Abstract
Purpose
Soil microorganisms are important in the cycling of plant nutrients. Soil microbial biomass, community structure, and activity are mainly affected by carbon substrate and nutrient availability. The objective was to test if both the overall soil microbial community structure and the community-utilizing plant-derived carbon entering the soil as rhizodeposition were affected by soil carbon (C) and nitrogen (N) availability.
Materials and methods
A 13C-CO2 steady-state labeling experiment was conducted in a ryegrass system. Four soil treatments were established: control, amendment with carboxymethyl cellulose (CMC), amendment with ammonium nitrate (NF), combined CMC and NF. Soil phospholipid fatty acid (PLFA) and 13C labeling PLFA were extracted and detected by isotope ratio mass spectrometer.
Results and discussion
The combined CMC and NF treatment with appropriate C/N ratio (20) significantly enhanced soil microbial biomass C and N, but resulted in lower soil inorganic N concentrations. There was no significant difference in soil PLFA profile pattern between different treatments. In contrast, most of the 13C was distributed into PLFAs 18:2ω6,9c, 18:1ω7c, and 18:1ω9c, indicative of fungi and gram-negative bacteria. The inorganic-only treatment was distinct in 13C PLFA pattern from the other treatments in the first period of labeling. Factor loadings of individual PLFAs confirmed that gram-positive bacteria had relatively greater plant-derived C contents in the inorganic-only treatment, but fungi were more enriched in the other treatments.
Conclusions
Amendments with CMC can improve N transformation processes, and the ryegrass rhizodeposition carbon flux into the soil microbial community is strongly modified by soil N availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil microorganisms are both a labile nutrient pool and an agent of the transformation and cycling of organic matter and plant nutrients in soils (Fierer et al. 2012). Soil C and N availability are key factors which modify the microbial community and thus affect organic matter decomposition and nutrient cycling (Chen et al. 2014). Changes in microbial population size, community structure, and activity are usually observed after amendment with organic matter or N fertilizer (Marschner et al. 2003; Bengtsson et al. 2003). Since soil microbial turnover rate and activity are highest when substrate input corresponds to stoichiometric C and N ratios (Hessen et al. 2004), combined inorganic N fertilizer with organic C treatment is usually used to increase soil nutrient availability and has been proven to be an effective method to reduce N losses and improve crop productivity (Palm et al. 1997; Zhang et al. 2012).
Root exudates are implicated as a key determinant of rhizosphere microbial biomass and activity, and can modify the biochemical interactions between plants and soil microorganisms (Prosser et al. 2006). Based on a meta-analysis of the available data, rhizodeposition is estimated to account for approximately 17 % of photoassimilate (Nguyen 2003). Rhizodeposition carbon compounds can strongly influence nutrient availability and microbial community structure due to their preferential use as substrates by microorganisms involved in nutrient transformations (Phillips et al. 2011). Soil C and N cycling are often coupled. The coupling of rhizodeposition C input to the functioning of microbial community can affect microbial growth and reproduction and be beneficial for soil N availability and plant N uptake (Paterson 2003). N fertilizer application reduces the competition for N between roots and soil microbes, and the amount of rhizodeposition C is decreased by increased N availability (Henry et al. 2005; Liljeroth et al. 1994).
The isotope labeling technique has been effectively used to track carbon flow and study the relative importance of rhizodeposition C and other C sources in determining soil microbial community structure and activity (West et al. 2006). Soil microbial communities can utilize both labile and recalcitrant carbon, including root exudates, soil organic matter, and plant litter. Phospholipid fatty acid (PLFA) analysis combined with stable isotope probing (SIP) can quantify the 13C assimilated into microbial populations and determine the sources of C used by soil microbes (Yao et al. 2015). PLFA-SIP has been applied to study the decomposition of plant residues and the assimilation of organic matter (Williams et al. 2006; Murase et al. 2006) as well as the incorporation of photosynthates into microbial communities (Lu et al. 2004; Yao et al. 2012).
