Abstract
The chemical structure, thermal denaturation and nanostructure of collagen, obtained from a cation-exchange separation of the mantle, fins and tentacles of jumbo squid (Dosidicus gigas), were comparatively studied. The main idea of this work, was to provide an in-depth understanding of the interdependence between pyridinoline (Pyr) content, helix chemical structure and nanostructure of squid collagen with squid tissue firmness. The tentacles required more shear force and its collagen presented the higher temperature and enthalpy of transition, than the mantle and fins. The tentacle firmness may be explained by the relatively higher imino amino acid content, proline and lysine hydroxylation degrees and Pyr content of its collagen. Moreover, among the regions studied, the collagen from the tentacles had a more intense β band chain. Also, the Fourier transform infrared analysis and Raman spectra, implied that the collagen in the tentacles, was more intermolecularly ordered than the mantle and fins. Consistent with these results, a comparative evaluation of the surface morphology of the three regions, with atomic force microscopy, suggested a more ordered collagen structure in the tentacles (lower roughness values). Based on the above, collagen from tentacles has a higher degree of molecular order that sustains a higher muscle firmness compared to that of other anatomical regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squid is considered as an important fishery resource in many countries’ fishing industries, due to its high popularity among consumers [1]. The edible body-regions of this organism that are marketed are the mantle, fins, and tentacles. However, as with any seafood products, one of the challenges is to inhibit the changes in toughness and texture that happen during squid processing. Regarding the association between texture and proteins, numerous studies have shown that properties of jumbo squid connective tissue proteins can be used as benchmarks of squid muscle quality during freezing or cooking processes [2,3,4,5,6,7,8,9].
Squid muscle connective tissue is stronger than that of fish [10] though, the major component of the connective tissues of both organisms is collagen [11]. Collagen is a complex protein that helps to maintain the union between cells [12]. Several types of collagen have been identified and the primary characteristic of all types are amino acid arrangements that are rich in proline and glycine. These arrays, form three chains that intertwine to create a triple helix that varies with composition and size [13]. Few studies about seafood muscle texture, have reported the interdependence of fillet firmness with the major cross-linking molecule, pyridinoline (Pyr), present in collagen structure. Li et al. [14] detected a significant correlation between Atlantic salmon fillet texture and Pyr concentration. Furthermore, Ramirez-Guerra et al. [9] proved that the textural behavior of squid mantle during ice storage, is related to Pyr concentration.
In cephalopods, the chemical composition of the muscle tissue depends, among other factors, on the anatomical region [15] due to the distinctive function muscles have in the live organism [16]. Accordingly, differences in collagen properties have been detected in the edible and non-edible anatomical regions of squid species [16,17,18,19]. Moreover, in the Japanese pufferfish (Fugu rubripes), the Pyr concentration has been shown to differ between the muscular and skin collagen [20].
The main focus of this study was to compare the thermal denaturation, Pyr concentration, chemical structure and nanostructure of the collagen extracted from various anatomical regions of jumbo squid (Dosidicus gigas). The possible correlation between the chemical structure and nanostructure of collagen, with tissue firmness, was discussed. This work provides an in-depth understanding of the interdependence between squid firmness, and collagen chemical structure and nanostructure, and may be useful for future technological developments, such as squid-based convenience products. To our knowledge, a comparative study of the thermal denaturation of collagen, Pyr content, collagen chemical structure, collagen nanostructure, and muscle firmness properties, among the edible anatomical regions of squid species, has not been carried out before.
Materials and Methods
Samples and Sample Preparation
Ten jumbo squid specimens were hand-captured, by jigging, and were purchased from local fisherman (Bahia Kino in the Gulf of California, Mexico; 28.75° N, 112.25° W; water temperature 15–18 °C), during fall 2015. The squid were transported on ice to the laboratory, within 8 h of capture. The organisms were gutted, and the mantles were separated from the fins and tentacles. Each anatomical region was skinned, and the firmness was immediately measured. Approximately 250-g portions of each anatomical region, were packed in polyethylene bags and stored frozen (−25 °C), for no more than 7 days, until collagen extraction.
All reagents used in this work were reagent grade (Sigma Chemical Co., St. Louis, MO, USA). The solvents used in the amino acid analysis, were liquid chromatographic grade.
