Abstract
Purpose
Hair analysis can provide effective information to prove drug-facilitated crimes (DFCs). Herein, an analytical procedure for obtaining evidence of DFCs stronger than with conventional segmental hair analysis is demonstrated for an actual case. A victim reported to the police that, approximately 1 month earlier, she had consumed a drink, fallen asleep, and then been assaulted.
Methods
Her hair strands were collected to examine whether drugs were detected from her hair. Drug screening by liquid chromatography–high-resolution mass spectrometry (LC–HR-MS) revealed a specific peak, derived from zolpidem, on the chromatogram obtained from the hair extract. Micro-segmental analysis using an internal temporal marker (ITM) was performed to estimate the day of zolpidem ingestion using sensitive LC–low-resolution tandem mass spectrometry (MS/MS). The victim ingested cold medicine as an ITM twice, with an interval of 21.0 days, to calculate the actual growth rate of her hair. Her hair strands were collected again 2 weeks after the second ITM ingestion. A hair strand was cut at 0.4-mm intervals, and the distribution curves of zolpidem and the ITM in a hair strand were plotted.
Results
The estimated day of zolpidem ingestion was consistent with the day of the incident that she had reported. The two-step hair analysis proved that she had ingested zolpidem on the day of the incident.
Conclusions
The combination of drug screening by LC–HR-MS and determination of the day of drug ingestion using micro-segmental analysis by sensitive LC–MS/MS would be useful for elucidating the relationship between the drug and the incident when investigating DFCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In drug-facilitated crimes (DFCs), drinks are secretly spiked with drugs which can cause drowsiness/hypnosis. When the victims fall asleep, the perpetrators commit crimes such as robbery and/or sexual assault. Therefore, only the perpetrator knows the type of drugs used in the crimes. Additionally, victims often hesitate to immediately report the incident to the police because they do not remember the situation clearly and/or have been psychologically traumatized. If victims come forward with their ordeal several weeks after the incident, there is no longer any evidence except for the victims’ biological samples. Although urine and blood are usually used to prove drug ingestion, the detection window for hypnotics ingested at single doses is only a few days [1,2,3]. Therefore, in most cases, it is impossible to detect drugs used in DFCs from victims’ urine and blood.
Hair is an effective specimen for proving drug ingestion a few weeks or more prior to collection. Additionally, segmental analysis reveals approximately how many months ago the drugs were ingested [3,4,5]. In conventional segmental hair analysis, dozens of hair strands are cut at intervals of several centimeters, and the hair segments from the same position are lumped together. Because most hypnotics, particularly benzodiazepines, can cause drowsiness even at very small doses (less than 1 mg) [6], many strands of hair must be collected from a victim’s scalp to sufficiently detect small amounts of drugs.
Generally, liquid chromatography (LC)–tandem mass spectrometry (MS/MS) with selected reaction monitoring (SRM) mode is the first choice for the detection of hypnotics. However, in investigations of DFCs, no one knows the type of drugs that the victim ingested. Target analysis using SRM may be insufficient to search for drugs unregistered under the LC–MS/MS conditions.
On the other hand, non-target analysis in LC–MS/MS usually requires a user’s library of retention time and mass spectra, which is built by analyzing many types of drug standards in LC and mass spectrometry (MS) conditions designated beforehand [7, 8]. High-resolution mass spectrometry (HR-MS), the full width at half maximum (FWHM) of which has recently been improved to over 100,000, is effective for drug screening because it enables us to search for drugs of interest without a mass spectral library and drug standards [9,10,11,12,13]. If the compositional formulae of the drugs of interest are known, we can detect specific peaks using exact masses corresponding to the drugs of interest after the HR-MS measurement. Therefore, LC–HR-MS was used for drug screening in this actual case to prevent overlooking any drugs in the victim’s hair. When some drugs are detected in a victim’s hair, it is important to elucidate the relationship between the detected drug(s) and the incident(s) which the victim remembered.
