Abstract
The objective of this study was to investigate the influence of interfacial composition and electrical charge on the in vitro digestion of emulsified fats by pancreatic lipase. An electrostatic layer-by-layer deposition technique was used to prepare corn oil-in-water emulsions (3 wt% oil) that contained droplets coated by (1) lecithin, (2) lecithin–chitosan, or (3) lecithin–chitosan–pectin. Pancreatic lipase (1.6 mg mL−1) and/or bile extract (5.0 mg mL−1) were added to each emulsion, and the particle charge, droplet aggregation, and free fatty acids released were measured. In the presence of bile extract, the amount of fatty acids released per unit amount of emulsion was much lower in the emulsions containing droplets coated by lecithin–chitosan (38 ± 16 μmol mL−1) than those containing droplets coated by lecithin (250 ± 70 μmol mL−1) or lecithin–chitosan–pectin (274 ± 80 μmol mL−1). In addition, there was much more extensive droplet aggregation in the lecithin–chitosan emulsion than in the other two emulsions. We postulated that lipase activity was reduced in the lecithin–chitosan emulsion as a result of the formation of a relatively thick cationic layer around each droplet, as well as the formation of large flocs, which restricted the access of the pancreatic lipase to the lipids within the droplets. Our results also suggest that droplets initially coated by a lecithin–chitosan–pectin layer did not inhibit lipase activity, which may have been because the chitosan–pectin desorbed from the droplet surfaces thereby allowing the enzyme to reach the lipids; however, further work is needed to establish this. This information could be used to create food emulsions with low caloric level, or to optimize diets for individuals with lipid digestion problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary fats may be consumed by humans in a variety of different forms, e.g., bulk fats, fats trapped in solid matrices, water-in-oil emulsions, or oil-in-water emulsions.[1] Nevertheless, most fats are broken down into oil-in-water emulsions in the mouth, stomach, and/or small intestine because of the mechanical stresses they experience and because of the presence of various surface-active and stabilizing components present in the food or secreted by the body, e.g., small molecule surfactants, bile salts, phospholipids, proteins, polysaccharides, and their derivatives.[2, 3] Hence, the substrate for digestion of dietary fats is usually fat droplets embedded in an aqueous medium, with the surfaces of the droplets being coated by a complex layer of surface-active material that may come from a variety of different sources.
In humans, the digestion of dietary fat begins in the stomach and is completed in the small intestine, where final absorption of the lipid digestion products occurs.[4−8] In the stomach, fat digestion is attributable to hydrolysis of the lipids by gastric lipase, whereas in the small intestine it is attributable to hydrolysis by pancreatic lipase. Before pancreatic lipase can catalyze fat digestion, it is necessary for it to adsorb to the surfaces of the fat droplets so that it can come into close proximity to the lipid substrate. It has been proposed that the adsorption of pancreatic lipase is facilitated by the presence of bile acids that are released from the gall bladder or directly from the liver, and by the presence of colipase that is released from the pancreas.[6, 9, 10] Hence, the composition, structure, and physicochemical properties of the interfacial layer surrounding the fat droplets should play an important role in determining the extent of enzyme binding to the emulsion surface and therefore the extent of lipolysis.[11]
The purpose of the present study was to use an in vitro digestion model to examine the influence of interfacial characteristics on the hydrolysis of emulsified fats by pancreatic lipase. In particular, we wanted to examine whether interfacial layers containing chitosan, an indigestible cationic polysaccharide naturally present in some foods, would retard lipid digestion. Our reason for doing this was that a number of feeding studies indicated that consumption of chitosan may interfere with lipid digestion and absorption.[12−15] One of the principle physicochemical mechanisms that has been proposed for this interference is the formation of a layer of chitosan around the surfaces of the emulsified fats as a result of electrostatic attraction between the cationic chitosan molecules and anionic fat droplets.[16, 17] The presence of a chitosan layer around the fat droplets may retard lipolysis because it is positively charged and relatively thick, and should therefore prevent the lipase from coming into close contact with the lipid substrate inside the fat droplets.
