Abstract
Crop residues and their derived biochar are frequently used for their potential to improve grain yield, soil fertility and carbon (C) sequestration. However, the effects of root are often overlooked, and the effects of chemical fertilizer (NPK) combined with root or its biochar on microbial community structure need further study. This study used 13C-labeled maize root, its biochar and soil with different fertilization for 8 years as materials and substrates. A 112-day incubation experiment was conducted to explore the effects of microbial community on the C processing. During incubation, the root-C (54.9%) mineralized significantly more than biochar-C (12.8%), while NPK addition significantly increased the root-C mineralization. Adding biochar alone did not significantly change the microbial community. Compared to the biochar treatment (BC), the root treatment (R) notably increased the contents of total phospholipid fatty acids (PLFAs), 13C-PLFA and the proportion of fungi and Gram-negative bacteria, but reduced the proportion of actinomycetes. The root mineralization was significantly correlated with the relative content of 13C-Gram-positive bacteria and 13C-fungi, while biochar mineralization was significantly correlated with the relative content of 13C-Gram-positive bacteria and 13C-actinomycetes. Notably, NPK addition significantly increased the contribution of biochar-C to PLFA-C pool, while decreasing the contribution of root-C. In summary, due to microbial adaptation to the lack of bioavailable C in biochar-amended soil, biochar can act as a buffer against the significant disturbance caused by NPK to microbial communities and native soil organic carbon (SOC), which contributes to the steady enhancement in soil C storage.
Graphical Abstract

Highlights
-
The addition of biochar alone for 8 consecutive years did not change the composition of the microbial community structure, but the total PLFA content increased significantly compared to the control.
-
NPK addition reduced the proportion of microbial assimilation of root-C, while increasing the proportion of microbial assimilation of biochar-C.
-
The effect of NPK on microbial biomass is short-lived, but the effect on microbial community structure is long-lasting.
-
Biochar has a stronger buffering effect on the drastic changes in microbial communities and native SOC caused by NPK.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil organic carbon (SOC), one of the Earth’s most substantial terrestrial C reservoirs, is pivotal for boosting soil fertility, combating global climate change, and upholding ecosystem stability (Bossio et al. 2020; Liang et al. 2017). Historically, plant residues were incorporated into agricultural soils not only to maintain soil structure, organic content, and moisture retention but to act as a vital energy substrate for microbial activities (Pan et al. 2016a; Powlson et al. 2011; Schmatz et al. 2016). However, the effects of roots routinely left in the soil after harvest are often overlooked despite their relevance. Biochar, originating from crop residues, is more resistant to chemical decomposition than its precursor and shows immense promise in boosting SOC accumulation, mitigating greenhouse gas emissions, and curbing both organic and inorganic pollutant bioavailability and accumulation (Khan et al. 2015; Lehmann et al. 2011; Stewart et al. 2013; Waqas et al. 2014). Discussions persist about the degradation pathways of crop roots and biochar in the soil, especially concerning their influence on microbial community configurations. Filling the knowledge void about this process, particularly under diverse fertility conditions, is crucial.
The dynamics of microbial communities are responsible for the metabolism of soil organic matter (SOM) (Waldrop and Firestone 2004) and regulate the formation and mineralization of SOM. Varied soil microorganisms, each with distinct life cycles and physiological traits, demonstrate differential utilization of substrates and metabolism of crop residues(Andresen et al. 2014; Fierer et al. 2007). Recalcitrant C is mainly tapped by K-strategists like certain fungi, attributed to their adaptability in nutrient-depleted environments and prowess in mineralizing resistant C (Terrer et al. 2021). Labile C can boost microbial communities dominated by r-strategists such as Gram-negative bacteria (Fontaine et al. 2011; Paterson and Sim 2013), which results in CO2 emissions and diminishes the fraction of residual C incorporated into SOM (Arcand et al. 2016; Li et al. 2019a; Liang et al. 2017). However, a recent study by Kramer et al. (2016) posited that bacterial utilization of labile C and fungal utilization of recalcitrant C sources were not distinctly separated during a decomposition experiment employing diverse plant C resource. For example, it has been reported that fungi are strong utilizers of labile C sources (Müller et al. 2017).Such finding underscores the considerable variability in microbial functions which has spurred continued discussions regarding the roles of specific microbial groups at varied decomposition stages (Strickland and Rousk 2010). Therefore, understanding the influence of the complexity of microbial function on residue decomposition is important for predicting SOM turnover.
