Abstract
Purpose
The key environmentally beneficial process that substantially removes reactive nitrogen from biosphere is a complete denitrification. The science of measuring and constraining nitrous oxide (N2O) emissions has advanced significantly; however, despite several attempts, in situ dinitrogen (N2) measurement is still a great challenge and is poorly understood due to the high atmospheric N2 background. This study aimed at estimating field-scale inferred N2 emissions using data of field N2O emissions and laboratory-measured N2O/(N2O + N2) ratios and correlating those emissions with the soil-environmental factors.
Materials and methods
Closed static chamber and He/O2 direct measurement methods were used at field and laboratory scale, respectively. For each treatment (varying N fertilizer rates), on each sampling date, N2O and N2 emissions were measured at laboratory and N2O emissions at field-scale, allowing the calculation of field-scale inferred N2 emissions.
Results
The results demonstrate that field-scale inferred cumulative N2 emissions were 1.35, 1.48 and 1.60 times greater than laboratory-measured cumulative N2 emissions in low nitrogen level (LNL), medium nitrogen level (MNL) and high nitrogen level (HNL) treatments, respectively. This suggests that estimating N2 emissions at the field-scale in agricultural soil could give more insight on N cycling processes. Moreover, N fertilizer application rates increased linearly both field and laboratory cumulative N2O and N2 emissions. Both positive and negative relationships between soil-environmental parameters and N2O, N2 and their N2O/(N2O + N2) ratios at field and laboratory-based indicate their heterogeneous roles in N2O formation and reduction processes.
Conclusion
The results provide complementary insights into field-scale N2 emissions in agricultural soil and help in closing the knowledge gap in the N balance. Linear relationships between the emissions (N2O and N2) and N fertilizer rates observed suggest that climate change mitigation options could be achieved by optimizing the N fertilization rates since N2O and N2 emissions are enhanced by increasing N inputs. As our results present the field-scale inferred N2 emissions, there is still a need to design a robust methodological approach that will enable researchers to directly quantify field N2 emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gaseous nitrogen (N) losses such as dinitrogen (N2) and nitrous oxide (N2O) emitted from soil are due to the reduction of nitrogen available in soil, potentially representing substantial loss of applied N fertilizers (Zistl-Schlingmann et al. 2019; Bizimana et al. 2021). Anthropogenic activities such as combustion processes and N fertilizer production have greatly increased reactive nitrogen (Nr) in the biosphere by a factor of two (Gruber and Galloway 2008; Butterbach-Bahl and Dannenmann 2011). The increased usage of N has ensured the increase of global food supply and security (Erisman et al. 2008); however, Nr losses resulted in hazardous environmental consequences, for instance, global warming, air pollution, N leaching in agricultural soils, eutrophication in water bodies, and depletion of ozone layer in stratosphere (Davidson and Seitzinger 2006; Rockström et al. 2009; Butterbach-Bahl et al. 2013). Complete denitrification is a major environmentally beneficial pathway which converts Nr into a N2, a stable molecule compared with other gaseous N losses. Denitrification is a reduction process of nitrate (NO3−) and nitrite (NO2−) through nitric oxide (NO) and nitrous oxide (N2O) to di-nitrogen (N2) (Firestone and Davidson 1989; Wang et al. 2011), facilitating the removal of accumulated Nr in the biosphere (Davidson and Seitzinger 2006). There are high uncertainties of around the quantification of how much Nr has been converted back into N2 via denitrification due to the lack of accurate or unbiased N2 measurement techniques against high atmospheric N2 interference (Galloway et al. 2008; Butterbach-Bahl et al. 2013).
Nitrous oxide is a major long-lived (around 100 years) anthropogenic greenhouse gas with 298 times greater potential for atmospheric global warming compared to carbon dioxide and also contributes to ozone depletion (Forster et al. 2007; Saggar et al. 2007; Ming et al. 2016). Atmospheric concentration of N2O has been increasing (NoAA, ESLR 2019) mainly due to intensification of land use and N fertilizer application (Park et al. 2012; Timilsina et al. 2020a). Global N2O emissions were averaged at 15.3–17.0 Tg of N2O-N per year (Tian et al. 2020; Davidson and Kanter 2014), with an average of 7.3 Tg of N2O-N per year from anthropogenic activities (Tian et al. 2020), of which most was contributed by fertilized agricultural soils, hence a significant anthropogenic N2O source. Moreover, N2O emissions produced mainly through nitrification and denitrification processes in agricultural soils were reported to contribute more than other sources (Butterbach-Bahl et al. 2013; Davidson and Kanter 2014), although large uncertainties still remain due to spatial and temporal variations (Davidson and Kanter 2014). Previous research reports N2O emissions from different sources (Tian et al. 2020; Timilsina et al. 2020b, c), while soil N2 emissions are still poorly understood which might be due to its methodological constraints that are hindered by the high atmospheric N2 background of 78% (Groffman et al. 2006).
Factors that influence nitrification and denitrification fall into two main categories from proximal to distal drivers (Wang et al. 2020; Araujo et al. 2021). Some of the major proximal drivers include soil NO3− content, soil ammonium (NH4+) content, C substrates, soil temperature, soil water-filled pore space and oxygen availability. They influence the activity of microbial community and crop yield. Major distal drivers include mean annual temperature, mean annual precipitation, soil pH, tillage and soil texture (Groffman et al. 1988; Paul 2007, Saggar et al. 2013, Butterbach-Bahl and Dannenmann 2011; Araujo et al. 2021). However, the magnitude and direction of relationships among these drivers and the emissions of N2O, N2, and their ratios of N2O/(N2O + N2) are still not well understood.
Many previous studies estimated N2O, N2, and N2O/(N2O + N2) ratios based on the acetylene (C2H2) inhibition method, 15N gas flux method, and He/O2 direct measurement method. In theory, C2H2 can block N2O reductase enzyme so that the N2 end product of denitrification can be measured as N2O emissions (Yoshinari et al. 1977; Qin et al. 2013). However, the C2H2 inhibition technique has presented some shortcomings of not only inhibiting the N2O reduction in denitrification but it can also inhibit nitrification, hence potentially underestimating N2 and N2O emissions (Butterbach-Bahl et al. 2013; Qin et al. 2013). Furthermore, 15N gas flux method also presents its shortcomings. For example, Kulkarni et al. (2014) compared the direct method to 15N isotope labeling method in measuring denitrification from forest soil; their results emphasized that it is hard to achieve uniform distribution of labeled N in soils which consequently reported uncertain denitrification. Furthermore, Ruser et al. (2006) reported high detection limit of 43 g N ha−1 day−1 that makes this method inappropriate especially in measuring low soil N2 fluxes. To overcome uncertainties and biases that occurred in nitrification and denitrification measurement, many researches have proposed the use of helium (He)/oxygen (O2) approach direct measurement method (Butterbach-Bahl et al. 2013; Qin et al. 2017; Friedl et al. 2020; Bizimana et al. 2021) because it does not change soil properties by adding extra substrates such as 15N-labeled substrates in the 15N isotope labeling method or C2H2 in C2H2 inhibition technique. Direct method has high sensitivity for measuring N2 fluxes with low detection limit which is less than 10 µg N m−2 h−1 (Butterbach-Bahl et al. 2002; Groffman et al. 2006) while it has shortcomings as it requires a long time to replace the soil core atmosphere with N2-free atmosphere.