We used an electrostatic layer-by-layer deposition method to create interfacial layers with different compositions, structures, and charges.[18, 19] This technique involves adsorbing charged biopolymers onto oppositely charged droplets to build up multiple layers around the fat droplets.
Materials and methods
Materials
Powdered lecithin was obtained from ADM-Lecithin (Decatur, IL, USA). Powdered chitosan (medium molecular weight; 75–85% deacetylation; viscosity of 1 wt% solution in 1 wt% acetic acid, 200–800 cps) and powdered pectin (extracted from citrus fruit, approx. 60% esterified) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bile extract (total bile salt content = 49 wt%; with 10–15% glycodeoxycholic acid, 3–9% taurodeoxycholic acid, 0.5–7% deoxycholic acid, 1–5% hyodeoxycholic acid, and 0.5–2% cholic acid; phosphatidylcholine 5 wt%; Ca2+ <0.06 wt%; CMC of bile extract 0.07 ± 0.04 mM) and lipase (from porcine pancreas, Type II) were obtained from Sigma-Aldrich. Hydrochloric acid (HCl), sodium hydroxide (NaOH), and acetic acid were purchased from Sigma-Aldrich. Corn oil (a typical edible oil) was purchased from a local supermarket and used without further purification. Distilled and deionized water was used for the preparation of all solutions.
Solution preparation
An emulsifier solution was prepared by dispersing 1.0 wt% lecithin in acetate buffer (100 mM acetic acid, adjusted to pH 3.0 with 1 M HCl). Chitosan and pectin solutions were prepared by dispersing 2.0 wt% powdered chitosan and 2.0 wt% powdered pectin in acetate buffer. At relatively low pH (<6.5), chitosan is positively charged and tends to be soluble in dilute aqueous solutions. Thus, we used chitosan dissolved in acetate buffer (pH 3.0, 100 mM) in this study to prepare the multilayered emulsions.
Emulsion preparation
Our objective was to prepare primary, secondary, and tertiary emulsions that had the same final oil concentration (3 wt%) so that we could compare them on an equal droplet concentration basis. This goal was achieved byusing the following preparation procedures.
Primary emulsion
The term “primary emulsion” is used to refer to the emulsion that contained fat droplets coated by lecithin only. A primary emulsion was prepared by homogenizing 3 wt% corn oil with 97 wt% lecithin solution (1.0 wt% lecithin dispersed in acetate buffer, pH 3) in a high-speed blender for 2 min (M133/1281-0; Biospec Products, Inc., ESGC, Switzerland) followed by three passes through a two-stage high-pressure valve homogenizer: 5,000 psi first stage, 500 psi second stage (LAB 1000; APV-Gaulin, Wilmington, MA, USA). The final composition of the primary emulsion was therefore 3 wt% corn oil and 0.97 wt% lecithin (pH 3).