Most 13C labeling PLFA studies in soil-plant systems have been based on the pulse-labeling technique (Butler et al. 2003; Treonis et al. 2004) but this technique does not label all plant C pools to the same degree, emphasizing current photosynthate (De Visser et al. 1997). The steady-state 13C labeling approach can determine the relative turnover activities of individual PLFAs and show a gradual shift in one direction during the labeling period (Paterson et al. 2011). In this study, we conducted a 13C-CO2 steady-state labeling experiment in a ryegrass system with different amendments. The objective was to test if both the overall soil microbial community structure and the community-utilizing plant-derived carbon entering the soil as rhizodeposition were affected by soil C and N availability.
2 Materials and methods
2.1 Soil and 13C steady-state labeling
A typical red clay soil (Ultisols) was collected from the plow layer (0–20 cm) in Longyou county (28°58′ N and 118°53′ E), Zhejiang Province, China, in September 2013. The air-dried soil was sieved through a 2-mm mesh. Some chemical and physical properties of the soil were as follows: pH (soil, water = 1: 2.5) 4.51, soil organic carbon 10.81-mg g−1 soil, total nitrogen 1.22-mg g−1 soil.
Ryegrass (Lolium perenne L.; cultivar ORE-TET) was grown in 250-ml containers, filled with 160-g air-dried soil, and rewetted to 50 % water holding capacity. In order to investigate the interactive effects of organic C and N fertilizer, four soil treatments were established: (1) control, CK; (2) amendment with carboxymethyl cellulose at the rate of 2000 mg C kg−1 soil, CMC; (3) amendment with ammonium nitrate (NH4NO3) at the rate of 100 mg total N kg−1 soil, NF; and (4) combined CMC and NF, CMC + NF. All the soils were amended with potassium dihydrogen phosphate (KH2PO4) at the rate of 50-mg P2O5 kg−1 soil. Seeds of ryegrass were germinated in Petri dishes containing deionized water at 30 °C in the dark, and, following germination, 50 seedlings were sown in each pot. Four days after sowing, 30 healthy seedlings were retained in each pot. The pots were placed in a growth chamber (day/night temperature, 30/25 °C; photoperiod, 12 h light; relative humidity of 80–90 %).
After 2 weeks’ growth, steady-state labeling of 13CO2 at 2.0 atom % excess and at ambient CO2 concentrations was introduced. All the pots were arranged in a random block structure in a Perspex labeling chamber, housed within a controlled environment room. The chamber had ports at each end as inlets/outlets for gas flows and two access doors that maintained air-tight seals when closed (Yao et al. 2012). The steady-state 13CO2 enrichment of the labeling chamber continuously aerated at a rate of 1.8 × 104 cm3 min−1 was achieved by routing the compressed air supply via a self-regenerating pressure swing adsorption CO2 scrubber unit. Soil water status was maintained by additions of deionized water every day during the 15 d labeling experiment; this was performed during the dark period in order to avoid affecting the 13C- signature of CO2 fixed by the plants.
Destructive harvests of plants and soils were taken after labeling for 0, 5, 10, and 15 days. Each treatment included three replicates. Soil was separated from roots and divided into two portions. The first portion was stored at 4 °C up to 7 days before microbial biomass C and N analysis, except that inorganic N (NH4 +_N and NO3 -_N) was immediately analyzed. The other portion was freeze-dried for PLFA analysis. Plant roots and shoots were separated and dried at 105 °C for 24 h, then weighed. The inorganic N was extracted from soil subsamples by shaking for 1 h in 1-M KCl (soil: solution, 1:10), filtering using a filter paper (Quantitative 203 grade filter paper, ϕ = 7 cm) and then detected colorimetrically using a micro-plate reader (Spectramax M5, Molecular Devices, USA) (Shand et al. 2008). Soil microbial biomass C and N were determined using the chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987; Wu et al. 1990). Extraction coefficients 0.45 and 0.54 were used for calculating microbial biomass C and N, respectively. The N content in plants was determined by macro elemental analyzer (Vario Max, Elementar, Germany).