Muscle Firmness Analysis
Muscle portions (1 cm × 1 cm × 1 cm) from each fresh anatomical region (mantle, fins and tentacles), obtained from 10 jumbo squid specimens, were subjected to firmness analysis. The shear force required to cut the muscle sample was measured using a TA-XT2 Plus Texturometer (Food Technology Corp., Sterling, VA, USA), equipped with a Warner-Bratzler shearing device, attached to a load cell (100 N), at a crosshead speed of 200 mm/min [9].
Extraction of Collagen
The connective tissue was extracted at 4 °C, by removing the myofibrillar and proteoglycan proteins from fresh muscle, using a 6 M urea solution containing 0.5 M sodium acetate (pH 6.8). Samples were stirred for 60 min and centrifuged at 39200×g for 40 min. The pellets were sequentially treated with 1 M Tris (pH 7.2, containing 0.05 M NaCl), 0.5 M acetic acid (1:5 w/v) and pepsin (10 mg/g tissue in 0.5 M acetic acid; 1:5 w/v). At each step, after stirring for 24 h, the samples were centrifuged at 39200×g for 40 min. Then, the collagen solutions were precipitated with 2 M NaCl and collected by centrifugation (39,200×g for 40 min). The precipitates were dissolved in 0.05 M acetic acid and dialyzed at 4 °C against water, using a cellulose membrane with a 10 kDa molecular weight cut-off. The collected samples were frozen at −40 °C and lyophilized [19].
The collagen was then purified by cation exchange column chromatography, using HiTrap CM Sepharose FF (GE Healthcare, Uppsala, Sweden) assisted by ÄKTApurifier chromatographic equipment (GE Healthcare, Uppsala, Sweden). Collagen samples (50 mg) were dissolved in 15 mL of 50 mM sodium acetate, pH 4.8, containing 6 M urea and, then, applied to a HiTrap CM FF column (5 × 1 mL), equilibrated with the same buffer. Fractions were eluted with a linear gradient of 0–0.5 M NaCl, in a total volume of 20 mL, at a flow rate of 1 mL/min. The effluent was monitored at 280 nm. The appropriate fractions were pooled and dialyzed against 0.05 M acetic acid, to prevent precipitation [21]. Finally, the sample was lyophilized and stored at −40 °C, for further analysis.
Amino Acid Profile
The amino acid composition of collagen was determined, using reverse-phase high-performance liquid chromatography (HPLC). The samples were hydrolyzed under reduced pressure in 6 M HCl, containing sodium thioglycolate (1:1 v/v), at 150 °C for 60 min. The hydrolyzed samples were derivatized at 60 °C for 5 min in the presence of 0.5 M citrate, containing 2 mg/mL 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) in methanol and applied to an HPLC system (Agilent Technologies Inc., Palo Alto, CA) [22]. Analyses were performed in triplicate and the results were expressed as the number of residues per 1000 residues.
Determination of Purified Collagen Content
The hydroxyproline (Hyp) content, calculated by HPLC [22], was used as an index of collagen. The mass of Hyp was converted to collagen using the eq. 1 [23].
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
In order to establish the purity of the obtained collagen, proteins were analyzed by SDS-PAGE [24] using a 5.5% acrylamide separating gel. Samples for electrophoresis were prepared homogenizing 10 mg of purified collagen in one mL of cold 0.05 M Tris, pH 7.4, containing 1% SDS. The homogenate was diluted 1:1 (w/v) in the sample buffer (0.5 M Tris-HCl pH 6.8, glycerol, 10% SDS, β-mercaptoethanol, 0.05% bromophenol blue), and a 20 μL aliquot of 12-μg protein was loaded onto a vertical electrophoresis gel, which was developed using a Mini Protean® III, Bio-Rad electrophoretic equipment (Hercules, CA). The electrophoretic separation was carried out according to manufacturer’s procedure (120 V for 90 min). After electrophoresis, the gels were stained with Coomassie Blue R-250. Five electrophoresis gel images, for each extracted collagen were analyzed, using a densitometer (Bio-Rad Model GS-700, Hercules, CA), to obtain representative and statistically valid results.
Determination of Pyridinoline (Pyr)
Fluorescence spectrophotometry (Cary Eclipse G9800A Agilent Technologies, USA) was performed to determine the Pyr content in the collagen samples. Collagen was dissolved (0.5 absorbance units at 280 nm) with 50 mM sodium acetate, containing 6 M urea, pH 4.8. The excitation wavelength was 295 nm and the emission signal was measured from 300−450 nm. The Pyr content was established from a standard Pyr (5−40 pmol) curve [25]. The Pyr content was estimated as moles per mole of collagen. The assays were performed in triplicate.