Secondary emulsion
The term “secondary emulsion” is used to refer to the emulsion that contained fat droplets coated by lecithin–chitosan. First, a primary emulsion was prepared by homogenizing 5 wt% corn oil with 95 wt% lecithin solution (1.0 wt% lecithin dispersed in acetate buffer, pH 3) in a high-speed blender for 2 min (M133/1281-0, Biospec Products, Inc., ESGC, Switzerland) followed by three passes through a two stage high-pressure valve homogenizer: 5000 psi first stage; 500 psi second stage (LAB 1000; APV-Gaulin). A secondary emulsion was prepared by mixing the primary emulsion with 2 wt% chitosan solution and buffer solution to obtain a final concentration of 3 wt% corn oil, 0.57 wt% lecithin, 0.06 wt% chitosan, and acetate buffer (pH 3.0). This system was sonicated for 2 min at a frequency of 20 kHz, an amplitude of 40%, and a duty cycle of 0.5 s (Model 500, Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA, USA) to disrupt flocs formed during preparation. These emulsions were initially prepared at pH 3 because an electrostatic attraction was needed between the cationic chitosan and the anionic lecithin-coated droplets.[18, 19]
Tertiary emulsion
The term “tertiary emulsion” is used to refer to the emulsion that contained fat droplets coated by lecithin–chitosan–pectin. A secondary emulsion (5 wt% corn oil, 0.45 wt% lecithin, 0.1 wt% chitosan, and acetate buffer, pH 3.0) was prepared as described above. A tertiary emulsion was then formed by diluting the secondary emulsion with an aqueous pectin solution: 3 wt% corn oil, 0.27 wt% lecithin, 0.06 wt% chitosan, 0.4 wt% pectin, and acetate buffer (pH 3.0). These emulsions were initially prepared at pH 3, because an electrostatic attraction was needed between the anionic pectin and the cationic lecithin/chitosan-coated droplets.[18, 19]
After preparation, the pH of each of the samples was adjusted to pH 7 with 1 M NaOH and then the samples were subjected to an in vitro digestion model and changes in their microstructure and physicochemical characteristics were measured (see below). It should be noted that even though the final oil concentrations were the same in each of the emulsions (3 wt%), there was a difference in the final lecithin concentrations: 0.97, 0.57, and 0.27 wt% for the primary, secondary, and tertiary emulsions, respectively.
Particle size measurements
Emulsions were diluted to a droplet concentration of approximately 0.002 wt% oil using buffer solution to avoid multiple scattering effects. The particle size distribution (PSD) of the emulsions was then measured by laser light scattering (Mastersizer X; Malvern Instruments Ltd., Malvern, UK). The instrument finds the PSD that gives the best fit between the experimental measurements and predictions made using light scattering theory (Mie theory). A refractive index ratio of 1.08 was used by the instrument to calculate the PSDs. The particle size measurements are reported as the mean diameter: \(d_{{43}} = {{\sum {n_{i} d^{4}_{i} } }} \mathord{\left/ {\vphantom {{{\sum {n_{i} d^{4}_{i} } }} {{\sum {n_{i} d^{3}_{i} } }}}} \right. \kern-\nulldelimiterspace} {{\sum {n_{i} d^{3}_{i} } }}\)or \(d_{{32}} = {{\sum {n_{i} d^{3}_{i} } }} \mathord{\left/ {\vphantom {{{\sum {n_{i} d^{3}_{i} } }} {{\sum {n_{i} d^{2}_{i} } }}}} \right. \kern-\nulldelimiterspace} {{\sum {n_{i} d^{2}_{i} } }}\)where n i is the number of droplets of diameter d i . Each individual particle size measurement was determined from the average of two readings made on the same sample.
ζ potential measurements
Emulsions were diluted to a droplet concentration of approximately 0.006 wt% oil using buffer solution prior to analysis. Diluted emulsions were then injected into the measurement chamber of a particle electrophoresis instrument (ZEM 5003, Zetamaster; Malvern Instruments, Worcs., UK), and the ζ potential was determined by measuring the direction and velocity of the droplets' movement in the applied electric field. Each individual ζ potential measurement was determined from the average of five readings made on the same sample.
Optical microscopy
Emulsions were gently agitated in a glass test tube before analysis to ensure that they were homogeneous. A drop of emulsion was then placed on a microscope slide and covered by a cover slip. The microstructure of selected emulsions was determined using optical microscopy (Nikon microscope Eclipse E400; Nikon Corporation, Japan). The images were acquired using a CCD camera (CCD-300-RC, DAGE-MTI, Michigan City, IN, USA) connected to a Digital Image Processing system (Micro Video Instruments Inc., Avon, MA, USA).