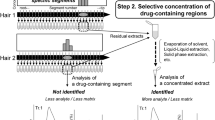
The information obtained by conventional segmental hair analysis that the victim ingested specific drugs a few months ago may be insufficient to prove DFCs. Micro-segmental hair analysis that we recently developed is a revolutionary procedure for estimating the day of drug ingestion. This method involves (1) cutting of a single hair strand into 0.4-mm segments, largely corresponding to the daily growth length [14], and (2) independent quantification of drugs in each 0.4-mm hair segment [15, 16] (Fig. 1). The method can display drug distribution in a hair strand. In order to estimate the day of drug ingestion accurately, the subject is asked to ingest over-the-counter medicines such as chlorpheniramine (CP) as an internal temporal marker (ITM) to mark a timescale within individual hair strands [17, 18]. The subject ingests the ITM twice at an interval greater than 2 weeks. Several weeks after the second ITM ingestion, three or more hair strands are donated to determine the day of drug ingestion. The positional relationship between the drug ingested unconsciously on an unknown day and the ITMs ingested intentionally on known days in a hair strand is used to determine the day of drug ingestion.
Herein, a hair analysis procedure is proposed for obtaining more powerful evidence in DFCs than using the conventional segmental hair analysis. This procedure involves drug screening by LC–HR-MS (quadrupole-Orbitrap) and highly sensitive qualitative analysis by low-resolution LC–MS/MS (tandem quadrupole) for determining the day of drug ingestion by micro-segmental analysis. In this article, a case of DFC is demonstrated in which the day of ingestion of zolpidem was successfully identified by the micro-segmental analysis.
Materials and methods
Materials
Stac® common cold medicine containing CP was purchased from SSP Co., Ltd. (Tokyo, Japan). CP-d6 maleate was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). CP maleate and the other reagents were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Acetonitrile, methanol and water were of LC/MS grade.
Hair collection to prove drug use
An Asian woman in her late teens reported to the police that, approximately 1 month earlier, she had consumed a drink offered by an acquaintance, fallen asleep, and been assaulted. To obtain evidence of drug use, 100 strands of black hair from the posterior vertex region near her scalp were cut with scissors. She stated that she had not ingested any medicine for therapeutic purposes for at least 2 months before the hair collection.
Drug screening using the conventional hair analysis
For drug screening, 20 out of the 100 hair strands were used. The hair strands were cut 10 cm long from the proximal cut end to examine drug use over the 10 months before. After cutting these 10-cm hair strands into pieces less than 2 cm long, they were placed in a 2-mL safe-lock tube and weighed. The surface of hair strands was washed by sonication in an aqueous solution of 1% sodium dodecyl sulfate for 1 min, followed by sonication alternately in water and methanol three times for 1 min each.
The method used to extract hypnotics in hair was validated previously [19,20,21]. A stainless-steel bullet was placed in the tube containing the hair strands, and an aqueous solution of 3 M ammonium phosphate (0.2 mL, pH 8.4) and acetonitrile (0.2 mL) was added. The hair was pulverized using a reciprocal shaker (Automill TK-AM5-S, Tokken, Inc., Kashiwa, Japan) at 25 Hz for 5 min. After the bullet was transferred into another tube, the bullet surface was washed with an aqueous solution of 3 M ammonium phosphate (0.1 mL, pH 8.4). The solution was then transferred to the tube containing the pulverized hair. The tube was centrifuged and the upper phase was transferred to another tube. The extract was dried under nitrogen flow, and the residue was dissolved with the initial mobile phase of the LC (0.1 mL) described below. The solution was filtered with a filtration device (Ultrafree MC, polytetrafluoroethylene, 0.2 μm; Merck KGaA, Darmstadt, Germany), and the filtrate (20 μL) was injected into the quadrupole-Orbitrap LC–MS/MS instrument described below.