The quality of organic matter is the most common limiting factor for microbial activity in the soil (Kuzyakov 2010; Potthast et al. 2010). Maize roots are composed of biopolymers with varying degrees of persistence, resulting in different microbial community structures adapted to the metabolism of various substrates. In contrast, biochar after pyrolysis is difficult to be used by microorganisms due to its inertness (Lehmann et al. 2011), and thus it has been reported that biochar’s addition has minimal (Pan et al. 2016b) or even no effects (Tian et al. 2016) on microbial community composition. However, biochar’s unique porous structure and high surface aromatization can modulate soil microbial communities (Atkinson et al. 2010; Chen et al. 2019). There have been some different reports stating that biochar notably impacts microbial community composition in different ways (Yang et al. 2022; Zhang et al. 2021). For example, biochar promoted fungal growth (Azeem et al. 2020) and greatly reduced bacterial abundance and total PLFA content (Wang et al. 2015b). In contrast, Jones et al. (2012) reported that biochar led to a shift from microbial to bacterial communities, but the dominance of Gram-positive (Mitchell et al. 2015) or Gram-negative bacteria (Gomez et al. 2014) was inconsistent. Such inconsistencies may arise from variations in biochar types, application quantity, soil types and plant system responses (Chen et al. 2017; Gomez et al. 2014; Gul et al. 2015). The inconsistency in the extent and direction of biochar’s effects on microbial communities creates barriers to exploring the functionality of biochar. Additionally, the microbial community regulates the decomposition of exogenous C and also receives its influence, and clarifying the relationship between them is important for understanding the C process.
Fertilization is a necessary measure to ensure crop yield, while also significantly affecting the biochemical processes of soil microorganisms. There have been studies highlighting that prolonged fertilizer application significantly modifies microbial community composition in agricultural soils (Carrara et al. 2021; Yuan et al. 2023; Zheng et al. 2016), thus compromising their stability (Ibrahim et al. 2020; Li et al. 2020; Pang et al. 2022). For instance, chemical fertilizer (NPK) addition significantly increased and decreased the diversity of bacteria and fungi in the top soil layer, affecting microbial community composition (Yuan et al. 2023). Compared to NPK applied alone, the combination of NPK and organic matters creates a different microbial community by providing a source of available nutrients and C source for a more active microbiota (Li et al. 2017). In a 6-year field trial, Wang et al. (2018) showed that the synergistic effect of biochar and NPK increased total microbial biomass and bacterial biomass. However, it has also been shown that microbial response to NPK may be weaker in biochar-amended soils (Watzinger et al. 2013) and the combination of biochar and NPK does not affect fungal populations (Dangi et al. 2020). This may be because biochar can help to maintain the stability of microbial community structure (Song et al. 2020). In addition, we speculate that the pattern of microbial community response to newly added exogenous C and NPK may be altered in soil amended with different fertilization practices over time due to adaptation to long-term substrate supply (Blagodatskaya and Kuzyakov 2013; Schimel et al. 2007). It’s essential to further explore the effects of NPK on the decomposition and transformation of diverse organic substrates in soil amended with different ways over time.
In this study, 13C-labeled maize root and its biochar were applied to brown soil with different fertilization regimes for up to 8 years for a 112-day incubation experiment. To study the fate of maize root and its derived biochar, the abundance of 13C in SOC and PLFAs was analyzed using a combination of biomarker and isotopic technology, and the response of soil microbial community structure and function to the addition of maize root, biochar and NPK was investigated. Our hypotheses are as follows. (1) The structure of the microbial community changes significantly during root decomposition, while the response to biochar addition may not be significant due to the difficulty of microbial utilization. (2) Microorganisms can assimilate more exogenous C in the case of NPK because of higher biomass and greater activity. (3) Irrespective of NPK application and exogenous materials’ quality, exogenous C would contribute more to fungal PLFA compared to other microbial communities due to the higher capacity of fungi to decompose recalcitrant C.
2 Materials and methods
2.1 Site description and biochar production
The soil was collected from the brown soil long-term test base (40°48′N and 123°33′E) in Shenyang Agricultural University, Liaoning Province, northeastern China (2013–present), where the experimental soil is a brown soil of loess origin with a clay loam soil texture (48% sand, 29% silt and 23% clay). The climate is temperate, characterized by humid-semi-humid monsoons. The average annual precipitation ranges from 574 to 684 mm, with an average annual temperature of 7.0–8.1 °C. The frost-free period lasts 147–164 days annually. To investigate the decomposition status of roots and their derived biochar, as well as their impact on microbial communities under varying fertility levels, we conducted indoor incubation experiments using six field soils (0–20 cm) with different treatments spanning up to eight years. The treatments included the treatments without chemical fertilizer: no fertilizer (CK), biochar (C), straw (S), and the treatments with chemical fertilizer: NPK-only (NPK), biochar and NPK (C + NPK) and straw and NPK (S + NPK). Maize straw and its derived biochar were utilized as organic materials. The soil was fertilized annually prior to planting starting in 2013. The application rate was 9 t·ha−1 for straw and 3 t·ha−1 for biochar. Before planting, urea, calcium superphosphate and potassium chloride were applied once, with application rates of 195 kg·ha−1 N, 90 kg·ha−1 P2O5 and 75 kg·ha−1 K2O. Subsequently, the tractor pulled the ridge (20 cm high, 60 cm wide), and the maize seeds were sown on the ridge at a spacing of 30 cm. The maize grew naturally throughout the season, without any human intervention. The crop was artificially harvested in early October and the maize remnants were removed from the field.