Pancreatic lipase-catalyzed hydrolysis of emulsions
The release of free fatty acids from the emulsified lipids as a result of lipase activity was monitored using a titration method, which is based on that used by previous workers.[11]
A 30-mL sample of emulsion (pH 3.0) was transferred into a clean amber bottle and the pH was adjusted to pH 7.0 by adding 1 N NaOH. The samples were preincubated for 10 min and then a 7.5-mL mixture of bile extract and/or pancreatic lipase (containing 60 mg pancreatic lipase and/or 187.5 mg bile extract in 100 mM acetate buffer solution) was added into each emulsion. Final concentrations of pancreatic lipase and bile extract in the reaction mixture were 1.6 and/or 5.0 mg mL−1, respectively. Finally, samples were incubated in a shaking bath (95 rpm) at 37°C for 2 h to mimic conditions in the intestinal tract.[20] Lipase activity was determined by measuring the amount of fatty acid released from an emulsion. After incubation, acetone was added into the reaction mixture to terminate the pancreatic lipase catalyzed hydrolysis of lipids.[21] Liberated fatty acid content was then determined by titrimetry. Several drops of phenolphthalein were added and the liberated fatty acid was titrated with 0.01 M NaOH. Standards containing 0–1,000 μmol oleic acid were prepared in emulsion, followed by titration with 0.01 M NaOH, and a standard graph was plotted. The amount of free fatty acid released from the emulsions was then determined from the standard graph. The lipase activity was expressed as μmol of fatty acid liberated from 1 mL of emulsion. To determine the amount of fatty acid present before the action of lipase, the amount of fatty acid of each emulsion with only bile extract was measured.
Data analysis
All measurements are reported as the average and standard deviation of measurements made on at least two freshly prepared samples.
Results and discussion
Interaction of bile extract and lipase with lecithin-coated droplets
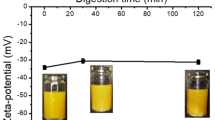
The purpose of these experiments was to investigate the ability of components from the bile extract and from the pancreatic lipase extract to adsorb to the surface of lecithin-coated droplets and alter emulsion properties and in vitro lipid digestion. An oil-in-water emulsion stabilized by lecithin was prepared at pH 3, then the pH was adjusted to 7 and bile extract and/or pancreatic lipase were added. The electrical charge (ζ potential) and the mean particle diameter (d 32) of the emulsions were measured after 24 h storage at room temperature. The ζ potential of the initial emulsions stabilized by lecithin was −39 mV at pH 7 (Figure 1), which was presumably due to the presence of anionic phospholipids from the lecithin that had adsorbed to the droplet surfaces. When bile extract was added to this emulsion, there was an increase in the magnitude of the negative charge on the droplets to −51 mV at pH 7, which is close to the ξ potential of a fat droplet saturated with pure bile extract (−54 ± 1 mV at pH 7, measured in a separate experiment). This suggested that bile extract adsorbed to the surfaces of the droplets and displaced some of the lecithin. Previous researchers have proposed that addition of bile salts to phosphatidylcholine-stabilized emulsions disrupts the packing of the phospholipids at the fat droplet surfaces, resulting in a mixed phospholipid/bile salt interface.[11] We also observed an increase in the magnitude of the negative charge on the droplets when pancreatic lipase was added alone or when bile extract and lipase were added together (Figure 1), which suggested that pancreatic lipase adsorbed to the surfaces of droplets coated with either lecithin and/or bile extract.