Differential Scanning Calorimetry (DSC)
The thermal profile of the collagen was analyzed using DSC, from 25−180 °C [19]. Thermal denaturation of collagen, was characterized by the enthalpy change (ΔH), estimated from the denaturation endotherm of collagen and maximum temperature of denaturation (T max), which were measured, using a 1020 Series DSC thermal analysis system (Perkin Elmer, Norwalk, CN). Collagen samples (4−5 mg) were placed in DSC hermetic cells, and an empty capsule was used as a reference. Data acquisition was performed on a PE Nelson model 1022 (Perkin Elmer). Determinations were performed in triplicate.
Fourier Transform Infrared (FTIR) Analysis
FTIR spectra of extracted collagen, were obtained from pellets, prepared with 0.2 mg lyophilized sample and 10 mg potassium bromide [26]. The spectra were recorded using an infrared spectrophotometer (Perkin Elmer FT-IR Spectrum GX), with an average of 16 scans, over a spectral range of 4000 to 400 cm−1, at a resolution of 4 cm−1. Four FTIR spectra for each extracted collagen were analyzed, to acquire reliable, characteristic and statistically valid results.
Raman Spectroscopy
The purified collagen was analyzed by Raman spectrometry, using a Perkin Elmer GX instrument [27]. The excitation was performed at 1064 nm, using a Nd:YAG laser. The backscattered light was collected at 180 °C. The equipment was adjusted to 100 mW and 128 scans at 4 cm−1, to avoid potential damage to the samples, due to the laser power. The bands of the Raman spectra were analyzed using the program equipment’s analysis. Three Raman spectra for each extracted collagen were analyzed, to acquire reliable, characteristic and statistically valid results.
Atomic Force Microscopy (AFM)
The morphological characteristics of each collagen obtained from the mantle, fins and tentacles, were visualized by AFM. Each fraction was solubilized in 50 mM sodium acetate, containing 6 M urea, pH 4.8, at 5 mg/mL, then, placed on a glass slide and dried at room temperature (20−25 °C) for 30 min. Subsequently, the samples were washed with about 10–20 mL of water and allowed to dry again. The AFM images were obtained, with an alpha300 RA (WITec), in non-contact mode and under atmospheric conditions. The field scans were 5 × 5 μm for each sample, with 65,536 points per image. The images were processed with the program ProjectFour v4.1. At least 10 samples of each extracted collagen were examined, to acquire trusty, characteristic and statistically valid results.
Statistical Analyses
Data were analyzed by one-way analysis of variance (ANOVA), to explore differences among the samples. Differences between least-squares means were examined by Tukey-Kramer’s honestly significant difference (HSD) test (p < 0.05, in all cases). Correlation values among firmness, proline and lysine hydroxylation degrees’ data, β-component (SDS-PAGE), Pyr content, maximum temperature, ratio of bands 1240/ 1451 (FTIR), ratio of bands 1270/1450 (RAMAN), and surface roughness (AFM) were also calculated. All analyses were carried out using PC-SAS version 6.08 (SAS Institute Inc., 1994). The results were presented as mean ± standard deviation.
Results and Discussion
Firmness
Muscle firmness was measured by recording the force required to penetrate the muscles of the mantle, fins and tentacles, respectively. The highest firmness (p < 0.05), was detected in the tentacles (86.5 N), followed by the fins (33.6 N) and mantles (29.6 N). The higher firmness detected in tentacles could be attributed to their specific functions during swimming mechanisms [16]. The firmness detected in mantle muscle concurred with that previously reported for fresh mantle of the same squid species [9].
Concentration of Collagen
The extracted collagen (expressed as grams of dry connective tissue per 100 g of each anatomical region), varied significantly (p < 0.05) among the evaluated anatomical regions. The greatest yield was obtained from the tentacles (20.3%), followed by the fins (12.7%) and mantle (7.7%). These results concurred with those previously obtained from the same squid species [19] and Todaropsis eblanae [16] but were higher than those found in Loligo patagonica, Illex argentinus and Illex coindetii [16, 28]. Several exogenous factors including the time of year, water temperature, pressure and diet, may influence the connective tissue extracted [16]. Concerning the percentage of collagen, the tentacles had a significantly higher concentration (p < 0.05) (91.3%) than the fins (85.5%) and mantle (83.1%). This trend agrees with previous works, which found that the muscle collagen content varies, depending on the anatomical region [11, 16, 19, 29, 30].