To achieve reliable results from the He/O2 direct measurement method, high gas tightness against atmospheric N2 contamination has been employed with N2-free atmosphere of soil intact cores in the vessels in laboratory setup experiment (e.g., Wang et al. 2020; Bizimana et al. 2021). Among the drawbacks of the He/O2 direct measurement method is that the method is limited to laboratory only that is why combining it with field-measured N2O emissions using static chambers could be used to give more insights into field-scale inferred N2 emissions. This suggests that estimating N2 emissions at the field-scale in agricultural soil could provide insight on N cycling process (Wang et al. 2020). In agricultural soil, among other factors, N fertilizer rates determine the N2O and N2 emissions at laboratory scale, while to date it is not clear how N fertilizer rates influence N2 emissions at field-scale, due to methodological limitations. Therefore, in the current study, we aimed (a) to quantify the temporal dynamics of laboratory-measured N2O, N2, and N2O/(N2O + N2) emission ratios using a direct method and field N2O emissions using static chambers under different N fertilizer rates; (b) to calculate field-scale inferred N2 emissions using field N2O emissions and laboratory-measured N2O/(N2O + N2) emission ratios; (c) to correlate field-measured N2O emissions, laboratory-measured N2O, N2, and field-scale inferred N2 emissions with the soil-environmental factors.
2 Materials and methods
2.1 Experimental site
The study field site is located at Luancheng Agro-ecosystem Experimental Station Hebei, China (37°53′N, 114°41′E and 50 m), which is in North China Plain (NCP). The site has a temperate semi-arid monsoon climate with more than 75% of precipitation occurring during the rainy season (July to September). The mean annual precipitation and temperature are 540 mm and 13 °C, respectively. The soil at this site is classified as a silt loam Haplic Cambisol. The crop system at the experimental site was continuous summer maize (Zea mays L.) and winter wheat (Triticum aestivum L.) crop rotation without a fallow period (Bizimana et al. 2021). The regional landscape is known as an alluvial flood plain of NCP in the piedmont of Taihang Mountains. At the study site, soil pH, total organic matter, and bulk density are 7.5 to 7.9, 17.1 to 22.3 (g kg−1), and 1.3 to 1.4 (g cm−3), respectively (Bizimana et al. 2021).
2.2 Experimental design and field management
At the experimental site, each plot had 70 m2 size with three plots per treatment. Urea-N was used as the N fertilizer in the cropping system. The cropping system was a winter wheat (Triticum aestivum L.) and summer maize (Zea mays L.) double-cropping system since 1998 in a complete randomized block design. Three levels of annual Urea-N application rates as 200 (low nitrogen level, LNL), 400 (medium nitrogen level, MNL), and 600 (high nitrogen level, HNL) kg N per hectare per year and control as unfertilized (UF) were designed with three replicates for each treatment. Half of the total annual N fertilizer rate was applied in the winter wheat season and the other half in summer maize season. The winter wheat N fertilizer was applied in two separate applications: one at planting in October and the other at stem elongation in early April. The summer maize N fertilizer was applied in one application, during the tasseling stage in August. Flood irrigation (ranging from 75 to 95 mm) was applied at the experimental field a few days after both basal and supplementation N fertilization for winter wheat. For summer maize, flood irrigation was also applied in June a few days after planting, and no irrigation was applied after N fertilization in August as there was enough rain. During basal fertilization, double superphosphate and potassium chloride were applied at 65 and 75 kg per hectare per year, respectively, to all treatments as additional mineral macronutrients. Soil rotary tillage (15 cm depth) was carried out annually after summer maize was harvested and before winter wheat was planted, returning the straw to the soil after being processed into small pieces. The current study was carried out since 14 April 2020 and ended on 13 March 2021 in the field and laboratory. The two different datasets used in this study were collected from the same treatments on the same sampling dates.
2.3 Field N2O flux measurement
The field investigation was conducted for 1 year period. The closed rectangular static chambers of 60 cm × 20 cm × 40 cm (L × W × H) were used for sampling N2O concentration. Three replicated chambers for each treatment were deployed, and gas samples were collected to measure N2O fluxes at the interval of 0, 20, 40, and 60 min. The static chambers were equipped with a sampling tube with a three-way stopcock, a thermometer, and a fan to ensure air circulation inside the chambers to avoid heating effect during gas sampling. Both sampling tube and thermometer were well sealed where they passed through the surface of the static chamber to prevent leakage. The chambers were fixed in between rows of crops to avoid crops growing in chambers, and they were fitted to the corresponding basal collars that were permanently left in their respective places between crop rows. After gastight closing each chamber, 40-ml gas samples were collected from its headspace using polypropylene syringes and pre-evacuated gas bags for N2O concentration measurement immediately after returning back to the laboratory. Between 8:00 a.m. and 11:00 a.m., all field N2O gas samples were extracted because N2O flux within this period approximates average daily fluxes (Reeves and Wang 2015), and therefore, this minimizes diurnal variation in the flux rates (Araujo et al. 2021). Gas chromatography (Agilent GC-6820, Agilent Technologies Inc., Santa Clara, CA, USA) equipped with electron capture detection (ECD) was used to measure N2O concentration. The calculation of N2O concentrations was based on the measurement of gas sample peak areas relative to reference standard peak areas that was run two times after every twelve gas samples. Coefficient of determination (R2) between time and headspace N2O concentration was used to decide whether N2O flux has to be rejected or considered. It has to be considered only when R2 is above 80%, and consequently, all sampling campaigns were considered in our current study.
N2O fluxes were calculated using the formula from (Timilsina et al. 2020a)
where F is the N2O flux (μg N2O-N m−2 h−1), M is the molecular weight of N2O-N, V is the chamber volume (m3), A is the surface area of soil occupied by the chamber base (m2), ΔC × Δt−1 is the rate of N2O accumulation in the chamber (ppbv N2O h−1), T is the air temperature (°C) inside the chamber, P is the atmospheric pressure (hPa), and P0 is standard atmospheric pressure.