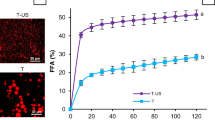
The activity of pancreatic lipase was quantified in terms of the amount of fatty acid released (μmol) from the emulsions after hydrolysis with pancreatic lipase (Figure 2). The amount of fatty acid released was higher for emulsions to which bile extract was added (250 ± 70 μmol mL−1) than for emulsions containing no bile extract (140 ± 70 μmol mL−1), which suggested that bile extract facilitated digestion, but was not absolutely necessary for digestion to occur. Previous researchers have also reported that the extent of lipolysis in lecithin-stabilized emulsions depended on the ratio of lecithin-to-bile salts in the system, with low levels of bile salts promoting enzyme activity and high levels inhibiting enzyme activity.[22] The inhibition observed at high levels has been attributed to the binding of lipase to nonadsorbed bile salts, which prevents it from adsorbing to the droplet surfaces.[11, 22, 23] In ourstudy, 5.0 mg mL−1 bile extract was added to the emulsions. The bile extract was composed of about 49% bile salts, with the main bile acid being glycodeoxycholic acid (MW = 468 Da) (see Materials and methods), hence the bile salt concentration was about 5.2 mmol L−1. This concentration is close to the values found in humans, e.g., bile salt concentrations measured in contents taken from the duodenum of human subjects were 2.5–6 mmol L−1 during fasting and 6.4–10.7 mmol L−1 postprandially.[5, 20]
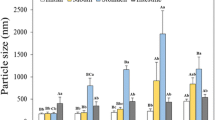
The particle size distribution (PSD) and mean particle diameter (d 32) of the lecithin-stabilized emulsions were determined from laser light scattering measurements (Figures 3 and 4), whereas the overall microstructure of the emulsions was determined by optical microscopy (Figure 5). In the absence of bile extract or lipase, the lecithin-stabilized emulsions had a uniform microstructure, a monomodal PSD, and a relatively small mean particle diameter (d 32 = 0.46 μm). The addition of bile extract to this emulsion had no noticeable affect on the microstructure, PSD, or mean particle diameter, which indicated that it did not promote droplet flocculation or coalescence. On the other hand, addition of lipase extract to the emulsions caused an appreciable increase in the mean particle diameter (d 32 = 0.73 μm), a pronounced change in the shape of the PSD, and a more textured microstructure (Figures 3, 4 and 5). There was a disappearance of relatively small droplets (d < 0.3 μm) in the PSD and an increase in the fraction of larger droplets (d 32 ∼ 1 μm). If there was no droplet aggregation in the emulsions, one would expect a progressive decrease in the mean droplet diameter with time as the lipids inside the droplets were digested and moved into the surrounding aqueous phase. The transport of digested lipids from the oil droplets into the aqueous phase could occur through a number of mechanisms[1]: (1) movement of individual fatty acid molecules from the droplet surface into the aqueous phase, (2) collision of an empty mixed micelle in the aqueous with the droplet surface leading to incorporation of some free fatty acids into the mixed micelle, and/or (3) spontaneous formation of a mixed micelle at the droplet surface through a “budding” mechanism. The fact that an increase in mean droplet diameter was actually observed in practice suggested that lipid digestion promoted some form of droplet aggregation. The most likely mechanism for droplet aggregation is coalescence, i.e., the merging of two or more individual droplets together to form a larger droplet.[1] Droplet coalescence may have been promoted by the increase in free fatty acids at the droplet surfaces, as these low hydrophile–lipophile balance (HLB) number surface active materials are not particularly effective at stabilizing oil-in-water emulsions.[1] The addition of bile and pancreatic lipase extracts in combination to the lecithin-stabilized emulsions led to the most dramatic change in microstructure, PSD, and mean particle diameter (Figures 3, 4, and 5). The general appearance of the emulsions became much coarser, a new population of relatively large particles (10 < d < 100 μm) was formed, and the mean particle diameter increased (d 32 = 1.3 μm). These results suggest that the combination of bile and lipase extracts had the most pronounced influence on the droplets in the emulsions, possibly because of more extensive digestion of the emulsified lipids and/or because they promoted greater droplet coalescence.
Interaction of bile extract and lipase with lecithin–chitosan-coated droplets
The objective of this section was to examine the influence of chitosan on the properties of lecithin-coated fat droplets and on fat digestibility. Primary and secondary emulsions were prepared at pH 3 and then adjusted to pH 7. The primary emulsion contained lecithin-coated fat droplets, whereas the secondary emulsion contained lecithin–chitosan-coated fat droplets. The electrical charge and mean particle diameter of the fat droplets were measured after the emulsions were stored for 24 h at room temperature.