Analytical conditions for drug screening
An Ultimate 3000 liquid chromatograph connected to a Q Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used. Chromatographic separation was achieved using an octadecylsilyl column (Hypersil GOLD C18, 50 × 2.1 mm i.d., particle diameter 5 μm; Thermo Fisher Scientific) at 40 °C. The mobile phase, delivered at a flow rate of 0.3 mL/min, consisted of an aqueous solution of 10 mM ammonium acetate and methanol. The proportion of methanol was changed over a linear gradient as follows: 0–2 min 10% methanol, 2–23 min 10–94% methanol, 23–26 min 94% methanol, 26–26.1 min 94–10% methanol, and 26.1–30 min 10% methanol. Electrospray ionization was performed in positive ionization mode. Spray voltage was 3 kV and capillary temperature was 350 °C. For drug screening, scan analysis with the Orbitrap analyzer was performed in a mass range of m/z 200–500 and resolving power of 140,000. To search for specific peaks in the hair extract, extracted ion chromatograms were drawn using the [M + H]+ of the drugs.
Peak identification
Forty hair strands from the specimens were used to identify the peaks detected in drug screening. The hair extract was prepared in the same manner as described above. Product ion analysis selecting the [M + H]+ of analytes as the precursor ions was performed under the same conditions as the scan analysis except for the use of a quadrupole mass filter. The positive control and negative control obtained using blank hair with or without spiking drug standards, respectively, were also analyzed.
For confirmatory analysis, the remaining specimens were treated in the same manner as for drug screening, and the extract was injected into a low-resolution but sensitive tandem quadrupole LC–MS/MS instrument (ACQUITY UPLC I-Class and Xevo TQ-S; Waters, Milford, MA, USA). The conditions are shown in supplementary material Table S1.
Micro-segmental analysis
To accurately estimate the day of ingestion of a targeted drug using the ITM, the victim was requested to participate in an experiment in which healthy subjects ingest over-the-counter medicines at normal doses and then donate their hair to be examined for drug distribution therein. The experiment had already been approved by the ethics committee at the National Research Institute of Police Science (Kashiwa, Japan). Informed consent was obtained from her for the experiment. According to our experimental protocol, the subject ingested tablets of Stac® common cold medicine containing CP maleate (2.5 mg) as the ITM twice with an interval of greater than 2 weeks. Subsequently, the subject selected one of the two hair collection methods. The first involved cutting approximately 10 hair strands near the scalp with scissors more than 4 weeks after the second ITM ingestion, whereas the second involved plucking three or more hair strands from the scalp more than 2 weeks after the second ITM ingestion. The subject selected the hair plucking method in this case. The subject had not ingested any other medicines except for Stac® common cold medicine for at least 3 months before the hair collection.
The detailed sample preparation was described in our previous reports [15,16,17,18]. Briefly, an individual hair strand was weighed and the full length was measured. The hair strand was washed as described above. The hair strand was attached straight on a double-sided tape on the stage of a tissue slicer equipped with a micrometer scale (Stoelting Co., Wood Dale, IL, USA), so that the hair growth direction was aligned at a right angle to the blade. The hair strand was cut at 0.4 mm from the end of hair root side, and the segment was placed in a 0.1-mL microtube using a tapered cotton swab. The stage was then moved by 0.4 mm to the hair root side using the micrometer scale. The procedure was repeated until a hair region expected to contain the targeted drug was segmented. A mixture of an aqueous solution of 5 mM ammonium acetate containing 0.05% formic acid (mobile phase A in Table S1) and acetonitrile (3:1, v/v) was used as the extraction solution. The extraction solution (100 μL) containing CP-d6 (4 pg/mL) as the internal standard was added to the tube containing the segment. The sample tube was sonicated at 23 kHz for 10 min and then maintained at approximately 22 °C in a dark place for 24 h. The supernatant (35 μL) was diluted with mobile phase A (35 μL). The solution (50 μL) was injected into the tandem quadrupole LC–MS/MS instrument (ACQUITY UPLC I-Class and Xevo TQ-S). The analytical conditions are summarized in Table S1.
The concentrations of analytes in each 0.4-mm hair segment were quantified. The weight of a 0.4-mm segment prepared from each hair strand was calculated by dividing the total weight of an individual hair strand by the full length and then multiplying by 0.4.