The 13C-labeled root was prepared with reference to Bei et al. (2013). The labeled root was washed with deionized water, dried at 105 °C for 2h and 75 °C for 24 h, and then cut into pieces of about 2 mm and set aside. Biochar was produced by slow pyrolysis of maize root under anaerobic conditions for more complete carbonization. After N2 purge, the 13C-labled root was placed in a muffle furnace, pyrolyzed at 450 °C for 20 min at an elevated rate of 22.5 °C·min−1, and then cooled by N2 purging and stored in a dryer for later use. Total ash content of the biochar was determined to be 40.1%. The basic physical and chemical properties of field treated soil and 13C-labeled root and biochar are shown in Table 1.
2.2 Experimental design
In 2021, six topsoil samples (0–20 cm) that had been treated in the field were chosen for a laboratory incubation experiment. The soil was air-dried and screened (< 2 mm), with fine roots, crop debris, and small stones carefully removed for backup. Before adding 13C-labeled root, its derived biochar and NPK, the soil equivalent to 60 g oven-dried soil weight was adjusted to 60% of the field maximum water holding capacity in a culture tank, and pre-incubated in a dark space at 25 °C for 14 days to stabilize the soil microbial community. After pre-incubation, labeled root, its derived biochar and/or fertilizer were mixed evenly into the soil. New treatments were formed: control (CK), maize root biochar (BC), maize root (R), NPK-only (NPK), maize root biochar with NPK (BC + NPK), and maize root with NPK (R + NPK). Each treatment was repeated three times. The cells were cultured at 25 °C in darkness for 112 days. During the incubation, the soil water content was maintained by weighing method with deionized water every 4–5 days and all the bottles were sealed with a preservative film with pinholes to maintain aerobic conditions. Details of the treatments are shown in Table 2.
2.3 Soil sampling
During the incubation period, destructive sampling was performed on each treatment sample on days 0, 7, 14, 28, 56 and 112. Part of the sample was stored in a refrigerator at − 80 °C for extraction of soil PLFAs content and its 13C abundance value to study microbial community changes, tracking the transfer of organic residue C to different microbial communities, and the remaining soil was air-dried for determination of soil pH, total C, N content and 13C abundance.
2.4 Chemical analysis
Soil moisture was measured by drying at 105 °C until a constant weight was achieved. The soil, maize root, and biochar were subsequently air-dried and ground. Total C, N content and 13C composition of soil and organic residues were analyzed using the EA-IRMS (Elementar Vario PYRO cube coupled with IsoPrime 100 Isotope Ratio Mass Spectrometer) instrument from Germany. Soil pH was measured with a pH ion meter, using soil: water at a 1:5 ratio. The δ13C values for each index were determined using an EA-IRMS (Elementar Vario PYRO cube coupled to IsoPrime 100 Isotope Ratio Mass Spectrometer, Germany).
2.5 Soil PLFA analysis
The extraction and quantification of phospholipid fatty acids were carried out according to the method proposed by Zelles (1999) as modified by Hamer et al. (2007). Briefly, lipids were extracted from 4 g freeze-dried soil with phosphate buffer solution, chloroform and methanol (0.8:1:2, v:v:v), transferred to the organic phase and concentrated under N2. After that, phospholipids were eluted with chloroform, acetone and methanol, and purified from neutral lipids and glycolipids by solid phase extraction column, and concentrated under N2. The purified phospholipids were methylated by methanol-toluene (1:1), 0.2 M KOH–methanol and n-hexane-chloroform (1:1) to derive their respective fatty acid methyl esters (FAMES). The solution was prepared by dissolving the sample in n-hexane and adding methyl nonadecanoate (19:0, Sigma) as an internal standard. The sample was then analyzed and quantified using Agilent 7890A gas chromatography equipped with MIDI peak recognition software (Version 4.5; MIDI Inc., Newark, DE, USA). The δ13C values of PLFA individuals were determined by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) using Thermo Scientific Trace GC Ultra attached to Finnigan MAT 253 IRMS (CuO / Pt Finnigan MAT Mark I combustion interface maintained at 850 °C).