Amino Acid Analysis
The amino acid compositions of the purified collagen from the mantle, fins and tentacles (Table 1), revealed a high prevalence of glycine (Gly), representing around 28% of the total collagen residues in all fractions. These Gly contents are typical for collagen. In addition, low proportions of leucine (Leu), Lysine (Lys) and serine (Ser) and high proportions of alanine (Ala) and hydroxyproline (Hyp) were detected. Morales et al. [16] observed the same amino acid distribution patterns, in the collagen isolated from the mantle and arms of some cephalopods.
Comparing the total imino amino acid [Proline (Pro) + Hyp] content among the anatomical regions, the collagen from the tentacles showed the highest value. This sample also showed the highest degree of Pro and Lys hydroxylation. Therefore, under the conditions of this study, this result demonstrated that the tissue collagen derived from the tentacles possessed a higher degree of crosslinkage than that of mantle and fins.
SDS-PAGE Electrophoresis
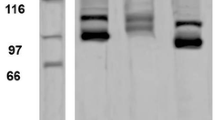
According to the electrophoretic patterns of the purified collagen samples obtained from each anatomical region (Fig. 1), the collagen comprised α-chains and inter- and intramolecular crosslinked components: β (dimer) and γ (trimer), indicating that lysine-derived crosslinks were present [31]. Comparable electrophoretic patterns of the mantle, fins and tentacles, have been reported for collagen type I of Illex coindetii, Todaropsis eblanae and Todarodes pacificus [16, 32]. The similarity in the electrophoretic migration and amino acid composition, suggested the presence of type I collagen in the analyzed tissues. The band optical intensity (OD) of the crosslinked chain (β dimer) was significantly higher in collagen (p < 0.05) from the tentacles (2128 OD) than fins (1851 OD) and mantle (1701 OD).
Pyr Content
Another considerable difference between the mantle, fins and tentacles, was detected in the Pyr content (p < 0.05). Consistent with the above-mentioned results, the collagen of the tentacles had a significantly higher Pyr content (p < 0.05) (2.09 mmol/mol collagen) compared to the fins (1.22 mmol/ mol collagen) and mantle (0.91 mmol/mol collagen). These differences indicated that the collagen from each anatomical region, differs in crosslinking degree, which is controlled by the number of bonds prone to form hydroxylamine (and likewise their solubility in salt solutions and buffers) [29]. The tentacles perform a grasping function in the live organism [16]. Therefore, its muscular collagen might require more Pyr than the mantle and fins.
As documented previously in fish and squid muscles [9, 14] the Pyr content is closely associated with squid muscle firmness. Pyr is formed through the oxidation of hydroxylysine (Hyl) by lysyl oxidase (LOX), and the activity of this enzyme has been shown to affect the physical strength of tissue fibers [3]. Our results showed the collagen derived from the tentacles had a higher Hyl content than that from the mantle and fins (Table 1). However, it is also possible that the LOX activity may vary in each body anatomical region studied, affecting its firmness and thermal stability. Therefore, further research on LOX, in squid tissue is needed.
DSC
DSC analysis of the collagen, produced one endothermic transition peak, with a denaturation temperature above 100 °C, in all instances, in concurrence with other reports [7, 19, 33]. The peak temperature (T max) is indicative of the stability of collagen during heating [34], whereas the ΔH is due to endothermic processes, such as the breaking of bonds [35]. The collagen with the highest T max and ΔH was derived from the tentacles (Table 2). Similarly, Torres-Arreola et al. [19] also reported that collagen from the tentacles showed both the highest T max and ∆H. These results imply that collagen from the tentacles has a more stable helix structure than the fin and mantle collagens [3, 26]. Unlike Pro, the Hyl content is closely correlated with the thermal stability of collagen [36]. However, the differences in hydroxylation (%) of Lys (Table 1), might suggest that Pyr itself contributed partially to the collagen thermal stability. Several factors that dictate the collagen thermal stability, such as LOX activity [3], are intertwined in a complex manner [37], requiring more studies to decipher the exact intercorrelations.