2.4 Laboratory measurement of N2O and N2 fluxes using direct method
The laboratory investigation was conducted for 1 year period. Direct measurement technique (He/O2 direct measurement method) was used to quantify trace N2O and N2 emissions by replacing intact soil core atmosphere with He/O2 in the double-layer cylinders. Gas samples were taken from the headspace of each inner cylinder by a robotic auto-sampler linking to gas chromatography. Three intact soil cores were randomly collected for each treatment, for laboratory-scale N2O and N2 flux measurement; on the same dates, gas samples were collected in field for measuring N2O fluxes. After soil sample collection, intact soil cores (10-cm depth and 19-cm diameter) were sealed well in double-layer cylinders (cylinder-in-cylinder setup) to avoid atmospheric N2 contamination while the soil core atmosphere was substituted by He/O2. The headspaces of both inner and outer cylinders were evacuated and filled with an artificial He (79%) and O2 (21%) 5 times in the laboratory to ensure a complete removal of ambient N2 so that direct trace N2O and N2 emissions from soil cores could be measured. More details about this method were described in previous studies (Qin et al. 2017; Bizimana et al. 2021). Briefly, the field conditions were simulated by placing double-layer cylinders outdoors after the first measurement of N2O and N2 emissions and wait for another measurement turn so that the double-layer cylinders could be brought back to laboratory again. For measuring N2O and N2, headspace concentrations were automatically sampled by a robotic sampler and sent to gas chromatography (GC, Agilent 7890A) which is equipped with electron capture detector (ECD) and a thermal conductivity detector (TCD), respectively. The fluxes of N2O and N2 were calculated based on our previous studies (Qin et al. 2017; Bizimana et al. 2021). Annual cumulative N2O or N2 emissions were calculated using linear interpolation while considering fluxes of three replicates during sampled days and the interval between sampling days.
2.5 Calculation of field-scale inferred N2 emissions
After all laboratory and field based data were ready, field-scale inferred N2 emissions were calculated using field N2O and laboratory-measured N2O/(N2O + N2) ratios, using the following formula (Wang et al. 2020) on the basis of individual measurements:
2.6 Measurement of soil parameters
On each gas sampling day, fresh soil from each treatment was sampled to measure soil variables. The soil was sampled from 10 cm depth using an auger soil cutter for all sampling occasions. The collected soil cores were kept in plastic bags and then transported to laboratory to be stored under 4 °C before soil variable analyses. Daily air temperature, soil temperature at 5 cm depth and precipitation records during our observational period were collected from the site experimental weather station. The soil moisture content (SWC) was determined using oven-drying (thermo-gravimetric) method at 105 °C for 24 h, and thereafter, the measured soil moisture was converted to water-filled pore space % (WFPS %):
Soil WFPS (%) was calculated from bulk density and gravimetric soil moisture content (%) using the default soil particle density of 2.65 g cm−3 based on the following equation:
where SWC is the soil moisture content (g g−1), BD is the soil bulk density (g cm−3), and PD is the soil particle density (g cm−3).
All visible particles such as crop roots and earthworms were removed from the soil samples; then, NO3− and NH4+ were extracted by shaking for 1 h a mixture of 10 g of the soil cores with 50 ml of 1 M KCl. Then, the soil extracts were filtered through Whatman 42 filter paper, and NO3− and NH4+ concentrations were measured using a UV spectrophotometer and Smartchem 140 automatic analyzer, respectively (Timilsina et al. 2020a). Soil dissolved organic carbon (DOC) was extracted by mixing 10 g of fresh soil cores with 50 ml deionized water. Centrifugation of the soil extracts for 10 min at 8000 rpm was done, and the mixture was finally filtered and determined by Elementar analyzer (Bizimana et al. 2021).
2.7 Statistical analysis
One-way ANOVA with Turkey’s test was used to compare cumulative N2O and N2 emissions among treatments. The difference was considered significant when P < 0.05. Pearson’s correlation coefficient was performed to check the dynamical effects of the soil parameters (NO3−, NH4+, WFPS, DOC and soil temperature) with the reported field- and laboratory-based N2O and N2 emissions and laboratory-measured N2O/(N2O + N2) ratios. All figures and statistical tests were done using Origin Pro8 software and R package.
3 Results
3.1 Soil-environmental parameters
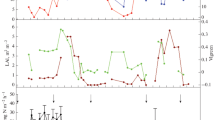
Mean daily precipitation, air temperature, and soil temperature at 5 cm depth showed a clear consistent pattern trend, higher in summer and lower in winter (Fig. S1a, b). Soil temperatures at 5-cm-depth throughout the study period ranged from − 3.2 to 33.6 °C (14.6 °C of mean) while the average air temperature was 14.4 °C (ranging from − 6.0 to 31.1 °C) (Fig. S1a, b). In the current study, annual precipitation was 421.2 mm while the highest daily rainfall (52.6 mm) was recorded in August 2020. The soil moisture ranged from 44.6 to 75.5% WFPS throughout all treatments (Fig. 1c). Soil exchangeable NO3−, NH4+, and DOC were in the range of 12.8 to 82.0 mg N kg−, 0.1 to 2.9 mg N kg−1, and 31.8 to 65.9 mg C kg−1 dried soil throughout all treatments, respectively (Fig. 1a, b, d). Highest soil mineral N concentrations were observed after N fertilization in August and October, 2020.
Soil parameters of top 10 cm taken during gas samplings include a NO3−-N and b NH4+-N (mg N kg−1 ds), c soil moisture in WFPS (%), and d DOC (mg C kg−1 ds) at the study site. Solid and dashed arrows denote N fertilizer application and flood irrigation events, respectively. Each data point represents arithmetic mean and standard error of three replicates
3.2 Laboratory-measured fluxes of N2O, N2, their ratios of N2O/(N2O + N2), and annual cumulative emissions
Laboratory-measured temporal N2O fluxes ranged from 0.31 ± 0.03 to 44.93 ± 4.82 g N ha−1 day−1 in all treatments while the average values were 1.32 ± 0.05, 6.7 ± 0.49, 10.87 ± 0.62, and 13.49 ± 0.4 g N ha−1 day−1 from UF, LNL, MNL, and HNL treatments, respectively (Fig. 2b). Throughout the study period, the peaks of N2O fluxes were observed after N fertilization in August and October, 2020. Average values of laboratory temporal N2 fluxes were 25.73 ± 0.21, 56.84 ± 1.1, 69.37 ± 2.99, and 77.82 ± 2.33 g N ha−1 day−1 from UF, LNL, MNL, and HNL treatments, respectively (Fig. 2c) with minimum and maximum values of 12.08 ± 0.53 to 139.03 ± 9.93 N ha−1 day−1, respectively, for all treatments. The N2 emissions peaked a few days after N fertilization in August and October, 2020. The laboratory ratios of N2O/(N2O + N2) were highest in HNL and lowest in UF treatments in the current study (Fig. 2d) while the average values were 0.055, 0.102, 0.119, and 0.129 from UF, LNL, MNL, and HNL treatments, respectively, with minimum and maximum of 0.03 to 0.255, respectively, for all treatments.
