Abstract
Purpose
Hair can be the only available specimen to prove drug-facilitated crimes (DFCs). However, it is difficult to identify drugs abused in DFCs, particularly benzodiazepines ingested in a single dose, using the conventional segmental hair analysis. In this study, an analytical procedure to identify a trace amount of drug in hair based on micro-segmental analysis was developed.
Methods
Hair was donated from a subject who had been administered midazolam in a single dose. First, tens of hair strands were analyzed according to our routine procedure. Drug screening and identification were performed using a quadrupole-Orbitrap liquid chromatography–tandem mass spectrometry (LC–MS/MS) instrument. Next, two hair strands were segmented at 0.4-mm intervals and multi-target selected reaction monitoring of each segment (micro-segmental analysis) was performed using a tandem quadrupole LC–MS/MS instrument. After distribution curves of midazolam-derived ions in each hair strand were constructed, the residual extracts from segments corresponding to drug-containing regions were collected selectively into one tube. The extract was concentrated using solid-phase extraction and then injected into the LC–MS/MS instrument for drug identification.
Results
We failed to identify drugs using our routine procedure, although a slight peak, predicted to arise from midazolam, was detected, while the peak detected in the concentrated extract from drug-containing hair regions (named “selective concentration”) was identified as midazolam.
Conclusions
The “selective concentration” enabled the identification of a trace amount of drug using several hair strands. The method would be helpful in proving DFCs even after it was impossible to identify drugs using conventional segmental hair analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypnotics and sedatives are often abused in crimes such as sexual assaults and robberies [1,2,3,4,5,6]. These drugs can cause drowsiness soon after single-dose ingestion. Unfortunately, victims that ingest such drugs unintentionally tend to report damages to the police long after the incident because of memory loss and psychological shock. Therefore, hair can be the sole biological specimen available to prove such drug-facilitated crimes (DFCs). In practical hair analysis using conventional segmental analysis (hair segments of several centimeters) to investigate DFCs, drugs sometimes go undetected or are unidentified in the victim’s hair, even though other evidence such as the victim’s statement, security camera footage, and the suspect’s personal belongings indicate the high possibility of a DFC. Notably, if a specific drug is ingested in high dose or repeatedly, its identification using conventional segmental hair analysis [1,2,3,4,5] is probable. However, in cases of single-dose ingestion of hypnotics and sedatives, particularly benzodiazepines, which can cause drowsiness in very small doses (1 mg or less) [7], it would be difficult to identify the drug even when a drug-related peak is detected.
A drug ingested in a single dose can be located in a specific hair region (several millimeters in length) in each hair strand, thereby reflecting the day of ingestion [8,9,10]. The rate of hair growth differs for the individual hair strands [11]. Thus, when a bundle of hair is considered (Supplementary material Fig. S1), the drug-containing region widens according to the differences in the growth rate from the time of drug ingestion of the individual hair strands. In conventional segmental hair analysis, tens of hair strands are normally used as the starting sample to increase the analyte amount, thereby resulting in an increase in the matrix amount. The segment length is determined by considering that head hair grows at an average rate of approximately 1 cm per month [11]. When hair strands are segmented at short intervals (several centimeters), the analyte spreads over a few segments, resulting in a decrease in both the analyte and matrix amounts in each segment. In contrast, when segmented at longer intervals, a large proportion of each segment is occupied by unnecessary non-drug-containing regions (hair matrices), while most of the analyte is contained in a specific segment. In such analyses, it is important to decrease the amount of matrix and increase the amount of analyte in an extract to obtain mass spectra of the drugs without extra ion peaks. Therefore, to identify a trace amount of drug in hair, it is required to make drug-containing regions in each hair strand visible and remove as many non-drug-containing regions as possible.
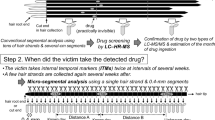
Micro-segmental analysis, which we previously developed [6, 10, 12,13,14], allows the visualization of the drug distributions in each hair strand by segmenting an individual hair strand at 0.4-mm intervals, corresponding to the daily average hair growth. In this study, this method was used to locate the drug-containing regions in each hair strand (step 1 in Fig. 1). Subsequently, the residual extracts from the drug-containing regions were selectively collected and concentrated to identify the trace amount of drug in the hair (step 2 in Fig. 1).