The 23 PLFA individuals were grouped as follows: 14:0, 15:0, 16:0, 17:0 and 18:0 were characterized as non-specific (general) PLFA; i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0 were characterized as Gram-positive bacteria; 16:1ω7c, 17:1ω8c, cy17:0ω7c, 18:1ω7c and cy19:0ω7c were characterized as Gram-negative bacteria; 16:1ω5c, 18:2ω6c and 18:1ω9c were characterized as fungi; 10Me 16:0, 10Me 17:0, 10Me 17:1ω7c, 10Me 18:0 were characterized as actinomycetes (Dungait et al. 2010; Olsson 1999; Pan et al. 2016a, 2016b; Potthast et al. 2010). The sum of all microbial PLFAs was characterized as total microbial biomass.
2.6 Calculations
For δ13C of each PLFA molecule, the mass balance equation is used to correct an additional C atom introduced during the methylation process:
where, n is number of C atoms, nc is the number of C atoms of the underived compound, nd is the number of C atoms of the derivatization reagent (methanol, nd = 1), and ncd is the number of C atoms of the corresponding derivatized compounds. The δ13Cc is the 13C abundance of the underivatized compound, the δ13Cd is the 13C abundance of the derivatization reagent (the δ13C value of methanol measured by GC / IRMS is − 29.33‰), and the δ13Ccd is the 13C abundance of the corresponding derivative compound.
The proportion of exogenous C in SOC and PLFAs (Fe) was calculated by the following formula:
where, δ13Ct and δ13C0 represent the δ13C value of the C pool of the root or biochar addition treatment and control soil (no organic materials addition), and δ13Cbiochar or root represents the δ13C value of the corresponding biochar or root.
The amount of root- and biochar-derived labeled C in each PLFA was calculated by formula:
where, CPLFA is the amount of C in each PLFA.
The ratio of exogenous C mineralization (Me) was calculated by the following formula:
where, Wsoil is the weight of soil in each treatment (60 g dry soil), and C (g kg−1) represents the content of SOC of each treatment. Abiochar or root (g) is the amount of initial C of the biochar or root.
2.7 Statistical analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL). The results were expressed as mean and standard deviation of three replicates. The ANOVA and Duncan test were used to compare the minimum significant differences between different sampling times and treatments at the 95% level. The relative abundance of individual PLFAs in total PLFA was calculated, and then normalized to unit variance using principal component analysis (PCA) to describe the soil microbial community structure under different treatments. All charts were drawn using Origin 2023b (Origin Lab Corporation, Northampton, USA).
3 Results
3.1 Mineralization rate of 13C-labeled maize root and biochar
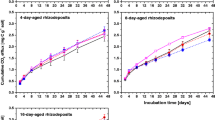
The rate of exogenous C mineralization sharply increased in the first 14 days and then gradually stabilized. The rate of exogenous C mineralization was significantly affected by the type of organic materials. On day 112 of the incubation, the root mineralization rate accounted for approximately 54.9% of the initially added root, whereas the biochar mineralization rate was significantly lower, at about 12.8% of the initially added biochar (p < 0.05, Fig. 1). NPK addition significantly increased the mineralization rate of root-C by 29.7%, but had no obvious effect on biochar-C mineralization (p > 0.05).
3.2 Dynamic changes of soil PLFA and microbial community structure
The application of organic materials and NPK notably augmented the total PLFA content in the early stage (p < 0.05), peaking on the seventh day and then gradually decreasing before stabilizing thereafter (Fig. 2). Throughout the incubation period, the total PLFA content in R and R + NPK treatments was significantly higher than BC and BC + NPK treatments (p < 0.05). Combining organic matter with NPK significantly increased the total PLFA content compared to using organic matter alone (0–28 days), but thereafter there were no significant differences and it was significantly higher compared to the treatments without organic matter (CK and NPK, p < 0.05). The variation trend of PLFA content in microbial community was similar to that of total PLFA content (Table. S1). Throughout the incubation period, the microbial community composition did not change significantly (p > 0.05) in the BC treatment compared to the CK treatment. However, the proportion of fungi and Gram-negative bacteria increased significantly in R treatment (p < 0.05), while actinomycetes and positive bacteria decreased. Compared to the NPK-free treatments, adding NPK significantly enhanced the proportion of bacterial PLFAs while reducing the proportion of fungal PLFAs (p > 0.05). Notably, the proportion of fungal PLFAs was significantly higher in root-added treatments than in biochar-added treatments, while the scenario for actinomycete PLFAs was reversed (Fig. S1).