The laboratory-measured cumulative N2O emissions increased linearly with increasing N fertilizer rates, and the average values were 0.45 ± 0.01, 2.30 ± 0.14, 3.92 ± 0.23, and 4.88 ± 0.20 kg N ha−1 year−1 in UF, LNL, MNL, and HNL treatments, respectively, and they were statistically different among all treatments (Fig. 3b). The average values of laboratory-measured cumulative N2 emissions were 9.11 ± 0.06, 20.36 ± 0.58, 24.51 ± 1.04, and 27.60 ± 0.88 kg N ha−1 year−1 in UF, LNL, MNL, and HNL treatments, respectively (Fig. 3c). They were statistically significant among treatments except for MNL and HNL treatments that were similar. The trend of N2 emissions increases linearly with increasing N fertilizer rates, as for N2O emissions.
3.3 Field-measured N2O fluxes, field-scale inferred N2, and their annual cumulative emissions
Field-measured N2O fluxes responded to N fertilizer rates in increasing order among treatments in conducive soil environmental conditions. The temporal average values were 2.03 ± 0.34, 8.15 ± 1.54, 11.55 ± 1.69, and 15.21 ± 2.02 g N ha−1 day−1 from UF, LNL, MNL, and HNL treatments, respectively (Fig. 2a). In this current study, two peaks were observed for the temporal field N2O fluxes, and both occurred after N fertilization in August 2020 and October 2020. Minimum and maximum field N2O emissions were 0.26 to 50.23 g N ha−1 day−1, respectively, for all treatments.
Differences in the field-measured cumulative N2O emissions were statistically significant among all treatments with a linear increase with increasing N fertilizer rates, and the average values were 0.64 ± 0.04, 3.16 ± 0.07, 4.42 ± 0.37, and 5.72 ± 0.22 kg N ha−1 year−1 in UF, LNL, MNL, and HNL treatments, respectively (Fig. 3a). Field-measured cumulative N2O emissions were 1.37, 1.12, and 1.17 times greater in LNL, MNL, and HNL treatments, respectively, compared to laboratory-measured cumulative N2O emissions.
Average values of field-scale inferred temporal N2 emissions were 43.27 ± 12.81, 72.16 ± 25.20, 97.24 ± 29.55, and 119.06 ± 36.44 g N ha−1 day−1 from UF, LNL, MNL, and HNL treatments, respectively. The minimum and maximum were 8.07 and 358.06 g N ha−1 day−1, respectively, for all treatments. Moreover, field-scale inferred cumulative N2 emissions were 14.64 ± 1.61, 27.58 ± 2.34, 36.39 ± 2.46, and 44. 42 ± 3.76 kg N ha−1 year−1 in UF, LNL, MNL, and HNL treatments, respectively (Fig. 4a, b). Field-scale inferred cumulative N2 emissions were 1.35, 1.48, and 1.60 times greater in LNL, MNL, and HNL treatments, respectively, compared to laboratory-measured cumulative N2 emissions. The influence of N fertilization rates was also observed in field-scale inferred N2 emissions identifying highest in HNL fertilized and lowest in unfertilized treatments.
3.4 Relationships between soil-environmental parameters and fluxes of N2O and N2 measured based on field and laboratory methods
Laboratory-measured N2O fluxes were positively correlated with NH4+ and WFPS in all fertilized treatments, and with NO3− in MNL only, while DOC and soil temperature showed no correlation with N2O fluxes in all treatments. Laboratory-measured N2 fluxes were positively correlated with WFPS and soil temperature in all treatments, with NO3− in LNL and MNL only, with NH4+ in all fertilizer treatments, while DOC showed no correlation with N2 fluxes in all treatments. Interestingly, laboratory-measured ratios of N2O/(N2O + N2) showed positive and negative relationships between NH4+ in LNL and MNL and soil temperature in UF, respectively (Fig. 5a). Furthermore, the field-measured N2O fluxes were positively correlated with NH4+, NO3−, WFPS, soil temperature, and DOC in all fertilized treatments except in HNL for DOC. Field-scale inferred N2 fluxes were positively correlated with soil temperature, NH4+, and NO3− in all fertilizer treatments, and with WFPS in UF and LNL only, while DOC showed no correlation with the field-measured N2O fluxes in all treatments (Fig. 5b).
Pearson’s partial correlation coefficients between soil parameters and fluxes of N2O, N2 and N2O/(N2O + N2) ratios measured based on direct laboratory method a; Pearson’s partial correlation coefficients between soil parameters and field-measured N2O fluxes and field-scale inferred N2 emissions b at *P < 0.05, **P < 0.01, ***P < 0.001 level of significance. Colors of red, green, blue, and maroon denote UF, LNL, MNL, and HNL treatments, respectively
3.5 Relationships between field and laboratory-measured fluxes of N2O and N2 in response to N fertilizer rates
N fertilizer application rates increased cumulative field-measured N2O emissions by 4.87, 6.81, and 8.82 times while cumulative laboratory-measured N2O emissions increased by 5.10, 8.66, and 10.79 times greater than UF in LNL, MNL, and HNL treatment, respectively. Moreover, temporal patterns of field-measured and laboratory-measured N2O emissions showed a positive linear relationship at P < 0.001 (Fig. 6a), highlighting that both methods used to estimate the temporal patterns of N2O emissions can respond to the conducive soil environment and N fertilizer rates.
N fertilizer application rates increased cumulative field-scale inferred N2 emissions by 1.88, 2.48, and 3.03 times while laboratory-measured N2 emissions increased by 2.23, 2.69, and 3.02 times greater than UF in LNL, MNL, and HNL treatment, respectively. Furthermore, temporal patterns of field-scale inferred N2 emissions were also positively related to laboratory-measured N2 emissions at P < 0.001 (Fig. 6b) highlighting that both were influenced by N fertilization rates.