The adsorption of chitosan to the surfaces of lecithin-coated droplets at pH 3 was confirmed using droplet charge measurements. At pH 3, the ζ potential of the droplets changed from −51 ± 1 mV in the absence of chitosan to +61 ± 1 mV in the presence of chitosan, which indicated that the cationic chitosan molecules adsorbed to the surfaces of anionic lecithin-coated droplets. In addition, there was no appreciable change in the mean particle diameter of the droplets when chitosan was added, indicating that the systems remained relatively stable at pH 3.
When the pH of the secondary emulsion was adjusted from 3 to 7, extensive droplet aggregation occurred and the system separated into an optically opaque “creamed” layer at the top and a transparent aqueous layer at the bottom. The creamed layer contained large particles (>0.1 mm) that were clearly visible to the human eye. It was not possible to make measurements of the mean particle size or ζ potential of the secondary emulsions at pH 7, because the aggregates formed were too large to be collected by pipette and placed in the measurement cells of either the laser diffraction or the electrophoresis instrument. The extensive droplet aggregation observed in these emulsions can be attributed to the fact that chitosan loses most of its positive charge at higher pH values, because the cationic functional groups (–NH3 +) on chitosan have a pK a value around pH 6.5.[24, 25] Hence, droplet aggregation can be attributed to the reduction in the electrostatic repulsion between the droplets at pH 7.
The influence of chitosan on the activity of pancreatic lipase was determined by measuring the amount of fatty acids released from the secondary emulsions in the presence and absence of bile extract (Figure 2). The amount of fatty acids released from the secondary emulsions in both the presence and absence of bile extract (38 and 0 μmol mL−1, respectively) was much smaller than that released from the primary emulsions (250 and 140 μmol mL−1, respectively). Presumably, the pancreatic lipase could not obtain access to the lipids within the droplets in the secondary emulsions, either because the droplets were surrounded by chitosan layers or because the droplets were trapped within large chitosan aggregates. This result clearly shows that chitosan is capable of reducing the digestibility of emulsified lipids.
Interaction of bile extract and lipase with lecithin–chitosan–pectin-coated droplets
As noted above, it was not possible to prepare stable secondary emulsions containing droplets coated by lecithin–chitosan layers at pH 7 because extensive droplet flocculation occurred. Another factor that may limit the utilization of secondary emulsions as food ingredients is that the fat droplets are surrounded by cationic chitosan, which has been shown to cause astringency when ingested because of its interaction with saliva proteins in the mouth.[26] We therefore examined the possibility of preparing tertiary emulsions containing droplets coated by lecithin–chitosan–pectin layers, because previous studies have shown that nonaggregated anionic droplets can be prepared at pH 7 using this combination of biopolymers.[27]
Tertiary emulsions containing droplets coated by lecithin–chitosan–pectin layers were prepared by adding pectin solution to a secondary emulsion at pH 3, and then adjusting to pH 7. The electrical charge and mean particle diameter of the tertiary emulsions were measured at pH 3 and 7. The adsorption of pectin to the surfaces of the lecithin–chitosan-coated droplets at pH 3 was confirmed by the droplet charge measurements. At pH 3, the ζ potential of the droplets changed from +60 ± 1 mV before pectin was added to −5 ± 1 mV after pectin was added, which indicated that the anionic pectin molecules adsorbed to the surfaces of the cationic lecithin–chitosan-coated droplets. In addition, there was no appreciable change in the mean particle diameter of the droplets when pectin was added (with d 32 going from 0.3 to 0.4 μm), which indicated that the tertiary emulsions were relatively stable at pH 3. When the pH was adjusted from 3 to 7, the ζ potential of the droplets went from −5 to −26 mV. An increase in solution pH should cause an increase in the negative charge on the pectin molecules (pK a ∼ 4.5) and a decrease in the positive charge on the chitosan molecules (pK a ∼ 6.5).