For the method development of hair analysis assuming a DFC, it is necessary to use authentic hair collected from subjects who ingested hypnotics and sedatives in a single dose. However, it is difficult to find such subjects, because (1) healthy subjects are not allowed to ingest prescription medicines, such as hypnotics and sedatives, for research purposes and (2) hypnotics and sedatives are typically prescribed to be taken daily for 2 weeks or more. Fortunately, we found a patient who was only administered one single dose of midazolam. Although midazolam, a prescription sedative and anesthetic with elimination half-life of 1.8–6.4 h [7], is administered in tablet or injection form under doctor supervision, it can be abused by medical staff and obtained illicitly [1,2,3,4, 15,16,17]. In this study, midazolam, administered as a single dose, was used as the model drug to mimic a DFC, and an analytical method to identify a trace amount of the drug in hair, based on micro-segmental analysis, was developed. To the best of our knowledge, this is the first report of hair analysis following single-dose ingestion of midazolam.
Materials and methods
Materials
Chlorpheniramine (CP)-d6 maleate was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Midazolam and the other reagents were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan). Acetonitrile, methanol, and water were of LC/MS grade.
Hair collection
For the method development, hair was donated from an Asian woman in her 30 s who had been administered a single dose of midazolam (ca. 3 mg) intravenously for medical examination approximately 2 months before the hair was collected. Her curly black hair, dyed brown, was cut with scissors from the posterior vertex region near her scalp. She stated that except for the single-dose of midazolam, she had not ingested hypnotics or sedatives for at least 1 year prior to the hair collection date. Hair treatment such as dyeing was not performed for her hair after midazolam administration. The experiment was performed based on approval by the ethics committee at the National Research Institute of Police Science (No. 2019005, Kashiwa, Japan). Informed consent was obtained from her for the experiment.
Conventional segmental hair analysis
Twenty hair strands were cut 3 cm long from the proximal cut end. The proximal hair segments were then cut in half, placed in a 2-mL safe-lock tube, and weighed (ca. 6 mg). Hair washing and drug extraction were performed using a previously described method [6]. Briefly, the hair was pulverized with a stainless-steel bullet after washing with 1% aqueous sodium dodecyl sulfate solution, purified water, and methanol. The drugs were extracted with acetonitrile. The extract (20 μL) was then injected into a high-resolution quadrupole-Orbitrap liquid chromatography–tandem mass spectrometry (LC–MS/MS) instrument (Ultimate 3000 liquid chromatograph connected to a Q-Exactive mass spectrometer; Thermo Fisher Scientific, Waltham, MA, USA). The analytical conditions are summarized in Supplementary material Table S1.
For the drug screening by scan analysis without a quadrupole mass filter, the extracted ion chromatograms were constructed using the [M + H]+ of the respective drugs to search for specific peaks in the hair extract. When a peak was detected, product ion analysis, selecting the [M + H]+ peak as the precursor ion, was performed under the same conditions as those used in the scan analysis, but with the use of a quadrupole mass filter. Moreover, the extract was injected into a tandem quadrupole LC–MS/MS instrument for confirmatory analysis, as described later.
Analytical conditions in a tandem quadrupole LC–MS/MS instrument
An Acquity UPLC I-Class liquid chromatograph and a Xevo TQ-S tandem quadrupole mass spectrometer (Waters Corp, Milford, MA, USA) were used for drug identification and quantification. Chromatographic separation was achieved using an octadecylsilyl column (Cadenza CD-C18 HT, 150 mm × 2.0 mm i.d., particle diameter 3 μm; Imtakt Corp., Kyoto, Japan) at 40 °C. The mobile phase consisted of an aqueous solution of 5 mM ammonium acetate and 0.05% formic acid (mobile phase A), and acetonitrile. The flow rate was changed as follows: 0–0.2 min 0.2 mL/min, 0.2–4 min 0.2–0.5 mL/min, 4.01–4.5 min 0.5–0.65 mL/min, 4.51–4.90 min 0.65–0.55 mL/min, and 4.91–5 min 0.2 mL/min. The proportion of acetonitrile was changed over a linear gradient as follows: 0–0.2 min 5% acetonitrile, 0.2–4 min 5–95% acetonitrile, 4–4.5 min 95% acetonitrile, and 4.5–5 min 5% acetonitrile. Electrospray ionization was performed in positive ionization mode with a capillary voltage of 3 kV and desolvation temperature of 650 °C.