2.2 PLFA analysis
Lipids were extracted from soil and PLFA, analyses followed the method of Bligh and Dyer (1959) as modified by Frostegård et al. (1993). From the freeze dried soil sub-samples, 2.0 g was extracted twice using a total of 22.8 ml of a chloroform-methanol-citrate buffer mixture (1:2:0.8 v/v/v), and the phospholipids were separated from neutral and glycolipids on a silicic acid column. The phospholipids were derivatized into fatty acid methyl esters (FAMES). Methylnonadecanoate fatty acid (19:0) was added as the internal standard. The concentration and 13C label of the FAMES were carried out by using a GC Trace Ultra with combustion column attached via a GC Combustion III to a Delta V Advantage isotope ratio mass spectrometer (Thermo Fisher Scientific, USA). Samples (1–2 μl) were injected in splitless mode (Column HP-5, 50-m length, 0.2-mm i.d., film thickness of 0.33 μm; Agilent Technologies Inc., Santa Clara, USA), and the running conditions were as described by Thornton et al. (2011).
The PLFA concentrations were determined based on the combined area of the peaks for all ions (m/z 44, 45 and 46) relative to the internal standard. The C isotope ratios were calculated from a CO2 reference gas (Thornton et al. 2011). The 13C value of the C added to each PLFA molecule during methylation to their corresponding FAMES was accounted for using a mass balance approach (Paterson et al. 2008). Nomenclature of fatty acids follows that used by Tunlid and White (1992). Briefly, the suffix c for cis refer to the geometric isomer. The prefixes i- and a- refer to iso- and anteiso-branched fatty acids. The suffix -10Me indicates a methyl group on the tenth carbon atom from the carboxyl end of the molecule. A cyclopropyl ring is indicated as cy-, and “OH” indicates a hydroxyl group. In total, 29 PLFAs were identified in these samples; however, only a subset of 22 PLFAs, comprising of about 95 % of the total PLFA concentration, were present in sufficient concentration for accurate isotope analysis. We therefore defined 13C incorporation into the soil microbial biomass as the total 13C incorporation into this subset of 22 PLFAs. The proportion of plant-derived labeled C in each PLFA was determined using a mass balance approach (Yao et al. 2012).
2.3 Statistical analysis
Soil chemical properties and PLFA data were compared by a one-way ANOVA. The significance of any difference was defined according to statistical convention at p < 0.05. The percentage distribution of 13C among PLFAs was calculated and used for principal component analysis, after generating a correction matrix to transform the data to unit variance. All statistical analyses were made using GenStat 16th Edition (VSN International, Oxford, UK).
3 Results
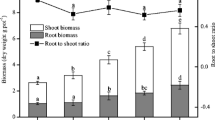
After 2 weeks’ growth before labeling, there was no significant difference in ryegrass biomass except for CK (Fig. 1). After labeling for 15 days, the CMC + NF treatment had the highest growth rate, and the dry weight was 0.37 g pot−1, which was significantly higher than the CK (0.24 g pot−1) and CMC (0.27 g pot−1) treatments. Compared to the NF treatment, the combined organic C with N fertilizer treatment improved plant biomass. We found that the NF treatment had the highest soil inorganic N content in the first 10 days of labeling. However, no significant differences in soil inorganic N concentration were observed among all the treatments at the end of the labeling period (Fig. 2). Interestingly, soil inorganic N concentration in the CMC + NF treatment was much lower than the inorganic-only fertilizer treatment in the first 10 days of labeling, and there was no significant difference in CK, CMC, and CMC + NF treatments during the whole labeling period.
Final microbial biomass C in the soils ranged from 144 to 228 μg C g−1 (Fig. 3). Application of N fertilizer significantly increased soil microbial biomass N. The combination of organic C with N fertilizer further increased soil microbial biomass such that the CMC + NF treatment had the highest value. Microbial biomass N in the CMC + NF treatment was 34.1 μg N g−1, which was much higher than the CK (21.1 μg N g−1), CMC (23.0 μg N g−1), and NF (28.9 μg N g−1) treatments.