4 Discussion
4.1 Relationships between laboratory-measured fluxes of N2O, N2, their ratios of N2O/(N2O + N2), and soil-environmental parameters
Laboratory-measured cumulative N2O and N2 emissions ranged from 0.4 to 4.8 and 9.1 to 27.6 kg N ha−1 year−1, respectively, for all treatments (Fig. 3b, c). These results are consistent with the range reported in literature. For example, Chen et al. (2019) found after applying N fertilizer to agricultural soil at 0 to 600 kg N ha−1 year−1 to maize and wheat crop rotation, and using the He/O2 approach, cumulative emissions of N2O and N2 were 0.4 to 2.2 and 8.3 to 24.6 kg N ha−1 year−1, respectively, while cumulative emissions of N2 and N2O were 7.2 and 2.4 kg N ha−1, respectively, after applying 260 kg N ha−1 during summer maize season (Wang et al. 2020). In grassland ecosystem, Zistl-Schlingmann et al. (2019) reported that N2 emissions were 16 to 21 kg N ha−1 accounting for 31 to 42% of the applied N fertilizer from slurry in southern Germany while N2O emissions were only 0.2 to 0.5 kg N ha−1; the study concluded that N2 emissions are overlooked as a key N loss component in grassland ecosystem. Stoichiometric ratios of N2O/(N2O + N2) ranged from 0.03 to 0.255, indicating that N2 emissions were dominant at the study site (Fig. 2d). Similar observations have been previously reported (Chen et al. 2019; Wang et al. 2020; Bizimana et al. 2021). In the current study, temporal N2O and N2 emissions were in a range of 0.31 to 44.93 and 12.08 to 139.03 g N ha−1per day−1, respectively (Fig. 2b, c), and the present findings are supported by previous literature (Werner et al. 2014; Chen et al. 2019; Wang et al. 2020; Bizimana et al. 2021).
Soil WFPS, soil temperature, DOC, NH4+, and NO3− have been previously reported as soil environmental variables that influence N2O and N2 emissions (Timilsina et al. 2020a; Wang et al. 2020; Bizimana et al. 2021; Senbayram et al. 2021). In the present study, positive, negative, or no relationships with the studied soil environmental variables have been found. Soil NH4+ and NO3− are well recognized as the substrates for both nitrification and denitrification in agricultural soils. N2O emissions were more intensively studied compared to N2, suggesting the need for more field- and laboratory-based studies in different ecosystems to elucidate the missing relevant N2 data for model development in agricultural soil. Previous studies have reported positive relationships between N2O emissions and NH4+ and NO3− substrates (Qin et al. 2012; Timilsina et al. 2020a). These findings are consistent to our current study, when examining individual treatments, NO3− and NH4+ (Fig. 1a, b) were found to enhance the fluxes of N2O. Concerning N2 fluxes reported in previous studies, only NO3− level was correlated to the N2 fluxes (Wang et al. 2013; Senbayram et al. 2021). However, in this study, positive relationships between N2 emissions and both NO3− and NH4+ were observed, indicating that N2 emissions were mainly from the denitrification process while the relationship between NH4+ and N2 emissions could be due to NH4+ available to be converted to NO3− then be completely denitrified.
Temperature is commonly known as a main factor regulating various biogeochemical processes. Previous studies reported positive and negative relationships between temperature and N2O and N2 emissions (Chen et al. 2019; Wang et al. 2020). In the present study, temperature is positively correlated to N2 emissions in all treatments (Fig. 5a), emphasizing that soil and air temperature are recognized to mainly affect microbial and enzymatic activities by favoring the N2 production pathway. The microbial activities for nitrification and denitrification are low when soil temperature is below 10 °C (Smith et al. 2010 and Hu et al. 2013). However, no relationship was observed between N2O emissions and temperature, and the reason is not known. A similar observation has been reported in previous literature (Chen et al. 2019).
Positive relationships were observed between N2 emissions and WFPS in all treatments (Fig. 5a), indicating that soil moisture plays a critical role in the N cycling processes. Creating anaerobic conditions in soil due to high WFPS led to suitable conditions for denitrification process through oxygen depletion (Hu et al. 2013; Butterbach-Bahl et al. 2013). Furthermore, nitrification is reported to occur when soil moisture is in the range of 20 to 60% WFPS (Peng et al. 2018) while denitrification is more favored at 60%WFPS and above (Paul 2007; Butterbach-Bahl et al. 2013). But, the range can be varied due to the differences of soil properties in various soil types. In the current study, average WFPS was in the range of 59.2 to 65.2% which favored the denitrification. Surprisingly, no correlation was observed between DOC (Fig. 5a) and either N2O or N2 emissions, indicating that availability of DOC in soil would not be a limiting factor as it was optimum at the study site. A similar observation was made in previous literature (Chen et al. 2019).
4.2 Factors affecting field-measured N2O and field-scale inferred N2 emissions
Field-measured annual cumulative N2O emissions ranged from 0.6 to 5.7 kg N ha−1 year−1, for all treatments (Fig. 3a). Previous studies reported similar N2O emissions from agricultural soil (Qin et al. 2012; Huang et al. 2017; Song et al. 2018; Wang et al. 2020). Nitrous oxide emissions increased linearly by increasing N fertilization rates in the current study; this may be due to the fact that N fertilization has mainly enhanced the emissions than other soil variables. Similar observations have been reported in previous literature (Qin et al. 2012; Huang et al. 2017). A conceptual model highlighting relationships between N fertilizer rates and direct N2O emissions was previously proposed (Kim et al. 2013). Three stages of the model were suggested. One suggested a linear relationship with direct N2O emissions before crops exceed N fertilizer level demand. The second suggested an exponential relationship with direct N2O emissions when crops exceed N fertilizer level demand. The third suggested that there could be stable state when SOC is limited. Nevertheless, when the emissions increased either linearly or exponentially, the best solution to substantially reduce N2O emissions is by optimizing the N fertilizer application rates (Huang et al. 2017; Qin et al. 2012; Bizimana et al. 2021). Furthermore, temporal N2O emissions ranged from 0.26 to 50.23 g N ha−1 day−1 for all treatments, indicating the contribution of N fertilization rates in agricultural soil (Fig. 2a). Similar ranges have been previously reported in literature (Qin et al. 2012; Huang et al. 2017; Song et al. 2018).
In the current study, field-measured N2O emissions correlated positively with NO3− and NH4+ (Fig. 5b), indicating that the source of the observed emissions was mainly from denitrification and possibly little from nitrification, and a growing body of literature confirmed the observation (Baggs et al. 2008; Baily et al. 2012; Timilsina et al. 2020a; Bizimana et al. 2021). Furthermore, it could be possible that less N2O emissions were contributed from nitrification due to the fact that there was low NH4+ concentration in the experimental soil.