Analytical validation for micro-segmental analysis
The method was validated using spiked hair segments according to a guideline on method validation from the Scientific Working Group for Forensic Toxicology (SWGTOX) [22]. The recovery of analyte from authentic hair segments was confirmed by soaking hair segments in an extraction solution for 24 and 72 h after sonication and comparing the concentrations of analyte in these solutions, because it was impossible to compare the recovery between different extraction methods using the same authentic hair segments.
Estimation of drug ingestion day
Distribution curves of the targeted drug and the ITM were constructed by assessing the drug concentration in each 0.4-mm hair segment, numbered from the root side to the tip side. The position of peak maximum was corrected using the weighted average of the three concentrations, which were obtained from a segment containing the peak maximum (n) and the next segments on both sides (n − 1 and n + 1) on the distribution curve (Fig. S1) as follows:
where n is the segment number containing peak maximum, Cn is the concentration in segment number n, and PD is the corrected position of the peak maximum corresponding to the ingestion of a targeted drug, which represents the distance from the end of the hair root side to a targeted drug peak. The number of days from ingestion of a targeted drug to hair collection (DD) was estimated based on peak maxima corresponding to ITM ingestions using the following formula:
where DITM1 and DITM2 represent the number of days from the ingestions of the first and second ITM to hair collection, respectively, and PITM1, and PITM2 represent the corrected positions of peak maxima corresponding to the ingestions of the first and second ITM, respectively.
Results and discussion
Drug screening using LC–HR-MS
According to the statement, the victim became drowsy immediately after she consumed the drink. Because there are over 100 approved drugs that can cause drowsiness [6, 23], it was impossible to target all the drugs using a general LC–MS condition. Therefore, scan analysis with a FWHM of 140,000 using HR-MS was performed in combination with a general LC condition. After the hair extract was analyzed, extracted ion chromatograms were drawn with data analysis software using the exact masses corresponding to the [M + H]+ of various drugs. When m/z 308.175 ± 0.001, which corresponds to the [M + H]+ of zolpidem, was used, a significant peak appeared on the chromatogram (Fig. 2a).
Qualitative analysis using two types of LC–MS/MS instruments
To identify the peak detected in drug screening, the remaining specimens were divided into halves, and two analytical samples were prepared independently in the same manner as for the drug screening. The first sample was analyzed using the quadrupole-Orbitrap LC–MS/MS instrument. Fragment ions characteristic of zolpidem were observed on the product ion spectrum of the detected peak (Fig. 2b). Both the retention time of the peak and the mass spectrum were consistent with those of the zolpidem standard (Fig. 2c, d). The other sample was also analyzed using the low-resolution tandem quadrupole LC–MS/MS instrument. The presence of zolpidem was also confirmed using the ratios of peak areas from two different ion transitions in the selected reaction monitoring mode (data not shown).
Two different analytical principles were used for the peak identification based on the guideline of the SWGTOX [22]. Although quadrupole-Orbitrap LC–MS/MS is generally inferior to tandem quadrupole LC–MS/MS in terms of sensitivity, the Orbitrap analyzer with good selectivity was able to detect ions with accurate masses characteristic of a targeted compound.
Ingestion of internal temporal markers
It was judged from the results of hair analysis that the victim had ingested zolpidem. However, there was no evidence that she had ingested zolpidem when the incident occurred. Conventional segmental hair analysis at intervals of several centimeters could estimate how many months had passed since the drug was ingested, but this analytical result was insufficient evidence to prove DFCs. Therefore, micro-segmental hair analysis was expected to elucidate the relationship between the drug and the incident. Ingesting ITMs before hair collection is required to estimate the day of drug ingestion accurately using micro-segmental analysis, as we previously reported [17, 18]. After the victim became a subject for our drug administration experiment approved by the ethics committee, she ingested ITMs twice with an interval of 21.0 days. Following 13.9 days after the second ITM ingestion, she plucked out several hair strands from her scalp and donated them for our experiment. Subsequently, she voluntarily submitted several more plucked hair strands to the police to investigate the DFC.