FTIR Analysis of Collagen
The FT-IR (3500−400 cm−1) spectra of the three collagen specimens (Fig. 2 ), represented those reported for other marine collagens [26, 38, 39]. The spectra of the collagens derived from jumbo squid mantle, fins, and tentacles, differed in the amide I and amide II peaks, which in the mantle, appeared at a lower frequency. A shift of these peaks to lower frequencies, implies a lower degree of molecular order [40]. Moreover, the ratio between amide III and the region near 1440−1460 cm−1, is associated with a helical structure (index ratio (IR) = % transmittance at 1240 cm−1/% transmittance at 1451 cm−1) [41, 42]. The IR values detected in the collagen of each anatomical region were 1.35 (mantle), 1.28 (fins) and 1.05 (tentacles). The IR values close to 1.0, detected in the collagen of the tentacles, confirmed that a considerable extent of intermolecular structure exists in this region [41]. This behavior coincides with the Pyr content, which was lower in the collagen samples derived from the mantle than the fins and tentacles. Therefore, it appears that there are fewer intermolecular cross-links in mantle collagen compared to that from tentacles and fins. In the present study, the IR values positively correlated with the squid muscle firmness. Thus, the ratio between amide III and 1440−1460 cm−1, should be considered as one of the explanatory variables, contributing to squid firmness in addition to the Pyr content.
Raman Spectroscopy Analysis of Collagen
Raman analysis was also applied to analyze the structure of the purified collagens. In general, relatively neutral bonds (CC, CH, C = C) are strong Raman scatterers. The differences in the shape and intensity of the bands, observed between the 1700–800 cm−1 regions of the Raman spectra of the obtained collagens (Fig. 3), were associated with the differences in the imino acid (Hyp and Pro) concentrations detected.
The Raman spectra indicated structural differences in the collagen from jumbo squid mantle, fins and tentacles. In all purified collagens a very intense band at 1670 cm−1 was present and attributed to amide I. The peak detected at 1270 cm−1 is assigned to amide III [27, 43]. Fewer intense bands were observed in the fin collagen than the mantle and tentacles. The absorption between the amide III and 1452 cm−1 bands demonstrates the existence of the helical structure [44]. As in FTIR, the ratio between the 1270/1450 cm−1 bands intensity, which is also associated with the helical structure [44] was 0.96, 0.64 and 0.46, for the tentacles, mantle and fin collagen, respectively. Lower values imply a less stable molecule [44]. Other considerable differences were in the Pro residue activity (830−910 cm−1) and the Hyp (845 cm−1). Among the three collagen specimens, these bands were more prominent in the collagen from the tentacles, in accordance with the amino acid profile. The thermal stability of collagen has been directly correlated with the content of imino amino acids (Pro + Hyp) [45]. In the present study, the Pro + Hyp content and the ratio of 1270/1450 cm−1 bands intensity ratio were strongly associated with the collagen thermal stability (Table 3). Thus, it is possible to suggest that due to the chemical structure characteristics of collagen from squid fins, its flesh in this region has a lower heat resistance than the mantle and tentacles, which is among the consumers and seafood producers’ concerns.
AFM of Collagen
The AFM results could lead to a more detailed understanding of the structure of collagen fibers and provide insightful knowledge of flesh firmness [46]. The collagen molecules contain many fibers that overlap, represented in the AFM images, as a surface roughness (SR) (Fig. 4). The apparent wide aggregated structure, indicated that the collagen was not completely hydrolyzed during the purification process. Sionkowska et al. [47] noted similar SR images in collagen from the tail tendon of rats. The SR values estimated, were significantly much larger in the collagen (p < 0.05) from the mantle (2701.1 nm) than fins (455.9 nm) and tentacles (304.5 nm). Roughness is often described as closely spaced irregularities [48]. Then, the highest irregular separate aggregates in the mantle collagen, indicated a weaker organization in this tissue and the results are consistent with the degree of molecular order, detected by FTIR. It was anticipated that a lower collagen order, would mean the fibers would be more likely to disassociate, resulting in less shear force required to cut the muscle. Nonetheless, squid texture has profound effects on customer acceptance of the seafood products and the collagen chemical structure usually contributes to the texture of seafood products [14, 28, 49].