Positive statistical relationships between field-measured N2O emissions and WFPS, temperature, and DOC (Fig. 5b) show that production and reduction of N2O could happen at the study site based on the fact that all essential conducive conditions were available for denitrification process. Temperature is an essential factor to accelerate microbial activities especially at above 10 °C (Hu et al. 2013), and it is evidential in our current study. Furthermore, WFPS (Fig. 1c) may possibly create anaerobic conditions for the denitrification process as previously reported (Butterbach-Bahl et al. 2013; Bizimana et al. 2021; Timilsina et al. 2022), and this is consistent with our present study. DOC (Fig. 1d) indicates that there is readily available carbon to be used by microorganisms in the soil for denitrification.
In the current study, the temporal mean values of field-scale inferred N2 emissions range from 43.27 to 119.06 g N ha−1 day−1 with minimum and maximum of 8.07 to 358.06 g N ha−1 day−1 (Fig. 4a). This suggests that estimating N2 emissions at the field-scale in agricultural soil could give more insight on N cycling processes. Moreover, field-scale inferred annual cumulative N2 emissions ranged from 14.64 ± 1.61 to 44. 42 ± 3.76 kg N ha−1 year−1 (Fig. 4b), indicating that even at field-scale, N2 emissions are dominant N losses. These results are supported by Wang et al. (2020), a study using a similar method, who reported that maize seasonal cumulative field-scale N2 emissions ranged from 2.7 to 9.6 kg N per hectare, which was higher compared to results from previously reported laboratory studies. Soil WFPS, temperature, NO3−, and NH4+ (Fig. 1a, b, c) positively correlated with field-scale inferred N2 emissions while no relationship was found with DOC (Fig. 5b); this might be due to the fact that readily available carbon was sufficient in soil and was not a limiting factor. Positive relationships between the N2 emissions and soil variables (WFPS, temperature and NO3−) are in line with findings in previous literature (Chen et al. 2019; Bizimana et al. 2021).
4.3 Relationship between field and laboratory-based measurement methods for N2O and N2 emissions in response to N fertilizer rates
At field-scale, closed static chambers were used to estimate N2O emissions; this method has been widely used in previous literature (Jury et al. 1982; Collier et al. 2014; Huang et al. 2017; Timilsina et al. 2020a), while the direct method to estimate gaseous N losses (N2O and N2) has been also previously used at laboratory (Butterbach-Bahl et al. 2002; Chen et al. 2019; Wang et al. 2020; Bizimana et al. 2021). The direct method is well known to accurately quantify these trace gases of N2O and N2 by minimizing the existing biases (Friedl et al. 2020; Wang et al. 2020) because currently, there is no in situ robust method to quantify N2 emissions due to its high atmospheric background that mostly causes contamination through the leakage (Groffman et al. 2006). In the current study, N fertilizer application rates increased cumulative field-based N2O emissions by 4.87, 6.81, and 8.82 times while cumulative laboratory-measured N2O emissions increased by 5.10, 8.66, and 10.79 times greater than UF in LNL, MNL, and HNL treatment, respectively. Moreover, field-measured cumulative N2O emissions were 1.37, 1.12, and 1.17 times greater than laboratory-measured cumulative N2O emissions in LNL, MNL, and HNL treatments, respectively. The influence of N fertilizer rates in both reported methods has been confirmed by the significant positive linear relationship that has been observed between temporal N2O emissions estimated using static chambers at field and N2O emissions obtained using direct method at laboratory (Fig. 6a). A recent meta-analysis reported a 76% increase of potential denitrification activity from long-term N fertilization as compared to non-fertilized control in cropland (Li et al. 2022).
N fertilizer application rates increased cumulative field-scale inferred and laboratory-measured N2 emissions by 1.88, 2.48, and 3.03 and 2.23, 2.69, and 3.02 times greater than UF in LNL, MNL, and HNL treatment, respectively. Furthermore, field-scale inferred cumulative N2 emissions were 1.35, 1.48, and 1.60 times higher than laboratory-measured cumulative N2 emissions in LNL, MNL, and HNL treatments, respectively. The higher N2O and N2 emissions observed in the field could be due to the fact that in the field conditions, the study considered the whole soil profile while in the laboratory, it represented 0–10 cm. This view is supported by the previous findings, that a significant amount of N2O and N2 is produced even in soil profile below 0–10 cm (Wang et al. 2014; Chen et al. 2018). A significant positive linear relationship between temporal field-scale inferred N2 emissions and laboratory-measured N2 emissions (Fig. 6b) suggests that estimating N2 emissions at the field-scale in agricultural soil could give more insight on N cycling processes. The key factor responsible for high agricultural N losses such as N2O and N2 is the lack of harmonization between crop N demand and soil N supply, with an average of around 50% of the applied N to soils not being taken up by crops (Snyder et al. 2009). In the current study, annual percentage cumulative field and laboratory-measured N2O emissions of annual N inputs applied were 1.58%, 1.10%, and 0.95% and 1.15%, 0.98%, and 0.81% in LNL, MNL, and HNL treatments, respectively, while annual percentage cumulative field-scale inferred and laboratory-measured N2 emissions of annual N inputs applied were 13.79%, 9.09%, and 7.40% and 10.18%, 6.12%, and 4.60% in LNL, MNL, and HNL treatments, respectively. Therefore, there is a need to reduce N losses (N2O and N2) by improving N use efficiency in agriculture since there are both trade-offs and synergies involved in managing the N cycle. Lowering N losses such as N2O and N2 emissions will require good N fertilizer management to increase the fraction of fertilizer taken up by crops, timing the fertilization, and optimizing the amount of N input by only supplying what the crops need (Bizimana et al. 2021). Moreover, in combination with direct He/O2 method, the indirect method (isotopic mapping approach) could give overview of a large field-scale N2O reduction to N2 emissions during denitrification in specific soil (Wu et al. 2019), thus enhancing knowledge on N2O and N2 emissions from agro-ecosystem.
Previous literature reported the inhibitory effect of N2O reduction to N2 due to high NO3− concentrations (over 40–50 mg NO3−-N kg−1 dry soil) mainly because NO3− is preferred as terminal electron acceptor than N2O (Firestone 1982; Senbayram et al. 2012). However, our current results clearly show that NO3− was not favorably utilized by denitrifiers over N2O as a terminal electron acceptor, hence resulting in high N2 emissions and low N2O/(N2O + N2) ratios throughout the study period. This may be because of the intensively irrigated soil, available carbon (as shown by high DOC) due to straw return, and even more abundance and increased activity of recently identified clade II nosZ possessing denitrifiers for N2O reductase enzymes. These Clade II nosZ possessing denitrifiers were reported to potentially consume N2O in soils and emit more N2 emissions (Hallin et al. 2018; Senbayram et al. 2021).
