era if such a system was to be established.
The CRP level is another commonly used inflammatory maker that has been studied as a predictive biomarker for mRCC patients treated with cytokine agents or TKIs [36, 37]. Interleukin-6, which mainly regulates the CRP synthesis in the liver, is thought to play important roles in stimulating angiogenesis [38] and inhibiting apoptosis of cancer [39], indicating that the CRP level reflects the tumor microenvironment and aggressiveness of tumors. In the present study, we showed for the first time that the CRP level was positively correlated with the NLR and that an elevated CRP level was significantly associated with a poor prognosis of nivolumab in mRCC patients, suggesting that the CRP level may also be a prognostic biomarker in mRCC patients treated with nivolumab, similar to the NLR.
In addition, the present study showed that the change in the NLR and CRP level but not their actual values were biomarkers for predicting the treatment response. Consistent with our results, a previous report on mRCC patients showed that a decreased CRP level was a parameter for predicting the anti-tumor effect of TKIs [23, 24]. In ICI treatment, Lalani et al. demonstrated an association between a decrease in the NLR and an objective response, although not significantly so [40]. However, the efficiency of using changes in inflammatory markers to predict the treatment response of ICIs in mRCC remains unclear, and further validation will be needed.
The present study had several limitations. First, the study was small in size and retrospective, with a relatively short follow-up duration. To validate our results, further prospective, large-scale studies will be needed. Second, the cut-off of the NLR and CRP level should be validated further for their future clinical application. In addition, the CRP level is not routinely evaluated in some hospitals [41]; as such, the CRP level will be most beneficial for predicting the treatment response in settings, where it is routinely evaluated, such as Japan. Furthermore, we showed a moderate correlation between the NLR and CRP. Thus, the results of the multivariate analysis of factors associated with for PFS and OS should be interpreted with care, because there may have been a multicollinearity problem [42].
In conclusion, we found that high values of the NLR and CRP level were significantly associated with a poor OS among mRCC patients treated with nivolumab. Regarding the treatment response, decreases in the NLR and CRP level but not their actual values were identified as significant factors for predicting an objective response. Our data suggest that the CRP level may be a useful marker for predicting the clinical outcome of ICI treatment, along with the NLR. These findings should be further investigated in a larger prospective cohort.
Abbreviations
- AE:
-
Adverse event
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CTLA-4:
-
Anti-cytotoxic T-lymphocyte antigen-4
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitor
- IMDC:
-
International metastatic renal cell carcinoma Database Consortium
- irAE:
-
Immune-related adverse event
- KPS:
-
Karnofsky performance status
- mRCC:
-
Metastatic renal cell carcinoma
- NLR:
-
Neutrophil-to-lymphocyte ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Anti-programmed death-1
- PFS:
-
Progression-free survival
- PD-L1:
-
Anti-programmed death-ligand 1
- TKI:
-
Multitargeted receptor tyrosine kinase inhibitor
References
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. New Engl J Med 373(19):1803–1813
Salgia NJ, Dara Y, Bergerot P et al (2019) The changing landscape of management of metastatic renal cell carcinoma: current treatment options and future directions. Curr Treat Options Oncol 20(5):41
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New Engl J Med 378(14):1277–1290
Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med 380(12):1116–1127
Motzer RJ, Penkov K, Haanen J et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med 380(12):1103–1115
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 366(26):2443–2454
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (Lond, Engl) 387(10027):1540–1550
Zhang T, Xie J, Arai S et al (2016) The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 7(45):73068–73079
Weide B, Martens A, Hassel JC et al (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 22(22):5487–5496
Kluger HM, Zito CR, Turcu G et al (2017) PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res 23(15):4270–4279
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Lino-Silva LS, Salcedo-Hernandez RA, Garcia-Perez L et al (2017) Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 27(2):140–144
Diem S, Schmid S, Krapf M et al (2017) Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung cancer (Amsterdam, Netherlands) 111:176–181
Nakaya A, Kurata T, Yoshioka H et al (2018) Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol 23(4):634–640
Bilen MA, Dutcher GMA, Liu Y et al (2018) Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin Genitourin Cancer 16(3):e563–e575
Oya Y, Yoshida T, Kuroda H et al (2017) Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 8(61):103117–103128
Ozawa Y, Amano Y, Kanata K et al (2019) Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol (Northwood, Lond, Engl) 36(4):33
Okuhira H, Yamamoto Y, Inaba Y et al (2018) Prognostic factors of daily blood examination for advanced melanoma patients treated with nivolumab. Biosci Trends 12(4):412–418
Heng DYC, Xie W, Regan MM et al (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 14(2):141–148
Guthrie GJ, Charles KA, Roxburgh CS et al (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88(1):218–230
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48(3):452–458
Templeton AJ, Knox JJ, Lin X et al (2016) Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol 70(2):358–364
Teishima J, Kobatake K, Kitano H et al (2016) The impact of change in serum C-reactive protein level on the prediction of effects of molecular targeted therapy in patients with metastatic renal cell carcinoma. BJU Int 117(6B):E67–74
Schmidt H, Bastholt L, Geertsen P et al (2005) Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 93(3):273–278
Atzpodien J, Royston P, Wandert T et al (2003) Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer 88(3):348–353
Teramukai S, Kitano T, Kishida Y et al (2009) Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 45(11):1950–1958
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10(15):4895–4900
Gooden MJ, de Bock GH, Leffers N et al (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103
Niederhuber JE (1997) Cancer vaccines: the molecular basis for t cell killing of tumor cells. Oncologist 2(5):280–283
Fogar P, Sperti C, Basso D et al (2006) Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 32(1):22–28
Clemente CG, Mihm MC Jr, Bufalino R et al (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77(7):1303–1310
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY) 313(5795):1960–1964
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New Engl J Med 348(3):203–213
Azab B, Bhatt VR, Phookan J et al (2012) Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 19(1):217–224
Casamassima A, Picciariello M, Quaranta M et al (2005) C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol 173(1):52–55
Beuselinck B, Vano YA, Oudard S et al (2014) Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int 114(1):81–89
Nagasaki T, Hara M, Nakanishi H et al (2014) Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer 110(2):469–478
Gado K, Domjan G, Hegyesi H et al (2000) Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell Biol Int 24(4):195–209
Lalani AA, Xie W, Martini DJ et al (2018) Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer 6(1):5
Walsh SR, Cook EJ, Goulder F et al (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91(3):181–184
Slinker BK, Glantz SA (1985) Multiple regression for physiological data analysis: the problem of multicollinearity. The American journal of physiology 249(1 Pt 2):R1–12
Author information
Authors and Affiliations
Contributions
KS, TT and KH designed the study. KS, TT, JF, KH acquired and analyzed the data. KS, TT and KH drafted the manuscript, and JF, NH, YN and MF revised it critically for important intellectual content. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study design was approved by the Research Ethics Committee of our institution (No. B190059), which was conducted in accordance with the Declaration of Helsinki. Informed consent to participate in the present study was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Suzuki, K., Terakawa, T., Furukawa, J. et al. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int J Clin Oncol 25, 135–144 (2020). https://doi.org/10.1007/s10147-019-01528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01528-5