Determination of the day of drug ingestion
The distribution curves of zolpidem and CP in a hair strand (hair no. 1) are depicted using micro-segmental analysis (Fig. 3). Zolpidem and CP are prominently detected in specific segments of the hair strand. Typical chromatograms for an extract from a 0.4-mm hair segment are shown in Fig. S2. CP was localized in two regions in the hair strand, reflecting two ingestions of CP with a 21.0-day interval. Zolpidem was localized in one region, implying that she had ingested zolpidem once.
Distribution curves of zolpidem and chlorpheniramine (CP) in a hair strand using micro-segmental analysis. The distribution curves of analytes in hair strand no. 1 are shown. PD, PITM1, and PITM2 represent the corrected positions of peak maxima corresponding to the ingestions of zolpidem, the first internal temporal marker (ITM), and the second ITM, respectively
The results of analytical validation using spiked hair segments indicated that this extraction method could satisfactorily quantify zolpidem at the concentration ranges in the actual hair segments (Table S2). Although the concentrations of zolpidem in hair soaked for 72 h were higher than those soaked for 24 h for each segment, the ratios were almost constant for any segment (Fig. S3). Therefore, the soaking time hardly affected the position of peak maximum.
The day of zolpidem ingestion was estimated using the positional relationship between the peaks of zolpidem and ITMs on the distribution curve. First, the actual growth rate of the analyzed hair strand was calculated using the distances between the two CP peaks ([PITM1 − PITM2]) and the known ingestion interval ([DITM1 − DITM2]). Second, the distance between the distal CP peak, corresponding to the first ingestion of CP, and the zolpidem peak ([PD − PITM1]) was measured. Third, the estimated number of days between zolpidem ingestion and the first ITM ingestion ([DD − DITM1]) was calculated using the actual hair growth rate and [PD − PITM1]. Afterwards, another hair strand (hair no. 2) was analyzed in the same manner as hair no. 1 (Table 1) to confirm the reproducibility of the estimated value. The numbers of days estimated between the two independent hair strands with ITMs were closely matched, although the growth rates of the two hair strands differed greatly. It was presumed that the victim had ingested zolpidem approximately 84 days before the hair strands were collected for the micro-segmental analysis. In fact, the day of the incident as reported by the victim to the best of her knowledge was 85 days before the hair collection. Therefore, the results of micro-segmental hair analysis supported her statement.
Because the victim’s hair strands were plucked out with the hair root, the number of days from zolpidem ingestion to hair collection could be approximately estimated using the distance from the hair root end to zolpidem peak (PD) and a general daily growth rate of hair (0.4 mm/day [14]). There was a difference of 25.1 days between hairs no. 1 and no. 2 in the days estimated without consideration of ITMs (PD/0.4) depending on the growth rate of individual hair strands. Additionally, when the estimated average values with and without ITMs were compared, the difference was 11.7 days (Table 1). Therefore, ITMs should be used to improve the estimation accuracy if informed consent is obtained from the victim.
Single hair analysis using matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS) can visualize the distribution of drugs in an individual hair strand [24,25,26,27,28,29]. However, it was difficult to quantify small amounts of drugs and determine the position corresponding to drug ingestion by MALDI-IMS. Kamata et al. [28] succeeded in distribution measurement for methoxyphenamine after its single administration by MALDI-IMS. Shima et al. [29] also successfully depicted a zolpidem-positive zone in hair resulting from its single 10-mg intake using MALDI-IMS. Additionally, they determined the amount of zolpidem in single hair specimens by LC–MS/MS. Although there are many reports about the detection of zolpidem in hair [4, 19, 21, 29,30,31], it was first demonstrated in the present study that micro-segmental hair analysis was effective in determining the day of zolpidem ingestion.