5 Conclusion
A closed static chamber technique was used in field to quantify N2O emissions, and direct measurement method in laboratory was used to estimate N2O and N2 emissions in order to jointly calculate field-scale inferred N2 emissions. The results demonstrate that field-scale inferred cumulative N2 emissions were 1.35, 1.48, and 1.60 times greater than laboratory-measured cumulative N2 emissions in LNL, MNL, and HNL treatments, respectively. This suggests that estimating N2 emissions at the field-scale in agricultural soil could give more insight on N cycling processes. Moreover, N fertilizer application rates increased linearly both field and laboratory cumulative N2O and N2 emissions. As N2O emissions contribute to global warming and N2 emissions decrease N use efficiency in agricultural soil, optimum N fertilization rates should be used according to plant needs so that climate change mitigation options could be achieved since both N2O and N2 emissions are increased by increasing N inputs. Both positive and negative relationships between soil-environmental parameters and N2O, N2 and their N2O/(N2O + N2) ratios at field and in laboratory-based indicate their important roles in N2O formation and reduction processes. Overall, to better understand the N cycling processes, there is still a need to design a robust methodological approach that will enable researchers to directly quantify field N2 emissions from agricultural soil.
References
Araujo PI, Piñeiro-Guerra JM, Yahdjian L, Acreche MM, Alvarez C, Alvarez CR, Piñeiro G (2021) Drivers of N2O Emissions from natural forests and grasslands differ in space and time. Ecosystems 24(2)
Baggs EM (2008) A review of stable isotope techniques for N2O source partitioning in soils: recent progress, remaining challenges and future considerations. Rapid Commun Mass Sp 22(11):1664–1672
Baily A, Watson CJ, Laughlin R, Matthews D, McGeough K, Jordan P (2012) Use of the 15N gas flux method to measure the source and level of N2O and N2 emissions from grazed grassland. Nutr Cycl Agroecosys 94(2–3):287–298
Bizimana F, Timilsina A, Dong W, Uwamungu JY, Li X, Wang Y, Hu C (2021) Effects of long-term nitrogen fertilization on N2O, N2 and their yield-scaled emissions in a temperate semi-arid agro-ecosystem. J Soils Sediments 21(4):1659–1671
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Env Sust 3(5):389–395
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos T Roy Soc B 368(1621):20130122
Butterbach-Bahl K, Willibald G, Papen H (2002) Soil core method for direct simultaneous determination of N2 and N2O emissions from forest soils. Plant Soil 240(1):105–116
Chen S, Wang F, Zhang Y, Qin S, Wei S, Wang S, Liu B (2018) Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ Microbiol 20(3):980–992
Chen T, Oenema O, Li J, Misselbrook T, Dong W, Qin S, Hu C (2019) Seasonal variations in N2 and N2O emissions from a wheat–maize cropping system. Biol Fert Soil 1–13
Collier SM, Ruark MD, Oates LG, Jokela WE, Dell CJ (2014) Measurement of greenhouse gas flux from agricultural soils using static chambers. J Vis Exp JoVE (90)
Davidson EA, Kanter D (2014) Inventories and scenarios of nitrous oxide emissions
Davidson EA, Seitzinger S (2006) The enigma of progress in denitrification research. Ecol Appl 16(6):2057–2063
Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W (2008) How a century of ammonia synthesis changed the world. Nat Geosci 1(10):636
Firestone MK, Davidson EA (1989) Microbiological basis of NO and NO production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, New York, 7–212
Firestone MK (1982) Biological Denitrification Nitrogen in Agricultural Soils 22:289–326
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Nganga J (2007) Changes in atmospheric constituents and in radiative forcing. Chapter 2. In Climate Change2007. Phys Sci Basis
Friedl J, Cardenas LM, Clough TJ, Dannenmann M, Hu C, Scheer C (2020) Measuring denitrification and the N2O/(N2O+N2) emission ratio from terrestrial soils. Curr Opin Env Sust 47:61–71
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892
Groffman PM, Altabet MA, Böhlke JK, Butterbach-Bahl K, David MB, Firestone MK, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16(6):2091–2122
Groffman PM, Tiedje JM, Robertson GP, Christensen S (1988) Denitrification at different temporal and geographical scales: proximal and distal controls. Adv Nitrogen Cycl Agric Ecosyst 174–192
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451(7176):293–296
Hallin S, Philippot L, Löffler FE, Sanford RA, Jones CM (2018) Genomics and ecology of novel N2O-reducing microorganisms. Trends Microbiol 26(1):43–55
Hu XK, Su F, Ju XT, Gao B, Oenema O, Christie P, Zhang FS (2013) Greenhouse gas emissions from a wheat–maize double cropping system with different nitrogen fertilization regimes. Environ Pollut 176:198–207
Huang T, Yang H, Huang C, Ju X (2017) Effect of fertilizer N rates and straw management on yield-scaled nitrous oxide emissions in a maize-wheat double cropping system. Field Crops Res 204:1–11
Jury WA, Letey J, Collins T (1982) Analysis of chamber methods used for measuring nitrous oxide production in the field. Soil Sci Soc Am J 46(2):250–256
Kim DG, Hernandez-Ramirez G, Giltrap D (2013) Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: a meta-analysis. Agr Ecosyst Environ 168:53–65
Kulkarni MV, Burgin AJ, Groffman PM, Yavitt JB (2014) Direct flux and 15N tracer methods for measuring denitrification in forest soils. Biogeochemistry 117(2–3):359–373
Li L, Yang M, Li J, Roland B, Du Z, Wu D (2022) Potential denitrification activity response to long-term nitrogen fertilization-a global meta-analysis. J Clean Prod 130451
Ming T, De_richter R, Shen S, Caillol S (2016) Fighting global warming by greenhouse gas removal: destroying atmospheric nitrous oxide thanks to synergies between two breakthrough technologies. Environ Sci Pollut Res 23(7):6119–6138
NoAA, ESLR. https://www.esrl.noaa.gov/gmd/hats/insitu/cats/conc.php?site=brw&gas=n2o. Accessed 1 Jun 2019