This work attempted to compare the nanostructure of collagen with flesh firmness. Important micro-properties of the cell fibers, like contour length, height and radius of the collagen monomer, were omitted in the analysis. Thus, such parameters must be included in future studies, to provide a more comprehensive analysis of squid collagen structural factors that determine the firmness of squid muscle. Presumably, an important part of data variability, would explain the thermal properties, chemical structure and nanostructure differences, among the main jumbo squid edible anatomical regions. Nevertheless, this experiment showed that the significant differences evident in the nanostructure of collagen fibers, must be considered in future works of the firmness properties of squid tissue, during cold management and thermal processing, including sensory analysis. The research has shown an association between the collagen chemical structure, nanostructure and firmness (Table 3) of squid tissue but a more detailed study on the properties of processing squid muscle, is still needed.
Conclusion
Under the conditions of this work, it was proven that each anatomic region from jumbo squid has different firmness. The T max and ∆H, indicated that the fibers of tentacle collagen, are stronger than that of the mantle and fins. The differences detected, are due to the different crosslinked helix structure among the collagens derived from the mantle, fins and tentacles. The surface morphology, examined with AFM, indicated a higher degree of molecular order in the tentacle collagen molecules, compared to the other anatomical regions, which was consistent with the results of muscle firmness and collagen thermodynamic values. These results may serve as a fundamental basis for the proper management and processing aspects, of jumbo squid tissues.
References
Food and Agriculture Organization of the United Nations, Globefish-analysis and information on world fish trade. (Market reports 2017), Available from: http://www.fao.org/in-action/globefish/market-reports/cephalopods/en/. Accessed 17 Mar 2017
Y.E. Ueng, C.J. Chow, Textural and Histological Changes of Different Squid Mantle Muscle during Frozen Storage. J Agric Food Chem 46(11), 4728–4733 (1998). https://doi.org/10.1021/jf9803278
M. Ando, M. Ando, M. Makino, Y. Tsukamasa, Y. Makinodan, M. Miyoshi, Interdependence Between Heat Solubility and Pyridinoline Contents of Squid Mantle Collagen. J Food Sci 66(2), 265–269 (2001). https://doi.org/10.1111/j.1365-2621.2001.tb11329.x
C. Ruiz-Capillas, A. Moral, J. Morales, P. Montero, The effect of frozen storage on the functional properties of the muscle of volador (Illex coindetii). Food Chem 78(2), 149–156 (2002). https://doi.org/10.1016/S0308-8146(01)00392-2
R.R. Olivas, O.R. Sández, N.F. Haard, R.P. Aguilar, J.M.E. Brauer, Eur Food Res Technol 219, 312 (2004)
M.E. Paredi, H.A. Roldan, M. Crupkin, Changes in myofibrillar proteins and lipids of squiD (ILLEX ARGENTINUS) during frozen storage. J Food Biochem 30(5), 604–621 (2006). https://doi.org/10.1111/j.1745-4514.2006.00088.x
A.Z. Valencia-Pérez, M.H. García-Morales, J.L. Cárdenas-López, J.R. Herrera-Urbina, O. Rouzaud-Sández, J.M. Ezquerra-Brauer, Food Chem 107, 137 (2008)
M. Raman, S. Mathew, JIARM 2, 452 (2014)
H.E. Ramirez-Guerra, M.J. Fimbres-Romero, A.E. Tapia-Vazquez, J.M. Ezquerra-Brauer, E. Márquez-Ríos, G.M. Suarez-Jimenez, W. Torres-Arreola, Relationship between lysyl oxidase activity, pyridinoline content and muscle texture during ice storage of jumbo squid (Dosidicus gigas). Int J Food Sci Technol 50(12), 2700–2706 (2015). https://doi.org/10.1111/ijfs.12951
I. Kołodziejska, Z.E. Sikorski, C. Niecikowska, Food Chem 66, 15 (1999)
Z.E. Sikorski, I. Kołodziejska, The composition and properties of squid meat. Food Chem 20(3), 213–224 (1986). https://doi.org/10.1016/0308-8146(86)90174-3