In micro-segmental hair analysis, the total time required to prepare and analyze 96 hair segments (a 3.84-cm hair strand) is approximately 2 days. The distributions of drugs in at least two hair strands are plotted to confirm whether the analyzed hair strands are in the anagen phase [14]. If the values estimated from the two hair strands are consistent with each other, it seems that further hair analysis is able to be omitted, because accidental coincidence rarely occurs in two hair strands selected randomly. It is better to determine when and which drug the victim ingested by one-step hair analysis (Fig. 1). However, micro-segmental analysis is not suitable for non-target analysis, because it is too difficult a task for rapid analysis with high sensitivity to quantify drugs from very small (ca. 4 μg/0.4-mm segment) and numerous (typically over 100) hair segments. Therefore, the drugs to be targeted for micro-segmental analysis are determined by drug screening using LC–HR-MS, followed by peak identification using two types of LC–MS/MS instruments (Step 1 in Fig. 1). There are advanced techniques for drug screening by LC–MS/MS such as data-dependent acquisition and multi-target analysis [8, 32]. However, these techniques may overlook some drugs co-eluted with matrix compounds and untargeted drugs. On the other hand, LC–HR-MS can search for drug peaks in data acquired previously using exact masses corresponding to the drugs of interest any time and can exclude interferences due to co-eluted matrix compounds, depending on the resolving power [9,10,11,12,13]. Drug screening by LC–HR-MS was very effective in detecting drugs in hair before micro-segmental analysis.
Conclusions
In DFCs, there is little evidence of drug use. The victim’s hair is often used as the only specimen to obtain some evidence of DFCs. Conventional segmental hair analysis can only show how many months ago a targeted drug was ingested. In comparison, our proposed procedure for hair analysis enables us to obtain stronger evidence. Drug screening by HR-MS and determination of the day of drug ingestion by micro-segmental analysis can prove that a victim ingested a specific drug on a specific day. The proposed procedure could elucidate the relationship between a drug and an incident in DFC investigations.
References
Karlonas N, Ramanavicius A, Ramanaviciene A (2014) Development of an SPE method for the determination of zaleplon and zopiclone in hemolyzed blood using fast GC with negative-ion chemical ionization MS. J Sep Sci 37:551–557. https://doi.org/10.1002/jssc.201300784
Kaartama R, Jarho P, Savolainen J, Kokki H, Lehtonen M (2011) Determination of midazolam and 1-hydroxymidazolam from plasma by gas chromatography coupled to methane negative chemical ionization mass spectrometry after sublingual administration of midazolam. J Chromatogr B 879:1668–1676. https://doi.org/10.1016/j.jchromb.2011.04.009
Johansen SS, Dahl-Sørensen R (2012) A drug rape case involving triazolam detected in hair and urine. Int J Legal Med 126:637–643. https://doi.org/10.1007/s00414-011-0654-6
Kim H, Lee S, In S, Park M, Cho S, Shin J, Lee H, Han E (2018) The correlation between concentrations of zolpidem and benzodiazepines in segmental hair samples and use patterns. Forensic Sci Int 282:13–23. https://doi.org/10.1016/j.forsciint.2017.10.044
Xiang P, Sun Q, Shen B, Chen P, Liu W, Shen M (2011) Segmental hair analysis using liquid chromatography-tandem mass spectrometry after a single dose of benzodiazepines. Forensic Sci Int 204:19–26. https://doi.org/10.1016/j.forsciint.2010.04.046
Drugs.com (2018) Triazolam dosage. https://www.drugs.com. Accessed 24 Mar 2019
Lin H-R, Liao C-C, Lin T-C (2014) Improved identification of multiple drugs of abuse and relative metabolites in urine samples using liquid chromatography/triple quadrupole mass spectrometry coupled with a library search. Rapid Commun Mass Spectrom 28:2043–2053. https://doi.org/10.1002/rcm.6997