Park S, Croteau P, Boering KA, Etheridge DM, Ferretti D, Fraser PJ, Trudinger CM (2012) Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat Geosci 5(4):261–265
Paul EA (2007) Soil microbiology, ecology, and biochemistry in perspective. In Soil Microbiology, Ecology and Biochemistry. Academic Press, pp 3–24
Peng Y, Wang G, Li F, Zhou G, Yang G, Fang K, Yang Y (2018) Soil temperature dynamics modulate N2O flux response to multiple nitrogen additions in an alpine steppe. J Geophys Res Biogeosci 123(10):3308–3319
Qin S, Clough T, Luo J, Wrage-Mönnig N, Oenema O, Zhang Y, Hu C (2017) Perturbation-free measurement of in situ di-nitrogen emissions from denitrification in nitrate-rich aquatic ecosystems. Water Res 109:94–101
Qin S, Wang Y, Hu C, Oenema O, Li X, Zhang Y, Dong W (2012) Yield-scaled N2O emissions in a winter wheat–summer corn double-cropping system. Atmos Environ 55:240–244
Qin S, Yuan H, Dong W, Hu C, Oenema O, Zhang Y (2013) Relationship between soil properties and the bias of N2O reduction by acetylene inhibition technique for analyzing soil denitrification potential. Soil Biol Biochem 66:182–187
Reeves S, Wang W (2015) Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system. Sci Total Environ 530:219–226
Rockström J, Steffen W, Noone K, Persson Å, Chapin FS, Lambin EF, Foley JA (2009) A safe operating space for humanity. Nature 461(7263):472–475
Ruser R, Flessa H, Russow R, Schmidt G, Buegger F, Munch JC (2006) Emission of N2O, N2 and CO2 from soil fertilized with nitrate: effect of compaction, soil moisture and rewetting. Soil Biol Biochem 38(2):263–274
Saggar S, Giltrap DL, Li C, Tate KR (2007) Modelling nitrous oxide emissions from grazed grasslands in New Zealand. Agr Ecosyst Environ 119(1–2):205–216
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Tillman RW (2013) Denitrification and N2O: N2 production in temperate grasslands: processes, measurements, modelling and mitigating negative impacts. Sci Total Environ 465:173–195
Senbayram M, Chen R, Budai A, Bakken L, Dittert K (2012) N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric Ecosyst Environ 147:4–12
Senbayram M, Wei Z, Wu D, Shan J, Yan X, Well R (2021) Inhibitory effect of high nitrate on N2O reduction is offset by long moist spells in heavily N loaded arable soils. Biol Fert Soil 1–14
Smith J, Wagner-Riddle C, Dunfield K (2010) Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl Soil Ecol 44(2):138–146
Snyder CS, Bruulsema TW, Jensen TL, Fixen PE (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric Ecosyst Environ 133(3–4):247–266
Song X, Liu M, Ju X, Gao B, Su F, Chen X, Rees RM (2018) Nitrous oxide emissions increase exponentially when optimum nitrogen fertilizer rates are exceeded in the North China Plain. Environ Sci Techol 52(21):12504–12513
Tian H, Xu R, Canadell JG, Thompson RL, Winiwarter W, Suntharalingam P, Yao Y (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586(7828):248–256
Timilsina A, Bizimana F, Pandey B, Yadav RKP, Dong W, Hu C (2020b) Nitrous oxide emissions from paddies: understanding the role of rice plants. Plants-Basel 9(2):180
Timilsina A, Dong W, Luo J, Lindsey S, Wang Y, Hu C (2020a) Nitrogen isotopic signatures and fluxes of N2O in response to land-use change on naturally occurring saline–alkaline soil. Sci Rep 10(1):1–13
Timilsina A, Zhang C, Pandey B, Bizimana F, Dong W, Hu C (2020c) Potential pathway of nitrous oxide formation in plants. Front Plant Sci 11:1177
Timilsina A, Oenema O, Luo J, Wang Y, Dong W, Pandey B, Hu C (2022) Plants are a natural source of nitrous oxide even in field conditions as explained by 15N site preference. Sci Total Environ 805:150262
Wang R, Feng Q, Liao T, Zheng X, Butterbach-Bahl K, Zhang W, Jin C (2013) Effects of nitrate concentration on the denitrification potential of a calcic cambisol and its fractions of N2, N2O and NO. Plant Soil 363(1):175–189
Wang R, Pan Z, Zheng X, Ju X, Yao Z, Butterbach-Bahl K, Huang B (2020) Using field-measured soil N2O fluxes and laboratory scale parameterization of N2O/(N2O+N2) ratios to quantify field-scale soil N2 emissions. Soil Biol Biochem 148:107904
Wang R, Willibald G, Feng Q, Zheng X, Liao T, Brüggemann N, Butterbach-Bahl K (2011) Measurement of N2, N2O, NO, and CO2 emissions from soil with the gas-flow-soil-core technique. Environ Sci Technol 45(14):6066–6072
Wang Y, Hu C, Ming H, Oenema O, Schaefer DA, Dong W, Li X (2014) Methane, carbon dioxide and nitrous oxide fluxes in soil profile under a winter wheat-summer maize rotation in the North China Plain. PloS One 9(6):e98445
Werner C, Reiser K, Dannenmann M, Hutley LB, Jacobeit J, Butterbach-Bahl K (2014) N2O, NO, N2 and CO2 emissions from tropical savanna and grassland of northern Australia: an incubation experiment with intact soil cores. Biogeosciences 11(21):6047–6065
Wu D, Well R, Cárdenas LM, Fuß R, Lewicka-Szczebak D, Köster JR, Bol R (2019) Quantifying N2O reduction to N2 during denitrification in soils via isotope mapping approach: Model evaluation and uncertainty analysis. Environ Res 179:108806
Yoshinari T, Hynes R, Knowles R (1977) Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem 9(3):177–183
Zistl-Schlingmann M, Feng J, Kiese R, Stephan R, Zuazo P, Willibald G, Dannenmann M (2019) Dinitrogen emissions: an overlooked key component of the N balance of montane grasslands. Biogeochemistry 143(1):15–30
Funding
This research was funded by the National Key Research and Development program of China (2021YFD1700901), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA26040103), and the Key Research and Development of Hebei Province (21323601D). Fiston Bizimana is grateful to the Alliance of International Science Organizations (ANSO) under the Chinese Academy of Sciences (CAS), for doctoral studies (Ph.D.) scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Hong Jie Di
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bizimana, F., Luo, J., Timilsina, A. et al. Estimating field N2 emissions based on laboratory-quantified N2O/(N2O + N2) ratios and field-quantified N2O emissions. J Soils Sediments 22, 2196–2208 (2022). https://doi.org/10.1007/s11368-022-03212-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03212-0