[19, 27] Hence, the increase in the net negative charge on the emulsion droplets may have been a result of the changes in electrical charge of the adsorbed chitosan and pectin molecules. On the other hand, it is also possible that the droplets became more negatively charged because both chitosan and pectin desorbed from the droplet surfaces (see below). Having said this, the ζ potential of the droplets in the primary emulsion was about −39 mV compared to about −26 mV in the tertiary emulsion, which suggested that at least some chitosan molecules remained adsorbed to the droplet surfaces in the tertiary emulsion. In addition, some droplet aggregation occurred in the tertiary emulsions when the pH was increased from 3 to 7, with d 32 going from about 0.3 to 3 μm and a coarse microstructure being observed by optical microscopy (Figure 6). There are a number of possible reasons for the droplet aggregation observed in the tertiary emulsions: anionic chitosan molecules may have caused bridging flocculation of cationic pectin-lecithin-coated droplets; nonadsorbed pectin and chitosan molecules may have promoted depletion flocculation; the electrical charge on the droplets may not have been sufficient to generate a strong electrostatic repulsion.[1]
After the tertiary emulsions had been brought to pH 7, we added bile extract and/or lipase and measured the change in particle charge, mean particle diameter, and free fatty acids released. There was a slight increase in the magnitude of the negative charge on the droplets in the tertiary emulsions when bile extract and lipase were added either separately or in combination (Figure 7), which suggested that these negatively charged components adsorbed to the surfaces of the emulsion droplets. The addition of the bile extract or lipase did not cause a large change in the mean particle diameter in the tertiary emulsions, with all d 32 values falling within the range 1–2 μm. Interestingly, the amount of free fatty acids produced when lipase wasadded to the tertiary emulsion was much greater than from the secondary emulsion, and was fairly similar to that produced by the primary emulsion (Figure 2). For the emulsion containing bile extract, around 274 μmol of fatty acid per milliliter of emulsion was released, whereas for the emulsion without bile extract around 125 μmol mL−1 was released. If the fat droplets were covered with a thick lecithin–chitosan–pectin interfacial layer, we would not have expected the lipase to be able to access the fat within the droplets, and would have expected a much lower amount of fatty acid generation. We therefore postulate that chitosan and pectin desorbed from the droplet surfaces at pH 7, leaving the fat within the lecithin-coated droplets exposed to the lipase. This would explain why the amount of free fatty acids produced in the emulsions was fairly similar in the primary and tertiary emulsions.
Conclusions
This study has provided some interesting insights into the importance of interfacial properties on the ability of pancreatic lipase to digest emulsified fats. For emulsions containing lecithin-coated droplets, we found that the amount of fatty acid released by hydrolysis was greater when lipase was added with bile extract than when it was added alone, which indicated that bile extract promoted fat digestion by lipase but was not absolutely essential. When the lecithin-coated emulsion droplets were covered with chitosan, the amount of fatty acids produced was substantially reduced, which was attributed to the fact that chitosan formed a protective layer around the droplets and that it promoted extensive droplet flocculation, both of which inhibited the ability of lipase to interact with the fat inside the droplets. On the other hand, the amount of fatty acids released from emulsions initially containing droplets coated by lecithin–chitosan–pectin layers was fairly similar to that in the emulsions containing droplets coated only by lecithin layers. Our hypothesis was that both pectin and chitosan desorbed from the droplet surfaces in the tertiary emulsions, thereby exposing the emulsified fats to lipase, but further work is needed to establish this. Our results have important implications for understanding the ability of chitosan to interfere with fat digestion. Under certain circumstances, chitosan may be effective at inhibiting lipid digestion (e.g., if it remains attached to the fat droplets or it promotes extensive droplet flocculation), but under other circumstances it may lose its effectiveness (e.g., if it formscomplexes with anionic biopolymers). This may influence the way that chitosan should be consumed by humans if it is going to be used to inhibit fat digestion.
References
D.J. McClements, Food Emulsions: Principles, Practice and Techniques (CRC Press, Boca Raton 2004).
D. Lairon, Eur J Clin Nutr 50, 125 (1996).
G.A. Van Aken, Coalescence mechanisms in protien-stabilized emulsion, In: Food Emulsions, edited by S. Friberg, K. Larsson and J. Sjoblom (Marcel Dekker, New York, NY 2004), 4th ed, chap 8.