Sum of PLFAs under different treatments over 112 d incubation (nmol g−1 dry soil). CK represents the control treatment (without organic materials and chemical fertilizer addition). NPK represents the addition of chemical fertilizer. R and BC represent root and biochar, respectively. The error bars represent the standard deviation of the mean (n = 3)
Principal component analysis (PCA) was created to illustrate the response of microbial communities to maize root, its derived biochar and fertilizer in different incubation periods (Fig. 3). The score plot (Fig. 3a) showed that the scores of all treatments in PC1 gradually increased with the prolongation of incubation time. The PLFA profiles of CK and BC treatments remained stable throughout the entire incubation period, whereas the community structure in other treatments underwent significant alterations during this period. The scores of biochar-amended treatments (BC and BC + NPK) on PC2 were significantly higher than those of root-amended treatments (R and R + NPK) and the scores of NPK addition treatments (NPK, BC + NPK and R + NPK) on PC1 were significantly higher than those of NPK-free treatments (CK, BC and R). The PLFAs loading plot (Fig. 3b) results identified 18:2ω6c, a15:0, and a17:0 as key explanatory variables for microbial alterations in R + NPK treatment, serving as indicators of fungi and Gram-positive bacteria. Similarly, PLFAs i15:0, 10Me 16:0 and 10Me 18:0 were pinpointed as indicators of Gram-positive bacteria and actinomycete, elucidating the observed microbial community shifts in CK and BC treatments. Cy17:0ω7c, cy19:0ω7c (indicators of Gram-negative bacteria), 17:0 10-methyl, 17:1ω7c 10-methyl (indicators of actinomycetes), i16:0 and i17:0 (indicators of Gram-positive bacteria) served to elucidate the primary microbial community alterations in NPK and BC + NPK treatments. The microbial community alterations in the R treatment were predominantly characterized by fungi (16:1ω5c, 18:1ω9c, 18:2ω6c), Gram-negative bacteria (16:1ω7c, 17:1ω8c), and Gram-positive bacteria (i14:0).
Principal component analysis (PCA) based on the relative proportion of individual PLFAs for different treatments. The score plot (a) shows the community structure changes over time in different treatments. Different colors represent different treatments and different symbols represent the sampling times. The loadings plot (b) shows the shift in dominant PLFAs
3.3 Microbial uptake of 13C-labeled root and biochar
The uptake of exogenous C by microorganisms was significantly influenced by various exogenous organic materials and NPK. Root- and biochar-C were rapidly assimilated by microorganisms during the incubation period, and the amount of assimilation decreased gradually over time with significant differences (p < 0.05, Table S2). Microbial utilization of root-C was greater compared to biochar-C, with total 13C-PLFA derived from R treatment averaging 1970 ng·g−1 and BC treatment averaging 14.7 ng·g−1. The assimilation of exogenous C by Gram-positive bacteria was at the highest-level during incubation (Fig.S2). In contrast, the assimilation of root-C by the fungi was at a high level on day 7 but then declined rapidly. The addition of NPK enhanced the microbial assimilation towards biochar-C, unlike the application of root alongside fertilizer, which only increased the microbial assimilation towards root-C on day 7 of the incubation period, but significantly reduced it for the remainder of the sampling duration.
Based on the contribution of exogenous C to the PLFAs of distinct microbial communities (Fig. 4), there were notable differences in the utilization of exogenous organic materials by microbial communities. In addition to the higher contribution of exogenous C to the PLFAs of Gram-negative bacteria in BC treatment on the 7th day, the fungal community in the other treatments was the most active microbial population using exogenous organic materials. The contribution of exogenous C to the PLFAs of fungi surpassed that of Gram-negative and Gram-positive bacteria, with actinomycete PLFAs displaying the lowest contribution. Notably, NPK addition significantly increased the proportion of exogenous C incorporated into the PLFA of the microbial community in the biochar-amended treatments, whereas the opposite trend was observed in the root-amended treatments except for the proportion of exogenous C incorporated into the fungal PLFA, which was only slightly elevated in the early stages (0–28 day).