The total PLFAs (± standard deviation) in the CK, CMC, NF, and CMC + NF treatments were 27.0 ± 4.7, 30.3 ± 1.9, 34.2 ± 4.0, and 38.9 ± 4.8 nmol g−1 soil, respectively, with no significant change during the labeling period. The total amount of PLFAs was significantly correlated with soil microbial biomass obtained from the fumigation-extraction method. Twenty-nine PLFAs with chain lengths from C14 to C19 were identified which varied significantly in their relative abundance (Fig. 4a). There was no difference in PLFA profile pattern based on principal component analysis (PCA) between different treatments and different labeling days.
At the end of labeling, the total 13C incorporation into the PLFA pools in the CK, CMC, NF, and CMC + NF treatments were 1633, 1855, 2488, and 2699 ng g−1 soil, respectively. The 13C was not evenly distributed among PLFAs. The highest relative abundances were observed for PLFAs 18:2ω6,9c and 16:0, followed by 18:1ω7c, and 18:1ω9c (Fig. 4b). PCA analysis of 13C PLFA data showed that the NF treatment was distinct from the other treatments in the first 5 and 10 days of labeling, but no real significant difference was found at the end of labeling (Fig. 5). In the first period of labeling (Fig. 5a), the NF treatment significantly separated along PC1, accounting for 59.5 % of the variation. Factor loadings of individual PLFAs on PC1 confirmed that i16:0 (score 0.154) and i17:0 (score 0.136) had relatively greater plant-derived C contents in the NF treatment. The loadings also confirmed that 18:2ω6,9c (score −0.835) was more enriched in the other treatments (Table S1, Electronic Supplementary Material).
Principal component analysis of the relative abundance (mol %) of13C PLFAs of individual soil samples after labeling 5 days (a), 10 days (b), and 15 days (c). CK control, CMC organic carbon alone, NF N fertilizer alone, CMC + NF combined organic carbon and N fertilizer. Values are means ± s.d. (n = 3)
4 Discussion
The combined impact of inorganic and organic fertilizers on soil organic matter content and crop yield has been stressed by many studies (e.g. Pan et al. 2009; Zhang et al. 2012). Compared to inorganic-only fertilizer treatment, the combination of organic carbon and inorganic N fertilizer treatment decreased soil inorganic N, but improved soil microbial biomass and plant growth (Fig. 1 and Fig. 3). Soil microorganisms determine N availability for plant uptake or loss mainly through the transformation processes of mineralization, nitrification, and immobilization. The balance between these processes can regulate the pool size of soil inorganic N and accordingly plant uptake (Shi et al. 2006; Herencia et al. 2008; Dinesh et al. 2010). Soil inorganic N was much lower in the CMC + NF treatment than in the NF treatment, indicating more inorganic N was incorporated into microorganisms (Cheng et al. 2014). Soil microbial biomass C and N increased with the amendment of CMC (Fig. 3), indicating that microbial N turnover rates were promoted and that inorganic N fertilizer can be more easily incorporated into soil microbial biomass. Soil C and N cycling are tightly coupled in soils. Barrett and Buke (2000) showed that soil carbon content alone can explain more than 60 % of the variation in potential N immobilization. Organic amendments typically increased soil microbial biomass through the supply of available C as a food source to the generally C-limited microbes in arable soils (Diacono and Montemurro 2010; Lazcano et al. 2013). The input of readily available organic compounds can change the predominant soil microbial type (Meidute et al. 2008) and N transformation processes (Bengtsson et al. 2003), and microbial activity is the highest when soil available C and N ratio matches microbial demands (Chen et al. 2014). In this study, the soil C/N ratio was quite low (8.9), and soil labile organic C was very limited for microbial N cycling. The enhanced microbial N turnover and plant productivity in the combination of CMC and inorganic N fertilizer treatment could be accounted for by the appropriate stoichiometric C and N ratios of the substrate input.