M. Armand, P. Borel, C. Dubois, M. Senft, J. Peyrot, J. Salducci, H. Lafont and D. Lairon, Am J Physiol 266, G372 (1994).
M. Armand, B. Pasquier, M. Andre, P. Borel, M. Senft, J. Peyrot, J. Salducci, H. Portugal, V. Jaussan and D. Lairon, Am J Clin Nutr 70, 1096 (1999).
H.L. Brockman, Biochimie 82, 987 (2000).
M. Armand, P. Borel, P. Ythier, G. Dutot, M. Melin, H. Senft, H. Lafont and D. Lairon, J Nutr Biochem 3, 333 (1992).
H. Mu and C.-E. Høy, Prog Lipid Res 43, 105 (2004).
S. Labourdenne, O. Brass, M. Ivanova, A. Cagna and G. Verger, Biochemistry 36, 3423 (1997).
C. Chapus, M. Rovery, L. Sarda and R. Verger, Biochimie 70, 1223 (1988).
M. Wickham, M. Garrood, J. Leney, P.D.G. Wilson and A. Fillery-Travis, J Lipid Res. 39, 623 (1998).
L.-K. Han, Y. Kimura and H. Okuda, Int J Obes 23, 174 (1999).
C.M. Gallaher, J. Munion, R. Hasslink, J. Wise and D.D. Gallaher, J Nutr 130, 2753 (2000).
S. Kobayashi, Y. Terashima and H. Itoh, Br Poult Sci 43, 270 (2002).
M. Sugano, S. Watanabe, A. Kishi, M. Izume and A. Ohtakara, Lipids 23, 187 (1998).
P. Faldt, B. Bergenstahl and P.M. Claesson, Colloids Surf A 71, 187 (1993).
C. Peniche, W. Aruelles-Monal, H. Peniche and N. Acosta, Macromol Biosci 3, 511 (2003).
S. Ogawa, E.A. Decker and D.J. McClements, J Agric Food Chem 51, 2806 (2003).
S. Ogawa, E.A. Decker and D.J. McClements, J Agric Food Chem 52, 3595 (2004).
D.A. Garrett, M.L. Failla and R.J. Sarama, J Agric Food Chem 47, 4301 (1999).
N. Saisuburamaniyan, L. Krithika, K.P. Dileena, S. Sivasubramanian and R. Puvanakrishnan, Anal Biochem 330, 70 (2004).
A. Lykidis, A. Antonis and P. Arzoglou, Comp Biochem Physiol B 116, 51 (1997).
A. Tavridou, A. Avranas and P. Arzoglou, Biochem Biophys Res Commun 186, 746 (1992).
F. Shahidi, J.K.V. Arachchi and Y.J. Jeon, Trends Food Sci Technol 10, 37 (1999).
G. Skjak-Braek, T. Anthonsen and P. Sandford, Chtin and Chitosan (Elsevier, London, 1989).
M.S. Rodriguez, L.A. Albertengo, I. Vitale and E. Agullo, JFood Sci 68, 665 (2003).
T. Aoki, E.A. Decker and D.J. McClements, Food Hydrocoll 19, 209 (2005).
Acknowledgments
This material is based upon work supported by the Cooperative State Research, Extension, Education Service, United State Department of Agriculture, Massachusetts Agricultural Experiment Station (project No. 831), by an United States Department of Agriculture, CREES, IFAFS Grant (Award Number 2001-4526) and an United States Department of Agriculture, CREES, NRI Grant (Award Number 2002-01655). This work was supported by the Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2004-214-M01-2004-000-10380-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mun, S., Decker, E.A., Park, Y. et al. Influence of Interfacial Composition on in Vitro Digestibility of Emulsified Lipids: Potential Mechanism for Chitosan's Ability to Inhibit Fat Digestion. Food Biophysics 1, 21–29 (2006). https://doi.org/10.1007/s11483-005-9001-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-005-9001-0