For the multi-target selected reaction monitoring (SRM), transitions for typical hypnotics and sedatives, including midazolam (m/z 326 > 291), were selected. For the confirmatory analysis of midazolam, SRM with two transitions (Table 1) and product ion scan analysis were performed. The ratio of the peak areas obtained from the two transitions was then compared with that of the drug standard. In the product ion scan analysis, the [M + H]+ of midazolam (m/z 326) was used as the precursor ion. The cone voltage and collision energy were 30 V and 25 eV, respectively.
Micro-segmental analysis
The detailed sample preparation is described in our previous report [10, 12]. Briefly, an individual hair strand was weighed and its full length was measured. After each hair strand was washed, it was cut at a 0.4-mm interval from the end of the hair root side using a tissue slicer equipped with a micrometer scale (Stoelting Co., Wood Dale, IL, USA), and each segment was placed in a 0.1-mL microtube. A mixture of mobile phase A and acetonitrile (3:1, by vol.) was used as the extraction solution. Subsequently, an extraction solution (100 μL) containing CP-d6 (4 pg/mL) as the internal standard (IS) was added to the tube containing the segment. The sample tube was then sonicated at 23 kHz for 10 min and then maintained at approximately 22 °C in a dark place until further analysis. The supernatant (35 μL) was transferred to a 96-well plate and then diluted with mobile phase A (35 μL), and the resultant solution (50 μL) was injected into the tandem quadrupole LC–MS/MS instrument. The analytical conditions were identical to those described above.
When peaks with the same retention times as those of the drug standards were detected during multi-target SRM, the analyte concentration in each 0.4-mm hair segment was measured. An analyte distribution curve was then constructed by assessing the analyte concentration in each 0.4-mm hair segment, numbered from the root to the tip side (step 1 in Fig. 1).
Validation of the quantitative analysis
The method was validated using spiked hair segments according to the method validation guidelines of the Scientific Working Group for Forensic Toxicology (SWGTOX) [18]. Moreover, analyte recovery from the subject’s 0.4-mm hair segments was confirmed by soaking the hair segments in an extraction solution for 24 and 72 h after sonication. The analyte-to-IS relative peak areas attained for these two different soaking times were then compared.
“Selective concentration” of the drug-containing regions
After part of the extract (25 μL out of 100 μL) from each 0.4-mm hair segment was used for micro-segmental analysis, the residual extracts were stored in each tube and each well of the 96-well plate. When drug-containing regions were found on the distribution curves, the extracts stored in the tubes and wells corresponding to the drug-containing regions were selectively collected in a 2-mL tube (Step 2 in Fig. 1). The collected extract (ca. 1 mL) was concentrated by solid-phase extraction (SPE). The analyte was extracted using an Oasis HLB cartridge with a 30-mg sorbent (Waters Corp.) according to the general protocol [19]. The sample (collected extract) was then diluted with 4% aqueous phosphoric acid solution (1 mL). After the cartridge was conditioned with methanol (1 mL) and water (1 mL), the sample solution was loaded onto the sorbent bed. The sorbent was washed with a 5% methanol aqueous solution (1 mL) and the analyte was then eluted with methanol (0.5 mL). After the elute was dried under nitrogen flow, the residue was dissolved in the initial LC mobile phase (100 μL). For confirmatory analysis of midazolam, the concentrated extract (50 μL) was injected into the tandem quadrupole LC–MS/MS instrument under the conditions described above.
Results
Drug identification by conventional segmental hair analysis
First, drug screening based on high-resolution MS was performed using a quadrupole-Orbitrap LC–MS/MS instrument (Fig. 2). When the extracted ion chromatograms at m/z 326.08547 ± 0.00163 (mass tolerance, 5 ppm) were constructed, a slight peak with the same retention time as that of the midazolam standard was detected in the hair extract (Fig. 2a, b). To confirm the mass resolution performance, the mass tolerance was increased to 15 ppm. However, when the extracted ion chromatogram was constructed at m/z 326.08547 ± 0.00489, the peak at 15.8 min was buried in many noise peaks (Fig. 2c). These results indicated the presence of large matrix peaks near m/z 326.08547, implying that it was difficult to detect the peak using low-resolution MS instruments such as quadrupole and ion trap instruments. Because the predicted midazolam peak was slight, product ion analysis was performed using m/z 326 as the precursor ion. Trace amounts of ion peaks with the same m/z as those of midazolam standard were detected in the hair extract (Fig. 2d, e). However, the ratio of the peak areas between the two transitions (m/z 326 > 291 and m/z 326 > 244) could not be calculated, because the peaks were too small. Additionally, it was impossible to obtain the same product ion spectrum as that of the midazolam standard from peak a of the hair extract (Fig. 2f, g).