Root- and biochar-C incorporation into microbial community PLFAs, expressed as a percentage of 13C-PLFA synthesized by each community to the PLFAs of distinct microbial communities. a–d represent BC, BC + NPK, R and R + NPK treatments, respectively. The error bars represent the standard deviation of the mean (n = 3)
3.4 Linking 13C-PLFA partitioning dynamics in microbial communities to exogenous C decomposition
In each treatment, microorganisms showed a heterogeneous distribution of synthesized 13C-PLFA among various communities and individual PLFA, reflecting varied patterns of microbial utilization of root- and biochar-C. Over the incubation duration, the average synthesis of general PLFA accounts for about 18.6–20.9% of the total 13C-PLFA (Table S2). The distribution ratio of 13C in Gram-positive bacteria was increased in each treatment. In BC treatment, the relative content of Gram-negative bacterial 13C-PLFA dropped markedly from 37% at the onset to 19% by the end of incubation. NPK application increased the distribution of 13C in fungi during the early stages of incubation but significantly decreased it at the end. During the incubation period, the distribution of exogenous C across the 22 individual PLFAs showed that the partitioning of 13C between the general and actinomycetes PLFA remained basically stable. The proportion of 13C incorporated into PLFA 16:0 was always at a high level during incubation, while that incorporated into actinomycete PLFA was always the lowest. On the seventh day of incubation, root-C was heavily incorporated into PLFA 18:2ω6c (fungi) and 16:0 (general), followed by a15:0, i15:0, a16:0, 16:1ω7c, cy17:0ω7c and 18:1ω9c. During the incubation, the allocation of root-C decreased in fungal community and gradually increased in bacterial community while the distribution of biochar-C was more stable (mainly in Gram-positive bacterial PLFA). The results of the regression analysis indicated a significant correlation between the root mineralization rate and the dynamics of the relative content of 13C-PLFA derived from root-C (13C-PLFA/TPLFAs, 13C-general (%), 13C-Gram-positive bacteria (%) and 13C-fungal (%)) (p < 0.01). Conversely, the biochar mineralization rate showed a significant increase with the increase in the relative content of biochar-derived 13C-PLFAs [13C-Gram-negative bacteria (%) and 13C-actinomycetes (%)] (p < 0.01). There was no linear relationship between the rate of exogenous C mineralization rate and Gram-negative bacteria (Figs. 5, 6).
4 Discussion
4.1 Effects of root and its derived biochar on soil microorganisms
In this study, we analyzed the total PLFA content across different treatments (Fig. 2). Aside from CK treatment, all treatments saw their PLFA content peak on the 7th day, which is in accordance with prior research showing that the PLFA content peaks within 7 days (Bai et al. 2016). During the incubation period, the soil amended with maize root exhibited a significantly higher PLFA content than biochar-amended soil (p < 0.05), which was attributed to the higher level of labile C content of the root provided more substrate for microbial growth. Whereas, there was no significant effect of biochar on PLFA content except for an increase on day 7. This may be due to the rapid mineralization of a small fraction of labile compounds in biochar within a few days (Smith et al. 2010), which can provide some of the energy and nutrients to the microorganisms (Bowen 2006; Farrell et al. 2013), yet the remaining fraction (about 97% of biochar-C) is the recalcitrant C that is challenging for microbial utilization (Wang et al. 2015a). Liu et al. (2024) has reported no direct effect of biochar application on microbial abundance. Notably, throughout the entire incubation period, the BC treatment displayed a significantly higher total PLFA content than the CK treatment (p < 0.05). We suggest that possibly because the long-term application of biochar (for up to 8 years) elevated organic C levels and enhanced nutrient retention and microbial accessibility on particular surfaces (Lehmann et al. 2011). Additionally, multi-year application of biochar might offer increased attachment sites for microorganisms (Awad et al. 2018; Dai et al. 2021), thereby accelerating microbial colonization (Singh et al. 2022).
PCA analysis revealed that there were no discernible differences in microbial community structures between BC and CK treatments (Fig. 3), echoing the findings of Pan et al. (2016b). Helfrich et al. (2015) concluded that biochar’s quality variation doesn’t sufficiently incite competition in soil microbial communities attuned to diverse organic matter. Meanwhile, Yuan et al. (2023) also noted that the addition of biochar is beneficial to maintain the stability of microbial community. In contrast, root addition significantly altered soil microbial community structure, which is consistent with our hypothesis (1). To be precise, root incorporation led to an increase in the relative abundance of fungal and Gram-negative bacterial PLFAs, while decreasing the relative abundance of Gram-positive bacterial and actinomycete PLFAs relative to the CK and BC treatments throughout the incubation period (Fig. S1). This may be attributed to the fact that fungi and Gram-negative bacteria likely prefer labile C from fresh exogenous residues, while Gram-positive bacteria opt for stable C in organic residues or tap into SOM over plant residues’ endogenous C (Li et al. 2019b; Liu et al. 2019; Zhang et al. 2020). The proportion of actinomycetes in the biochar-amended treatments was significantly higher than that in the root-added treatments, which may be related to the strong adaptation of actinomycetes to recalcitrant C metabolism (Yuan et al. 2023).
This study demonstrated that different microbial community structures were formed in soil amended with distinct organic materials for 8 consecutive years. The complexity of root components and the differing resistance to decomposition properties support different decomposition kinetics and longer decomposition cycles. Differences in metabolic capacity and competition for substrates lead to dynamic changes in microbial community composition in root-amended soil. Concurrently, the longer decomposition cycle ensures a continuous supply of available C for microorganisms, thereby augmenting microbial biomass. In contrast, since the majority of biochar-C is not available to microorganisms (13C-PLFA derived from biochar-C averaged only 14.7 ng·g−1, Table S2), the microorganisms continue to adopt the strategy of mineralizing native SOC, which has a negligible effect on microbial abundance and community structure. Nevertheless, the porous structure and extensive surface area of the biochar facilitated the adsorption of SOC and nutrients, while providing habitats for microorganisms, resulting in a slow yet sustained increase in microbial populations.