Dresen S, Ferreirós N, Gnann H, Zimmermann R, Weinmann W (2010) Detection and identification of 700 drugs by multi-target screening with a 3200 Q TRAP LC-MS/MS system and library searching. Anal Bioanal Chem 396:2425–2434. https://doi.org/10.1007/s00216-010-3485-2
Helfer AG, Michely JA, Weber AA, Meyer MR, Maurer HH (2017) Liquid chromatography-high resolution-tandem mass spectrometry using Orbitrap technology for comprehensive screening to detect drugs and their metabolites in blood plasma. Anal Chim Acta 965:83–95. https://doi.org/10.1016/j.aca.2017.03.002
Pan M, Xiang P, Yu Z, Zhao Y, Yan H (2019) Development of a high-throughput screening analysis for 288 drugs and poisons in human blood using Orbitrap technology with gas chromatography-high resolution accurate mass spectrometry. J Chromatogr A 1587:209–226. https://doi.org/10.1016/j.chroma.2018.12.022
Fabresse N, Larabi IA, Stratton T, Mistrik R, Pfau G, Lorin de la Grandmaison G, Etting I, Grassin Delyle S, Alvarez JC (2018) Development of a sensitive untargeted liquid chromatography-high resolution mass spectrometry screening devoted to hair analysis through a shared MS2 spectra database: a step toward early detection of new psychoactive substances. Drug Test Anal https://doi.org/10.1002/dta.2535
Yanini A, Esteve-Turrillas FA, de la Guardia M, Armenta S (2018) Ion mobility spectrometry and high resolution mass-spectrometry as methodologies for rapid identification of the last generation of new psychoactive substances. J Chromatogr A 1574:91–100. https://doi.org/10.1016/j.chroma.2018.09.006
Han B, Min H, Jeon M, Kang B, Son J (2019) A rapid non-target screening method for determining prohibited substances in human urine using liquid chromatography/high-resolution tandem mass spectrometry. Drug Test Anal 11:382–391. https://doi.org/10.1002/dta.2495
Kintz P, Salomone A, Vincenti M (2015) Hair analysis in clinical and forensic toxicology. Academic Press, Cambridge
Kuwayama K, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Inoue H (2016) Three-step drug extraction from a single sub-millimeter segment of hair and nail to determine the exact day of drug intake. Anal Chim Acta 948:40–47. https://doi.org/10.1016/j.aca.2016.10.029
Kuwayama K, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Inoue H (2018) Different localizations of drugs simultaneously administered in a strand of hair by micro-segmental analysis. Drug Test Anal 10:750–760. https://doi.org/10.1002/dta.2259
Kuwayama K, Nariai M, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Abe H, Iwase H, Inoue H (2018) Accurate estimation of drug intake day by micro-segmental analysis of a strand of hair using internal temporal markers. J Appl Lab Med 3:37–47. https://doi.org/10.1373/jalm.2017.025346
Kuwayama K, Nariai M, Miyaguchi H, Iwata YT, Kanamori T, Tsujikawa K, Yamamuro T, Segawa H, Abe H, Iwase H, Inoue H (2018) Micro-segmental hair analysis for proving drug-facilitated crimes: evidence that a victim ingested a sleeping aid, diphenhydramine, on a specific day. Forensic Sci Int 288:23–28. https://doi.org/10.1016/j.forsciint.2018.04.027
Miyaguchi H, Kuwayama K (2015) Comparison of sample preparation methods for zolpidem extraction from hair. Forensic Toxicol 33:159–164. https://doi.org/10.1007/s11419-014-0256-3
Miyaguchi H, Kuwayama K (2017) Enantioselective determination of (R)-zopiclone and (S)-zopiclone (eszopiclone) in human hair by micropulverized extraction and chiral liquid chromatography/high resolution mass spectrometry. J Chromatogr A 1519:55–63. https://doi.org/10.1016/j.chroma.2017.08.058
Miyaguchi H (2013) Determination of zolpidem in human hair by micropulverized extraction based on the evaluation of relative extraction efficiency of seven psychoactive drugs from an incurred human hair specimen. J Chromatogr A 1293:28–35. https://doi.org/10.1016/j.chroma.2013.04.007