Chromatograms and product ion spectra of midazolam standard and hair extracts using a quadrupole-Orbitrap LC–MS/MS instrument. Extracted ion chromatograms of midazolam at m/z 326.08547 ± 0.00163 (a), hair extract at m/z 326.08547 ± 0.00163 (b), and hair extract at m/z 326.08547 ± 0.00489 (c) in the scan analysis. Extracted ion chromatograms of midazolam (d) and hair extract (e) in product ion analysis using m/z 326 as the precursor ion. Product ion spectra of midazolam (f) and peak a (g)
Next, the hair extract remaining after quadrupole-Orbitrap LC–MS/MS analysis was injected into a tandem quadrupole LC–MS/MS instrument. Among the typical hypnotics and sedatives, such as triazolam and brotizolam, in the multi-target SRM, a peak was only detected at the midazolam-derived transition. SRM using two transitions (Tr. 1, m/z 326 > 291 and Tr. 2, m/z 326 > 244) and product ion scan analysis were performed to confirm that this peak was derived from midazolam (Fig. 3). The ratio of the peak areas at the two transitions (Tr. 2/Tr. 1 = 0.346) was different from that of the midazolam standard (Tr. 2/Tr. 1 = 0.262). This difference (32%) did not fulfill the criteria for drug identification as adopted in many working groups (the maximum relative tolerances are typically 20–30% [20,21,22,23]). In the product ion scan analysis, a trace amount of the midazolam-derived peak (peak a in Fig. 3e) was detected on the extracted ion chromatogram. However, the product ion spectrum was not consistent with that of the midazolam standard, because large matrix-derived ions such as m/z 220 and m/z 194 could not be removed, even by subtracting the background. Ultimately, we failed to identify the small peak using the conventional segmental hair analysis.
Chromatograms and product ion spectra of midazolam standard hair extracts using a tandem quadrupole LC–MS/MS instrument. Chromatograms attained from SRM (a) and product ion scan analysis using m/z 326 as the precursor ion (b) of midazolam. Product ion spectrum (m/z 326 >) of midazolam (c). Chromatograms attained from SRM (d) and product ion scan analysis using m/z 326 as the precursor ion (e) of the hair extract. Product ion spectrum (m/z 326 >) of peak a (f)
Drug identification by micro-segmental hair analysis
First, a preliminary study was performed to establish whether midazolam-derived peaks could be detected at specific hair segments in a single hair strand by micro-segmental analysis. Each of the 0.4-mm hair segments was soaked in the extraction solution for 24 h, and portions of the extracts were then analyzed using multi-target SRM. Because slight midazolam-derived peaks were detected at some hair segments, each 0.4-mm hair segment was soaked in the residual extraction solution for an additional 48 h. Thus, when the residual extracts were analyzed, the peak intensities were significantly enhanced (Supplementary material Fig. S2). However, the 72-h soaking time was adequate to locate the drug-containing hair regions in this study, because the position of the segments with high intensities did not change. Analytical validation using spiked hair segments was sufficient to detect midazolam at the concentration ranges present in the actual hair segments (Table 2). The matrix effect in the micro-segmental analysis was much smaller than that in the conventional segmental analysis. This implied that decreases in the matrix amounts reduced ion suppression because of the very smaller sample amounts (ca. 0.004 mg per 0.4-mm hair segment) and gentle extraction (soaking of the hair segment in the solution) employed.
Next, two hair strands were subjected to micro-segmental analysis under the validated conditions and the distribution curves of the midazolam-derived ions (m/z 326 > 291) in each hair strand were then constructed (Fig. 4). The regions in which the presence of midazolam was expected (drug-containing regions) were located at approximately 2 cm from the proximal end of both hair strands. Drug identification using one 0.4-mm hair segment with the highest drug intensity, which corresponded to the residual extract from segment No. 56 of hair 1, was next performed (Fig. 5a–c). In SRM, the ratio of the peak area at the two transitions for midazolam (Tr. 2/Tr. 1 = 0.349) did not fulfill the criteria for drug identification. In the product ion analysis, although a peak (peak a in Fig. 5b) with the same retention time as that of midazolam was detected, the product ion spectrum was not consistent with that of midazolam. Therefore, it was impossible to identify the peak using only one hair segment with the highest drug intensity.