4.2 Microbial assimilation of exogenous organic C
The microbial uptake of root-C was observed to be 34–152 times greater than biochar-C (Table S2), which is related to the significant difference in the proportion of labile and resistant C fraction between root and biochar. Consistent with the trend of total PLFA content, 13C-PLFA content peaked on the 7th day, indicating the rapid assimilation of labile C by microorganisms. As the proportion of resistant components in organic materials increases with time (Abiven et al. 2005), the microorganisms’ utilization of substrates shifts from labile C to resistant C (Xu et al. 2020), and the assimilation of exogenous C gradually decreased. While some studies have suggested that microbial accessibility might control organic C turnover (Dungait et al. 2012; Han et al. 2016; Schmidt et al. 2011), our study demonstrates that the recalcitrance of organic C remains a challenge for microbial utilization, especially given the significant disparities in the physical and chemical attributes of biochar and its precursors (Abiven et al. 2005; Puget and Drinkwater 2001).
Different microbial communities exhibit varied patterns in the assimilation of organic materials. The contribution of both root- and biochar-C in fungal PLFA was higher than the other microbial communities (Fig. 4), suggesting that fungi use exogenous organic C more efficiently to build their own biomass, which is in line with our hypothesis (3). This is related to the secretion of extracellular enzymes by fungi, which give them a high capacity to degrade complex compounds (Genre et al. 2020; Yang et al. 2022). Additionally, fungi can adopt saprophytic nutritional strategies (Nilsson et al. 2018) and absorb nutrients at the soil-residue interface through hyphae (Acosta‐Martínez et al. 2008), bolstering their competition for exogenous organic C.
The assimilation of root-C was initially dominated by fungi and bacteria, but the dominant position of fungi gradually decreased with the consumption of liable C and root-C was gradually enriched in bacterial PLFAs due to the higher biomass (Table S2 and Fig. 5). In contrast, no fungal dominance of biochar-C assimilation was observed in the early stages, which may be due to the fungal preference for lignin C over pyrolytic C (Santos et al. 2012). This finding also aligns with the results of Ippolito et al. (2014), indicating that the labile C substrate provided by biochar may be more conducive to the growth of fast-growing bacteria than fungi. Notably, the assimilation of exogenous C by Gram-positive bacteria was basically at the highest level in each treatment due to its ability to decompose recalcitrant C (Kramer and Gleixner 2008; Santos et al. 2012). The rapid decrease in the distribution of 13C in Gram-negative bacterial PLFAs (especially in BC treatment) may highlight the sensitivity of Gram-negative bacteria to rapid C sources. The assimilation of exogenous C by actinomycetes was always the lowest, which may be related to the lower biomass and the physiological adaptability of actinomycetes to the degradation of C-rich resistant substances (O’Neill et al. 2009). Our study suggests that fungi effectively assimilate exogenous C, credited to their proficient degradation capability, whereas Gram-positive bacteria become the main actor of exogenous C assimilation due to their high biomass and the ability to decompose recalcitrant C.
4.3 The effect of NPK
In this study, we observed a swift increase in microbial biomass shortly after NPK addition, but this promotive effect diminished as time progressed (Fig. 2). Furthermore, the PLFA content of the treatments with the same organic material had no significant difference regardless of NPK addition, suggesting that NPK application may only have a short-term positive effect on microbial biomass. This was further supported by the fact that there was no significant difference in PLFA content between NPK and CK treatments in the incubation’s later stage. Moreover, NPK addition significantly altered the microbial community composition during incubation (Fig. S1). Research has shown that applying N influences bacterial and fungal diversity, resulting in instability of the microbial community and pronounced effects on the composition of soil microbes (Carrara et al. 2021; Ibrahim et al. 2020; Li et al. 2020; Pang et al. 2022). In the NPK-applied groups, fungal PLFAs decreased notably, while bacterial PLFAs saw a significant rise compared to the NPK-free groups, reflecting differential responses of bacteria and fungi to inorganic fertilizer (Ge et al. 2016). In the same vein, Strickland and Rousk (2010) have shown that introducing N shifts communities from fungal-dominated to bacterial-dominated. Totally, the enhancement of microbial biomass by NPK is short-term, whereas the effect on microbial community structure is long-lasting.