Similar to soil microbial biomass, application of inorganic N fertilizer or organic amendment (CMC) increased the amount of PLFA. The whole soil PLFA and 13C-PLFA were significantly correlated with soil microbial biomass and plant yield. Available C and N are two key limiting factors for soil microbial growth (Flavel and Murphy 2006). Both inorganic N fertilizer and organic fertilizers can stimulate microbial processes, increase soil microbial biomass, and crop productivity by improved nutrient availability (Sarathchandra et al. 2001; Liu et al. 2010). Generally, different types of amendments differ in organic matter composition or C/N ratio, and this in turn, can affect the transformation rate and change microbial community structure (Marschner et al. 2003). Differences in microbial composition in soil with chemical and organic fertilization were identified based on the analysis of 16S rRNA gene libraries (Gu et al. 2009; Su et al. 2015). Interestingly, there was no significant change in soil microbial community structure with the addition of different amendments based on PLFA profile pattern. This result suggested that changes in microbial biomass and nutrient availability do not always involve a change in microbial community structure (Yao et al. 2000; Franco-Otero et al. 2012), since even the same species of microorganisms can carry out different functions and activities. Due to the complexity and heterogeneity of C sources in soil-plant ecosystems, including root exudates, soil organic matter, and plant litter, specific microbial groups can feed on different organic compounds, and the diversity in labile C sources is likely to support the similar microbial PLFA pattern observed in the present study. On the other hand, PLFA patterns do not provide high-resolution phylogenetic information or detailed species composition (Frostegård et al. 2011). The limitation of the low resolution in PLFA analysis may be another reason for no significant difference in PLFA patterns. Although different amendments had no significant effect on the soil PLFA pattern, some specific PLFA indicators changed in the different treatments. The ratio of cy17:0/16:ω7c, which is an indicator of environmental stress (Frostegård et al. 2011), was much higher in the inorganic-only fertilizer treatments (1.7) than in the other treatments (1.2–1.3). This result may suggest a high level of inorganic N has a stress effect in the C-limited soil.
The microbial PLFA profile derived from root exudates was distinct from the whole soil PLFA profile, which suggested that a subset of the microbial population assimilated ryegrass root derived C (Fig. 5). Most of the 13C (>60 %) was distributed into four straight chain fatty acids, 18:2ω6,9c, 16:0, 18:1ω7c, and 18:1ω9c, in all the soil treatments. This suggested that microbial groups containing these PLFAs were relatively more active and competitive for the available substrates released from ryegrass roots than microbial groups indicated by total PLFA which received little 13C. The observed differences in the whole soil PLFA and 13C-PLFA profiles are in agreement with Butler et al. (2003) who showed that most plant-derived C was incorporated into 18:2ω6,9c, followed by 16:0 and 18:1ω7c. Carbon substrate is the main reason to select for specific microbial groups and to change the composition of the soil microbial community (Ros et al. 2006; Zhong et al. 2010). The high heterogeneity of available C sources in soil may result in a more even distribution in whole soil PLFAs than in 13C PLFAs. The most common PLFAs in eukaryotic soil microorganisms, predominantly fungi, include 18:2ω6,9c, 16:0 and 18:1ω9c. Nearly all PLFA-SIP experiments suggest that these PLFAs have a high incorporation of plant rhizodeposition C (Butler et al. 2003; Lu et al. 2004; Yao et al. 2012). However, since plant root usually contain the PLFAs 18:2ω6,9c, and 18:1ω9c (Lovell et al. 2001; Lu et al. 2004), some input of PLFAs derived from fine ryegrass roots cannot be completely excluded.
In PLFA-SIP analysis, one important advantage is to be able to determine the sources of C used by soil microorganisms based on 13C-PLFA turnover. Most 13C-labelled rhizodeposition C was distributed into characteristically fungal (18:2ω6,9c) and gram-negative bacterial (18:1ω7c) PLFAs, and their rates of increase in δ13C were very high (>4‰ d−1), suggesting that these PLFAs are derived from microbes mainly reliant on rhizodeposition. In contrast, the relative abundance of i15:1, a17:0 and i17:0 (characteristic of gram-positive bacteria) was higher in whole soil PLFA than that in 13C-PLFA (Fig. 4). The rates of δ13C increase were relatively slow (<2‰ d−1), suggesting that these PLFAs were mainly derived from microbes more reliant on soil organic matter. The high 13C enrichment and turnover rate of 18:2ω6,9c and 18:1ω7c confirmed that it is fungi and gram-negative bacteria that preferentially utilize plant-derived labile C sources. Similar clear distinctions have been observed between communities degrading soil- and litter- derived C, with proportionally more fungi and general microorganisms utilizing litter derived C and more gram-positive bacteria utilizing soil-derived C (Creamer et al. 2015).