K. Gelse, E. Pöschl, T. Aigner, Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55(12), 1531–1546 (2003). https://doi.org/10.1016/j.addr.2003.08.002
J.H. Muyonga, C.G.B. Cole, K.G. Duodu, Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem 85(1), 81–89 (2004). https://doi.org/10.1016/j.foodchem.2003.06.006
X. Li, R. Bickerdike, E. Lindsay, P. Campbell, D. Nickell, A. Dingwall, I.A. Johnston, Hydroxylysyl Pyridinoline Cross-Link Concentration Affects the Textural Properties of Fresh and Smoked Atlantic Salmon (Salmo salarL.) Flesh. J Agric Food Chem 53(17), 6844–6850 (2005). https://doi.org/10.1021/jf050743+
R. Kreuzer, in Cephalopods: handling, processing and products, (FAO, 1984), p. 108
J. Morales, P. Montero, A. Moral, Isolation and partial characterization of two types of muscle collagen in some cephalopods. J Agric Food Chem 48(6), 2142–2148 (2000). https://doi.org/10.1021/jf990711k
S. Mizuta, R. Yoshinaka, M. Sato, M. Sakaguchi, Isolation and partial characterization of two distinct types of collagen in the squid <i>Todarodes pacificus</i>. Fish Sci 60(4), 467–471 (1994). https://doi.org/10.2331/fishsci.60.467
A. Thanonkaew, S. Benjakul, W. Visessanguan, Chemical composition and thermal property of cuttlefish (Sepia pharaonis) muscle. J Food Compos Anal 19(2-3), 127–133 (2006). https://doi.org/10.1016/j.jfca.2005.04.008
W. Torres-Arreola, R. Pacheco-Aguilar, R.R. Sotelo-Mundo, O. Rouzaud-Sández, J.M. Ezquerra-Brauer, CyTA-J Food 6, 10 (2008)
M. Ando, Y. Nakagishi, K. Yoshida, M. Nakao, Y. Nakagawa, Y. Makinodan, Y. Tsukamasa, K. Kawasaki, Pyridinoline concentrations in muscular and skin collagen of fish and relationship between collagen solubility and pyridinoline concentration in fish muscular collagen. Fish Sci 72(5), 1104–1108 (2006). https://doi.org/10.1111/j.1444-2906.2006.01262.x
M. Nishimoto, S. Mizuta, R. Yoshinaka, Characterization of molecular species of collagen in muscles of Japanese amberjack, Seriola quinqueradiata. Food Chem 84(1), 127–132 (2004). https://doi.org/10.1016/S0308-8146(03)00183-3
F.A. Vázquez-Ortiz, G. Caire, I. Higuera-Ciapara, G. Hernández, High performance liquid chromatographic determination of free amino acids in shrimp. J Liq Chromatogr Relat Technol 18(10), 2059–2068 (1995). https://doi.org/10.1080/10826079508013960
AOAC, Official Methods of Analysis, Gaithersberg, MD: Association of Official Analytical Chemist, 17th ed. (2000)
U.K. Laemmli, Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227(5259), 680–685 (1970). https://doi.org/10.1038/227680a0
N. Arakawa, M. Kim, M. Otsuka, An Improved High-Performance Liquid Chromatographic Assay for the Determination of Pyridinoline in Connective Tissues. J Nutr Sci Vitaminol 38(4), 375–380 (1992). https://doi.org/10.3177/jnsv.38.375
P. Singh, S. Benjakul, S. Maqsood, H. Kishimura, Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem 124(1), 97–105 (2011). https://doi.org/10.1016/j.foodchem.2010.05.111
T. Ikoma, H. Kobayashi, J. Tanaka, D. Walsh, S. Mann, Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int J Biol Macromol 32(3-5), 199–204 (2003). https://doi.org/10.1016/S0141-8130(03)00054-0
Z.E. Sikorski, J.A. Borderias, in Seafood Proteins, ed. by Z.E. Sikorski, B.S. Pan, F. Shahidi (Springer, Boston, 1994), p. 58. https://doi.org/10.1007/978-1-4615-7828-4_5
M. Sadowska, Z.E. Sikorski, Collagen in the tissues of squid illex argentinus and loligo patagonica - contents and solubility. J Food Biochem 11(2), 109–120 (1987). https://doi.org/10.1111/j.1745-4514.1987.tb00116.x
J.E. Chan-Higuera, R.M. Robles-Sánchez, A. Burgos-Hernández, E. Márquez-Ríos, C.A. Velázquez-Contreras, J.M. Ezquerra-Brauer, Squid by-product gelatines: Effect on oxidative stress biomarkers in healthy rats. Czech J Food Sci 34(2), 105–110 (2016). 10.17221/320/2015-CJFS