Chromatograms and product ion spectra of hair extracts based on micro-segmental analysis. Chromatograms attained from SRM (a) and product ion scan analysis using m/z 326 as the precursor ion (b) of hair extract from a 0.4 mm segment (segment No. 56 of hair 1 in Fig. 4). Product ion spectrum (m/z 326 >) of peak a (c). Chromatograms attained from SRM (d) and product ion scan analysis using m/z 326 as the precursor ion (e) of the concentrated extract from 10 segments of two hair strands (segment Nos. 52–57 of hair 1 and segment Nos. 43–46 of hair 2 in Fig. 4). Product ion spectrum (m/z 326 >) of peak b (f)
Drug identification by “selective concentration”
In this technique, each hair segment was soaked in the residual extraction solutions in each tube until the drug-containing hair regions were determined. Additionally, the residual extract after micro-segmental analysis was retained in the 96-well plate. The residual extracts corresponding to 10 hair segments with comparatively high intensities (segment Nos. 52–57 of hair 1 and segment Nos. 43–46 of hair 2) as shown in Fig. 4 were collected into a 2-mL tube.
After the selectively collected solution was subjected to SPE, the concentrated extract was injected into the tandem quadrupole LC–MS/MS instrument (Fig. 5d–f). In SRM, the peak areas in the concentrated extract (Fig. 5d) displayed an approximately 15-fold increase over those observed in the 0.4-mm segment corresponding to segment No. 56 of hair 1 (Fig. 5a). The ratio of the peak areas at the two transitions (Tr. 2/Tr. 1 = 0.307) fulfilled the criteria for drug identification. In the product ion scan analysis, a peak (peak b in Fig. 5e) with the same retention time as that of midazolam was detected and the product ion spectrum (Fig. 5f) was consistent with that of midazolam (Fig. 3c).
Discussion
Identification of a trace amount of drug using conventional segmental hair analysis
In most DFCs, only the suspects know what drugs were used. Considering practical drug testing, we, therefore, investigated whether any drug could be detected in the subject’s hair using our routine procedure, even though we knew that she had been administered a single therapeutic dose of midazolam. A quadrupole-Orbitrap LC–MS/MS instrument was used for drug screening. High-resolution MS has the advantage of non-target screening, because it can extract ions with precise masses from already acquired data. Multi-target SRM using a tandem quadrupole LC–MS/MS instrument is used as a general drug screening method in most laboratories [1, 4, 5, 24, 25]. This technique enables tens of analytes to be monitored simultaneously and detects any peaks with designated transitions more sensitively than high-resolution MS instruments. Notably, in this study, although both quadrupole-Orbitrap LC–MS/MS instrument and tandem quadrupole LC–MS/MS instrument were used, it was impossible to identify a trace amount of drug in the subject’s hair sample using the conventional segmental hair analysis.
Determination of drug-containing hair regions by micro-segmental analysis
The regions in which the presence of midazolam was expected were determined using micro-segmental analysis (Fig. 4). The positions agreed with the time of midazolam administration (approximately 2 months prior to hair collection). It was considered that the small difference in the locations of the two hair strands reflected the difference in hair length, from the root to the cut end, during hair collection (Fig. S1) and the difference in the growth rate of the individual hair strands [6, 8,9,10,11, 13, 14]. The maximal intensity in hair 1 was higher than that in hair 2. Thus, the difference in peak intensity may reflect the activity of the individual hair roots, although the uptake mechanism has not been yet elucidated.
Under the drug extraction conditions, the peak areas during 72-h soaking process were higher than those observed during 24-h soaking (Fig. S2). We previously examined the soaking times for over-the-counter medicines, such as CP and dihydrocodeine, in 0.4-mm hair segments [10]. Most of these tested medicines were extracted completely by soaking in the extraction solution for 24 h. However, benzodiazepines may be difficult to be extracted from hair under these conditions [26,27,28] and a more efficient drug extraction method for various drugs in micro-segmental hair analysis is currently under investigation.