Scientific Working Group for Forensic Toxicology (2013) Scientific working group for forensic toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J Anal Toxicol 37:452–474. https://doi.org/10.1093/jat/bkt054
KEEG (2018) KEGG drug database. https://www.kegg.jp/kegg/drug/. Accessed 24 Mar 2019
Kernalléguen A, Enjalbal C, Alvarez J-C, Belgacem O, Léonetti G, Lafitte D, Pélissier-Alicot A-L (2018) Synthetic cannabinoid isomers characterization by MALDI-MS3 imaging: application to single scalp hair. Anal Chim Acta 1041:87–93. https://doi.org/10.1016/j.aca.2018.09.036
Flinders B, Beasley E, Verlaan RM, Cuypers E, Francese S, Bassindale T, Clench MR, Heeren RMA (2017) Optimization of sample preparation and instrumental parameters for the rapid analysis of drugs of abuse in hair samples by MALDI–MS/MS imaging. J Am Soc Mass Spectrom 28:2462–2468. https://doi.org/10.1007/s13361-017-1766-0
Wang H, Wang Y (2017) Matrix-assisted laser desorption/ionization mass spectrometric imaging for the rapid segmental analysis of methamphetamine in a single hair using umbelliferone as a matrix. Anal Chim Acta 975:42–51. https://doi.org/10.1016/j.aca.2017.04.012
Beasley E, Francese S, Bassindale T (2016) Detection and mapping of cannabinoids in single hair samples through rapid derivatization and matrix-assisted laser desorption ionization mass spectrometry. Anal Chem 88:10328–10334. https://doi.org/10.1021/acs.analchem.6b03551
Kamata T, Shima N, Sasaki K, Matsuta S, Takei S, Katagi M, Miki A, Zaitsu K, Nakanishi T, Sato T, Suzuki K, Tsuchihashi H (2015) Time-course mass spectrometry imaging for depicting drug incorporation into hair. Anal Chem 87:5476–5481. https://doi.org/10.1021/acs.analchem.5b00971
Shima N, Sasaki K, Kamata T, Matsuta S, Wada M, Kakehashi H, Nakano S, Kamata H, Nishioka H, Sato T, Tsuchihashi H, Miki A, Katagi M (2017) Incorporation of zolpidem into hair and its distribution after a single administration. Drug Metab Dispos 45:286–293. https://doi.org/10.1124/dmd.116.074211
Cui X, Xiang P, Zhang J, Shi Y, Shen B, Shen M (2013) Segmental hair analysis after a single dose of zolpidem: comparison with a previous study. J Anal Toxicol 37:369–375. https://doi.org/10.1093/jat/bkt035
Kintz P, Alvarez JC, Deveaux M, Dumestre V, Gaillard Y, Gaulier JM (2014) Conflicting hair testing results can have an impact in courts: interpretation of single exposure to zolpidem. J Anal Toxicol 38:304–305. https://doi.org/10.1093/jat/bku014
Hebert AS, Thöing C, Riley NM, Kwiecien NW, Shiskova E, Huguet R, Cardasis HL, Kuehn A, Eliuk S, Zabrouskov V, Westphall MS, McAlister GC, Coon JJ (2018) Improved precursor characterization for data-dependent mass spectrometry. Anal Chem 90:2333–2340. https://doi.org/10.1021/acs.analchem.7b04808
Acknowledgements
This work was supported by JSPS KAKENHI grant number 15K08894.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical approval
The victim was asked to participate in an experiment in which healthy subjects ingest over-the-counter medicines at normal doses and then donate their hair to be examined for drug distribution. The experiment was approved by the ethics committee at the National Research Institute of Police Science (Kashiwa, Japan). The victim willingly accepted the request in order to contribute to advances in criminal investigation and forensic toxicology. Informed consent was obtained to have the victim become a subject for the experiment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuwayama, K., Miyaguchi, H., Iwata, Y.T. et al. Strong evidence of drug-facilitated crimes by hair analysis using LC–MS/MS after micro-segmentation. Forensic Toxicol 37, 480–487 (2019). https://doi.org/10.1007/s11419-019-00472-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00472-3