The δ13C values of soil PLFA increased during the steady-state labeling indicating that root-derived C is an important source of readily available C for soil microorganisms. Both the total 13C incorporation into PLFAs and plant biomass were the highest in the CMC + NF treatment. The results suggested that C substrate availability is the most important factor for microbial growth in this soil (Nguyen 2003). The importance of root exudation in regulating the size and composition of the microbial community is well known (Broeckling et al. 2008). In response to exudates, changes in microbial population and activity may stimulate a microbial demand for other nutrients, which can be met by increasing enzyme synthesis and promoting N transformations (Mergel et al. 1998; Dijkstra et al. 2009). Yin et al. (2013) demonstrated that root exudation can influence soil organic matter decomposition and microbial N cycling. Principal component analysis on the relative abundance of 13C-PLFAs showed that inorganic N fertilizer had a significant effect on the incorporation of 13C-labeled rhizodeposition C into soil microbial communities, implying an influence of plant root andmineral N interactions on soil microbial communities. Our current results provide knowledge of how different amendments affect the utilization of ryegrass rhizodeposition by soil microorganisms. The PLFAs i16:0 and i17:0, which are indicators of gram-positive bacteria (Butler et al. 2003), showed the highest 13C enrichment in the NF treatment, while 18:2ω6,9c, the indicator of fungal PLFA (Frostegård et al. 2011), was more enriched in the other treatments. The lower 13C enrichment of PLFA18:2ω6,9c in the NF treatment suggested that fungi generally exhibit a lower utilization of rhizodeposition C than soil-derived C with high inorganic N content. The previous studies found that inorganic fertilizers can suppress the utilization of rhizodeposition C by Actinobacteria but increase the utilization by Fibrobacteres (Ai et al. 2015). The changes in 13C-PLFA pattern implied that the increase in soil N availability can decrease the dependence of rhizosphere microorganisms on plant-derived C sources. The effect of soil available N on the soil microbial community assimilating rhizodeposition C may be due either to the altered quality and quantity of root exudates (Kuzyakov et al. 2002) or to a change in microbial C use efficiency (Shi et al. 2006; Ai et al. 2015).
5 Conclusions
The results of this study have demonstrated that the application of ammonium nitrate combined with CMC significantly enhanced soil microbial biomass and N transformation processes, which are mainly achieved by the appropriate C and N ratio of substrate input. On the other hand, the impact of ryegrass roots on the microbial community structure is strongly modified by soil N availability. The increase in N availability can reduce the dependence of soil microorganisms on rhizodeposition C. Further work is needed to elucidate the changes in detailed species composition and phylogenetic structure using high-resolution nucleic acid-based techniques.