“Selective concentration” of the extracts
The total volume of the selectively collected solution was approximately 1 mL, and the aqueous solution comprised ammonium acetate, formic acid, and acetonitrile. To inject the largest possible amount of analyte into the LC–MS/MS instrument, it is necessary to concentrate the solution. Thus, 1 mL of the analyte-free extraction solution was preliminarily evaporated using an evaporator. Solvent evaporation was not adopted as the concentration method, because it took over 1 h to completely remove the solvent using this method. Thus, SPE was next studied using an extraction solution containing midazolam (0.1 pg/mL). The general Oasis HLB protocol was employed to afford a midazolam extraction rate of 96.0 ± 10.5% (n = 3). The SPE method was adopted to concentrate the solution collected selectively in this study because of its simplicity and high extraction efficiency. Liquid–liquid extraction can also be used as a concentration method. However, it was not examined in this study, because the extraction procedure, including pH adjustment, multiple transfer of the organic solvent, and evaporation, was considered to be more complicated than the SPE procedure. Although the general Oasis HLB protocol can be applied to various drugs [19, 29,30,31], the concentration method used for a selectively collected extraction solution should be optimized for every targeted drug, considering the ease of procedure and extraction efficiency of the analyte.
The approximately 15-fold increase in the peak areas in Fig. 5d, over those presented in Fig. 5a, agreed with the sum of peak areas corresponding to the 10 selected segments (Fig. 4). The results indicated that the proposed “selective concentration” method successfully increased the analyte amounts without adding unnecessary hair matrices derived from non-drug-containing hair regions. In this study, the selective collection of 10 segments from two hair strands was sufficient for drug identification. However, in practical DFC cases, the number of hair strands and segments to be used for drug identification should be changed according to the height and width of the drug-containing regions in each distribution curve.
Applications of the “selective concentration” method
For practical application of the developed method in DFC cases, it is important to adopt the method using authentic hair samples from subjects with minimum hypnotic/sedative ingestion history. After the “selective concentration” method was developed, we found another patient (An Asian man in his 40 s) who had been administered midazolam twice in a 9-day interval (ca. 3 mg each), approximately 4 months before. Thus, the developed method was applied to the subject’s hair to confirm its validity. His black straight hair was plucked out from the posterior vertex region near his scalp, and the two strands were subjected to micro-segmental analysis (Supplementary material Fig. S3). Drug-containing regions were located in the distribution curves of the midazolam-derived ions in the respective hair strands, reflecting the administration history of the subject. Moreover, a product ion spectrum identical to that of midazolam was successfully obtained from the concentrated extract corresponding to the drug-containing regions.
One of the advantages of the “selective concentration” process is the capability of identifying drugs using only a few hair strands. However, it is not recommended to collect only a few hair strands from victims, expecting the “selective concentration” method will be the first method used for drug identification. This is because micro-segmental analysis is laborious work [6, 14] and conventional segmental analysis is helpful to narrow down the targeted drugs and estimate the drug amount prior to “selective concentration”. For special cases, where only a few hair strands are available [32], Step 1 in Fig. 1 may be the first choice for drug identification. This procedure can be used for not only midazolam but also the other analytes. Although this method is not successful in all cases, it is worth trying in cases where drug identification using routine hair analysis has failed.
Conclusions
In this study, we developed an advanced analytical method to enhance the possibility of drug identification in cases where it was impossible to identify drugs using the conventional segmental hair analysis. First, micro-segmental analysis was performed using several hair strands. Next, the residual extracts corresponding to the drug-containing regions from each hair strand were selectively collected. After the extract was concentrated, the concentrate was used for drug identification. A series of procedures was then employed to increase the analyte amounts without increasing the number of extra hair matrices. The “selective concentration” method shows great potential for application to prove the use of a specific drug in DFCs, particularly in cases where hair samples from victims who ingested a single dose of a drug are collected long after the incident or where the number of hair strands for analysis is limited.