S. Kimura, Y. Miyauchi, N. Uchida, Comp. Biochem. Physiol. Part B, Biochem. Mol Biol 99, 473 (1991)
P. Kittiphattanabawon, S. Nalinanon, S. Benjakul, H. Kishimura, J. Chem. (2015)
W.S. Otwell, D.D. Hamann, Textural characterization of squid (loligo pealei lesuer): scanning electron microscopy of cooked mantle. J Food Sci 44(6), 1629–1635 (1979). https://doi.org/10.1111/j.1365-2621.1979.tb09104.x
D.A. Ledward, R. Chizzolini, R.A. Lawrie, Int J Food Sci Technol 10, 349 (1975)
G. Bruylants, J. Wouters, C. Michaux, Curr Med Chem 12 (2011, 2005)
A. Miki, S. Inaba, T. Baba, K. Kihira, H. Fukada, M. Oda, Structural and physical properties of collagen extracted from moon jellyfish under neutral pH conditions. Biosci Biotechnol Biochem 79(10), 1603–1607 (2015). https://doi.org/10.1080/09168451.2015.1046367
C.A. Miles, N.C. Avery, V.V. Rodin, A.J. Bailey, The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J Mol Biol 346(2), 551–556 (2005). https://doi.org/10.1016/j.jmb.2004.12.001
R. Duan, J. Zhang, X. Du, X. Yao, K. Konno, Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem 112(3), 702–706 (2009). https://doi.org/10.1016/j.foodchem.2008.06.020
M.H. Uriarte-Montoya, H. Santacruz-Ortega, F.J. Cinco-Moroyoqui, O. Rouzaud-Sández, M. Plascencia-Jatomea, J.M. Ezquerra-Brauer, Giant squid skin gelatin: Chemical composition and biophysical characterization. Food Res Int 44(10), 3243–3249 (2011). https://doi.org/10.1016/j.foodres.2011.08.018
K.J. Payne, A. Veis, Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 27(11), 1749–1760 (1988). https://doi.org/10.1002/bip.360271105
A.M.D. Guzzi Plepis, G. Goissis, D.K. Das-Gupta, Dielectric and pyroelectric characterization of anionic and native collagen. Polym Eng Sci 36(24), 2932–2938 (1996). https://doi.org/10.1002/pen.10694
F. Pati, B. Adhikari, S. Dhara, Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour Technol 101(10), 3737–3742 (2010). https://doi.org/10.1016/j.biortech.2009.12.133
B.G. Frushour, J.L. Koenig, Raman scattering of collagen, gelatin, and elastin. Biopolymers 14(2), 379–391 (1975). https://doi.org/10.1002/bip.1975.360140211
M. Unal, H. Jung, O. Akkus, Novel raman spectroscopic biomarkers indicate that postyield damage denatures bone's collagen. J Bone Miner Res 31(5), 1015–1025 (2016). https://doi.org/10.1002/jbmr.2768
P.L. Privalov, Stability of proteins. Adv Protein Chem Struct Biol 35, 1 (1982). https://doi.org/10.1016/S0065-3233(08)60468-4
H. Yang, Y. Wang, S. Lai, H. An, Y. Li, F. Chen, Application of atomic force microscopy as a nanotechnology tool in food science. J Food Sci 72(4), R65–R75 (2007). https://doi.org/10.1111/j.1750-3841.2007.00346.x
A. Sionkowska, J. Skopinska-Wisniewska, M. Gawron, J. Kozlowska, A. Planecka, Chemical and thermal cross-linking of collagen and elastin hydrolysates. Int J Biol Macromol 47(4), 570–577 (2010). https://doi.org/10.1016/j.ijbiomac.2010.08.004
T.R. Thomas, Rough Surfaces, 2nd edn. (Imperial College Press, London, 1999), pp. 1–9
Z.E. Sikorski, D.N. Scott, D.H. Buisson, R.M. Love, The role of collagen in the quality and processing of fish. Crit Rev Food Sci Nutr 20(4), 301–343 (1984). https://doi.org/10.1080/10408398409527393
Acknowledgments
This research was supported by CONACYT, under grant 180214. Author Sarabia-Sainz acknowledges the doctoral fellowship received from CONACYT. The authors are also grateful to Ivan Tolano, for his valuable help with the DSC measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sarabia-Sainz, H.M., Torres-Arreola, W., Márquez-Ríos, E. et al. Interrelation of Collagen Chemical Structure and Nanostructure with Firmness of three Body Regions of Jumbo Squid (Dosidicus gigas). Food Biophysics 12, 491–499 (2017). https://doi.org/10.1007/s11483-017-9505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-017-9505-4