References
Ai C, Liang GQ, Sun JW, Wang XB, He P, Zhou W, He XH (2015) Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol Biochem 80:70–78
Barrett JE, Buke IC (2000) Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol Biochem 32:1707–1716
Bengtsson G, Bengtson P, Månsson KF (2003) Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35:143–154
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol 37:911–917
Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (2008) Root exudates regulate soil fungal community composition and diversity. Appl Environ Microb 74:738–744
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microb 69:6793–6800
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Global Change Biol 20:2356–2367
Cheng Y, Wang J, Zhang JB, Mary B, Cai ZC (2014) The mechanisms behind reduced NH4 + and NO3 − accumulation due to litter decomposition in the acidic soilof subtropical forest. Plant Soil 378:295–308
Creamer CA, de Menezes AB, Krull ES, Sanderman J, Newton-Walters R, Farrell M (2015) Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol Biochem 80:175–188
De Visser R, Vianden R, Schnyder H (1997) Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation: assessment by 13C and 15N steady state labelling. Plant Cell Environ 20:37–46
Diacono M, Montemurro F (2010) Long-term effects of organic amendments on soil fertility: a review. Agron Sustain Dev 30:401–422
Dijkstra FA, Bader NE, Johnson DW, Cheng WX (2009) Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability? Soil Biol Biochem 41:1080–1087
Dinesh R, Srinivasan V, Hamza S, Manjusha A (2010) Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour Technol 101:4697–4702
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG (2012) Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci U S A 109:21390–21395
Flavel TC, Murphy DV (2006) Carbon and nitrogen mineralization rates after application of organic amendments to soil. J Environ Qual 35:183–193
Franco-Otero VG, Soler-Rovira P, Hernández D, López-de-Sá EG, Plaza C (2012) Short-term effects of organic municipal wastes on wheat yield, microbial biomass, microbial activity, and chemical properties of soil. Biol Fertil Soils 48:205–216
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Gu Y, Zhang X, Tu S, Lindström K (2009) Soil microbial biomass, crop yield, and bacterial community structure as affected by long-term fertilizer treatments under wheat-rice cropping. Eur J Soil Biol 45:239–246
Henry F, Nguyen C, Paterson E, Sim A, Robin C (2005) How does nitrogen availability alter rhizodeposition in Lolium multiflorum Lam.during vegetative growth? Plant Soil 269:181–191
Herencia JF, Ruiz JC, Melero S, Garcia Galavís P, Maqueda C (2008) A short-term comparison of organic v. conventional agriculture in a silty loam soil using two organic amendments. J Agric Sci 146:677–687
Hessen DO, Agren GI, Anderson TR, Elser JJ, De Ruiter PC (2004) Carbon, sequestration in ecosystems: the role of stoichiometry. Ecology 85:1179–1192
Kuzyakov Y, Siniakina SV, Ruehlmann J, Domanski G, Stahr K (2002) Effect of nitrogen fertilisation on below-ground carbon allocation in lettuce. J Sci Food Agr 82:1432–1441
Lazcano C, Gómez-Brandón M, Revilla P, Domínguez J (2013) Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol Fertil Soils 49:723–733
Liljeroth E, Kuikman P, Van Veen JA (1994) Carbon translocation to the rhizosphere of maize and wheat and influence on the turnover native soil organic matter at different soil nitrogen levels. Plant Soil 161:233–240
Liu E, Yan CR, Mei XR, He WQ, Bing SH, Ding LP, Liu Q, Liu S, Fan TL (2010) Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 158:173–180
Lovell CR, Bagwell CE, Czako M, Marton L, Piceno YM, Ringelberg DB (2001) Stability of a rhizosphere microbial community exposed to natural and manipulated environmental variability. FEMS Microbiol Ecol 38:69–76
Lu Y, Murase J, Watanabe A, Sugimoto A, Kimura M (2004) Linking microbial community dynamics to rhizosphere carbon flow in a wetland rice soil. FEMS Micro Biol Ecol 48:179–186
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
Meidute S, Demoling F, Bååth E (2008) Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol Biochem 40:2334–2343
Mergel A, Timchenko A, Kudeyarov V (1998) Role of plant root exudates in soil carbon and nitrogen transformation. In: Box JE (ed) Root Demographies and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems. Section I: Global patterns of carbon allocation. Springer Science + Business Media, Berlin, pp 43–54