References
Chèze M, Duffort G, Deveaux M, Pépin G (2005) Hair analysis by liquid chromatography-tandem mass spectrometry in toxicological investigation of drug-facilitated crimes: report of 128 cases over the period June 2003-May 2004 in metropolitan Paris. Forensic Sci Int 153:3–10. https://doi.org/10.1016/j.forsciint.2005.04.021
Lee S, Han E, In S, Choi H, Chung H, Chung KH (2011) Determination of illegally abused sedative-hypnotics in hair samples from drug offenders. J Anal Toxicol 35:312–315. https://doi.org/10.1093/anatox/35.5.312
Rust KY, Baumgartner MR, Meggiolaro N, Kraemer T (2012) Detection and validated quantification of 21 benzodiazepines and 3 “z-drugs” in human hair by LC-MS/MS. Forensic Sci Int 215:64–72. https://doi.org/10.1016/j.forsciint.2011.07.052
Irving RC, Dickson SJ (2007) The detection of sedatives in hair and nail samples using tandem LC-MS-MS. Forensic Sci Int 166:58–67. https://doi.org/10.1016/j.forsciint.2006.03.027
Villain M, Concheiro M, Cirimele V, Kintz P (2005) Screening method for benzodiazepines and hypnotics in hair at pg/mg level by liquid chromatography-mass spectrometry/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 825:72–78. https://doi.org/10.1016/j.jchromb.2004.12.036
Kuwayama K, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Inoue H (2019) Strong evidence of drug-facilitated crimes by hair analysis using LC–MS/MS after micro-segmentation. Forensic Toxicol 37:480–487. https://doi.org/10.1007/s11419-019-00472-3
Drugs.com, https://www.drugs.com. Accessed 20 Jul 2020
Kamata T, Shima N, Sasaki K, Matsuta S, Takei S, Katagi M, Miki A, Zaitsu K, Nakanishi T, Sato T, Suzuki K, Tsuchihashi H (2015) Time-course mass spectrometry imaging for depicting drug incorporation into hair. Anal Chem 87:5476–5481. https://doi.org/10.1021/acs.analchem.5b00971
Shima N, Sasaki K, Kamata T, Matsuta S, Wada M, Kakehashi H, Nakano S, Kamata H, Nishioka H, Sato T, Tsuchihashi H, Miki A, Katagi M (2017) Incorporation of zolpidem into hair and its distribution after a single administration. Drug Metab Dispos 45:286–293. https://doi.org/10.1124/dmd.116.074211
Kuwayama K, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Inoue H (2018) Different localizations of drugs simultaneously administered in a strand of hair by micro-segmental analysis. Drug Test Anal 10:750–760. https://doi.org/10.1002/dta.2259
Kintz P, Salomone A, Vincenti M (2015) Hair analysis in clinical and forensic toxicology. Academic Press, Cambridge
Kuwayama K, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Inoue H (2016) Three-step drug extraction from a single sub-millimeter segment of hair and nail to determine the exact day of drug intake. Anal Chim Acta 948:40–47. https://doi.org/10.1016/j.aca.2016.10.029
Kuwayama K, Nariai M, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Abe H, Iwase H, Inoue H (2018) Accurate estimation of drug intake day by micro-segmental analysis of a strand of hair using internal temporal markers. J Appl Lab Med 3:37–47. https://doi.org/10.1373/jalm.2017.025346
Kuwayama K, Nariai M, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Abe H, Iwase H, Inoue H (2018) Micro-segmental hair analysis for proving drug-facilitated crimes: evidence that a victim ingested a sleeping aid, diphenhydramine, on a specific day. Forensic Sci Int 288:23–28. https://doi.org/10.1016/j.forsciint.2018.04.027
Colucci AP, Gagliano-Candela R, Aventaggiato L, De Donno A, Leonardi S, Strisciullo G, Introna F (2013) Suicide by self-administration of a drug mixture (propofol, midazolam, and zolpidem) in an anesthesiologist: the first case report in Italy. J Forensic Sci 58:837–841. https://doi.org/10.1111/1556-4029.12053
Kintz P, Villain M, Dumestre V, Cirimele V (2005) Evidence of addiction by anesthesiologists as documented by hair analysis. Forensic Sci Int 153:81–84. https://doi.org/10.1016/j.forsciint.2005.04.033
Cirimele V, Kintz P, Doray S, Ludes B (2002) Determination of chronic abuse of the anaesthetic agents midazolam and propofol as demonstrated by hair analysis. Int J Legal Med 116:54–57. https://doi.org/10.1007/s004140100240