Murase J, Matsui Y, Katoh M, Sugimoto A, Kimura K (2006) Incorporation of 13C-labeled rice-straw-derived carbon into microbial communities in submerged rice field soil and percolating water. Soil Biol Biochem 38:3483–3491
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396
Palm CA, Myers RJK, Nandwa SM (1997) Combined use of organic and inorganic nutrient sources for soil fertility maintenance and replenishment. In: Buresh RJ (ed) Replenishing soil fertility in Africa. American Society of Agronomy, Madison, pp 193–217
Pan GX, Zhou P, Li ZP, Smith P, Li LQ, Qiu DS, Zhang XH, Xu XB, Shen SY, Chen XM (2009) Combined inorganic/organic fertilization enhances N efficiency and increases rice productivity through organic carbon accumulation in a rice paddy from the Tai Lake region, China. Agr Ecosyst Environ 131:274–280
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Paterson E, Osler G, Dawson LA, Gebbing T, Sim A, Ord B (2008) Labile andrecalcitrant plant fractions are utilized by distinct microbial communities insoil: independent of the presence of roots and mycorrhizal fungi. Soil Biol Biochem 40:1103–1113
Paterson E, Sim A, Osborne SM, Murray PJ (2011) Long-term exclusion of plant-inputs to soil reduces the functional capacity of microbial communities to mineralise recalcitrant root-derived carbon sources. Soil Biol Biochem 43(9):1873–1880
Phillips R, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Prosser JI, Rangel-Castro JI, Killham K (2006) Studying plant–microbe interactions using stable isotope technologies. Curr Opin Biotech 17:98–102
Ros M, Klammer S, Knapp B, Aichberger K, Insam H (2006) Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag 22:209–218
Sarathchandra SU, Ghani A, Yeates GW, Burch G, Cox NR (2001) Effect of nitrogen and phosphate fertilizer on microbial and nematode diversity in pasture soils. Soil Biol Biochem 33:953–964
Shand CA, Williams BL, Coutts G (2008) Determination of N-species in soil extracts using microplate techniques. Talanta 74:648–654
Shi W, Yao H, Bowman D (2006) Soil microbial biomass, activity and nitrogen transformations in a turfgrass chronosequence. Soil Biol Biochem 38:311–319
Su JQ, Ding LJ, Xue K, Yao HY, Quenxen J, Bai AJ, Wei WX, Wu JS, Zhou JZ, Tiedje JM, Zhu YG (2015) Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol Ecol 24:136–150
Thornton B, Zhang ZL, Mayes RW, Högberg MN, Midwood AJ (2011) Can gas chromatography combustion isotope ratio mass spectrometry be used to quantify organic compound abundance? Rapid Commun Mass Spectrom 25:2433–2438
Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P (2004) Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36:533–537
Tunlid A, White DC (1992) Biochemical analysis of biomass, community structure, nutritional status and metabolic activity of microbial communities in soil. In: Stotzky G (ed) Soil biochemistry. Marcel Dekker, New York, pp 229–262
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
West JB, Bowen GJ, Cerlingc TE, Ehleringer JR (2006) Stable isotopes as one of nature’s ecological recorders. Trends in Ecol Evol 21:408–414
Williams MA, Myrold DD, Bottomley PJ (2006) Carbon flow from 13C-labeled straw and root residues into the phospholipid fatty acids of a soil microbial community under field conditions. Soil Biol Biochem 38:759–768
Wu JS, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Yao HY, He ZL, Wilson MJ, Campbell CD (2000) Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb Ecol 40:223–237
Yao HY, Thornton B, Paterson E (2012) Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol Biochem 53:72–77
Yao HY, Chapman S, Thornton B, Paterson E (2015) 13C PLFAs: a key to open the soil microbial black box? Plant Soil 392:3–15
Yin HJ, Li YF, Xiao J, Xu ZF, Cheng XY, Liu Q (2013) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Global Change Biol 19:2158–2167
Zhang AF, Liu YM, Pan GX, Hussain Q, Li LG, Zheng JW, Zhang XH (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351:263–275
Zhong W, Gu T, Wang W, Zhang B, Lin X, Huang Q, Shen W (2010) The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326:511–522
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41301251), the Ningbo Natural Science Foundation (2014A610101), and the Fujian Natural Science Foundation (2014I0007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Wang, J., Pan, F. et al. Soil nitrogen availability alters rhizodeposition carbon flux into the soil microbial community. J Soils Sediments 16, 1472–1480 (2016). https://doi.org/10.1007/s11368-015-1337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1337-6