Scientific Working Group for Forensic Toxicology (2013) Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J Anal Toxicol 37:452–474. https://doi.org/10.1093/jat/bkt054
Waters Corporation, Oasis Solid-Phase Extraction Products, https://www.waters.com/webassets/cms/library/docs/720001692en.pdf. Accessed 28 June 2020
World Anti-Doping Agency Laboratory Expert Group (2015) WADA technical document—TD2015IDCR: minimum criteria for chromatographic-mass spectrometric confirmation of the identity of analytes for doping control purposes. WADA, Montreal
Narciso J, Luz S, Bettencourt da Silva R (2019) Assessment of the quality of doping substances identification in urine by GC/MS/MS. Anal Chem 91:6638–6644. https://doi.org/10.1021/acs.analchem.9b00560
SOFT/AAFS Forensic Laboratory Guidelines 2006 Version (2006) http://www.soft-tox.org/files/Guidelines_2006_Final.pdf. Accessed 28 June 2020
Elliott SP, Stephen DWS, Paterson S (2018) The United Kingdom and Ireland association of forensic toxicologists forensic toxicology laboratory guidelines (2018). Sci Justice 58:335–345. https://doi.org/10.1016/j.scijus.2018.05.004
Kahl KW, Seither JZ, Reidy LJ (2019) LC-MS-MS vs ELISA: validation of a comprehensive urine toxicology screen by LC-MS-MS and a comparison of 100 forensic specimens. J Anal Toxicol 43:734–745. https://doi.org/10.1093/jat/bkz066
Perez ER, Knapp JA, Horn CK, Stillman SL, Evans JE, Arfsten DP (2016) Comparison of LC-MS-MS and GC-MS analysis of benzodiazepine compounds included in the drug demand reduction urinalysis program. J Anal Toxicol 40:201–207. https://doi.org/10.1093/jat/bkv140
Miyaguchi H (2014) Determination of sedative–hypnotics in human hair by micropulverized extraction and liquid chromatography/quadrupole-Orbitrap mass spectrometry. Anal Methods 6:5777–5783. https://doi.org/10.1039/C4AY00505H
Morini L, Vignali C, Polla M, Sponta A, Groppi A (2012) Comparison of extraction procedures for benzodiazepines determination in hair by LC-MS/MS. Forensic Sci Int 218:53–56. https://doi.org/10.1016/j.forsciint.2011.10.013
Madry MM, Kraemer T, Baumgartner MR (2018) Systematic assessment of different solvents for the extraction of drugs of abuse and pharmaceuticals from an authentic hair pool. Forensic Sci Int 282:137–143. https://doi.org/10.1016/j.forsciint.2017.11.027
Khelfi A, Azzouz M, Abtroun R, Reggabi M, Alamir B (2018) Determination of chlorpromazine, haloperidol, levomepromazine, olanzapine, risperidone, and sulpiride in human plasma by liquid chromatography/tandem mass spectrometry (LC-MS/MS). Int J Anal Chem 2018:5807218. https://doi.org/10.1155/2018/5807218
Pérez-Alcaraz A, Borrull F, Aguilar C, Calull M (2019) Enantioselective determination of cathinones in urine by high pressure in-line SPE-CE. Electrophoresis 40:1762–1770. https://doi.org/10.1002/elps.201900072
Castilla-Fernández D, Moreno-González D, Beneito-Cambra M, Molina-Díaz A (2019) Critical assessment of two sample treatment methods for multiresidue determination of veterinary drugs in milk by UHPLC-MS/MS. Anal Bioanal Chem 411:1433–1442. https://doi.org/10.1007/s00216-019-01582-y
Kuwayama K, Nariai M, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Abe H, Iwase H, Inoue H (2019) Estimation of day of death using micro-segmental hair analysis based on drug use history: a case of lidocaine use as a marker. Int J Legal Med 133:117–122. https://doi.org/10.1007/s00414-018-1939-9
Acknowledgements
This work was supported by JSPS KAKENHI grant number 19K10700.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at the National Research Institute of Police Science (Approval No. 2019005, Kashiwa, Japan) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuwayama, K., Miyaguchi, H., Kanamori, T. et al. Development of the “selective concentration” analytical method for drug-containing hair regions based on micro-segmental analysis to identify a trace amount of drug in hair: hair analysis following single-dose ingestion of midazolam. Forensic Toxicol 39, 156–166 (2021). https://doi.org/10.1007/s11419-020-00553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-020-00553-8