The results demonstrated that in the case of NPK addition, the microbial community had different modes of utilization of two exogenous C (Table.S2 and Fig.S2). Microbial uptake of biochar-C significantly increased after fertilization, while the uptake of root-C only increased on the 7th day of incubation, which is not in accordance with our hypothesis (2). Several factors could explain this observation. Firstly, we suggest that NPK addition provided the microorganisms with generous inorganic nutrients for growth and development, thereby simultaneously enhancing their uptake capability for both root-C and native SOC. Yet, as the labile C in root is consumed, the fraction of resistant C rises, which makes microorganisms change the strategy of using root-C to the utilization of SOC. It has been reported that microorganisms prefer to use SOC with high C content to maintain their internal element balance after N application (Chen et al. 2020). The microbial uptake of native SOC amplifies, consequently diminishing the assimilation ratio of root-C (Moore-Kucera and Dick 2008). Secondly, there is no doubt that the soil perennially amended by maize residues contains more incompletely decomposed plant residue-derived compounds, which provide more alternative C sources for microorganisms. In contrast, with perennial application of biochar, the depletion of labile C in soil and fewer C sources for microorganisms to choose from make the microbial community adaptive to the utilization of recalcitrant C. The NPK addition provides energy for the microbial community to decompose recalcitrant C and thus increases the absorption of biochar-C.
4.4 Linking C process to microbial community dynamics and NPK
Due to the significant differences in the labile C fractions, the mineralization rate of root was considerably higher than that of biochar. As the labile C content decreases, microbial utilization of exogenous C becomes more difficult, so we observed a gradual slowing down of mineralization and a subsequent decrease in the content of total PLFA and 13C-PLFA. NPK addition significantly increased the mineralization rate of root but not that of biochar, since NPK can provide nutrients and power for microorganisms to support further decomposition of root composed of biopolymers with varying degrees of persistence, but cannot overcome the extreme recalcitrance of biochar. Unexpectedly, the mineralization rate of biochar in this study was 12%, which is somewhat high. Wang et al. (2015a) estimated that the labile C pool was about 3% of biochar. It may be because the biochar used in this study was prepared by slow pyrolysis and short residence time, so the proportion of liable C in biochar was higher. Studies have shown that slow pyrolysis leads to a more complete carbonization process favoring the formation of carbonaceous biochar (Huang et al. 2013; Keiluweit et al. 2010), while shorter residence time usually results in higher content of labile C (Zornoza et al. 2016).
There is a significant opposite correlation between the root mineralization rate and the allocation of root-C in fungal and Gram-positive bacterial communities (Fig. 6), representing the shift in the contribution of microbial communities during root decomposition. Since maize root contains a large amount of cellulose and hemicellulose and fungi are increased as the main participants in cellulose decomposition, there was a large allocation of root-C to the saprophytic fungal PLFA in the initial stage of incubation (particularly in 18:2ω6c, Fig. 5). Some studies have shown that saprophytic fungi dominate the decomposition of newly added residues (De Deyn et al. 2011; Theuerl and Buscot 2010) and then the filamentous hyphae of fungi promote subsequent bacterial growth by breaking down complex substrates into readily available compounds (Bai et al. 2016). Due to the preference of Gram-positive bacteria for recalcitrant C, they become the main decomposer after fungi. In contrast, the mineralization rate of biochar increased significantly with higher allocation of biochar-C to Gram-positive bacteria and actinomycetes. Farrell et al. (2013) reported that Gram-positive bacteria can utilize the bioavailable C fraction in biochar. Furthermore, Gram-positive bacteria and actinomycetes are effective in mineralizing recalcitrant C (Fontaine et al. 2011; Waldrop and Firestone 2004) due to their extracellular enzymes degrading resistant compounds more readily (Brant et al. 2006).
NPK application provides sufficient nutrients for microbial proliferation in the short term, increasing the decomposition of exogenous C and SOC. However, as the proportion of recalcitrant C in root increases during mineralization, microorganisms gradually shift to excavating native SOC and this may create uncertainty about C storage in soil. In contrast to the abundance of bioavailable C fractions in plant residue-amended soil, which is lower in soil with perennial biochar application. Moreover, due to the low biomass, the uptake of native SOC by microorganisms is quite limited. We suggest that the scarcity of bioavailable C fractions in biochar and the soil amended with biochar over time is the fundamental reason why biochar can maintain the stability of microbial communities and steadily increase soil C sequestration. These results provide new insights into understanding microbial community dynamics and fertilizer effects on the decomposition of root, biochar and native SOC.
5 Conclusions
This study highlighted the response of microbial community structure to newly added organic materials in soils amended with diverse organic matter for 8 consecutive years. The differential soil PLFA index stemmed chiefly from the contrasting chemical stability of C and the addition of NPK. Maize root mineralisation was significantly associated with fungi and Gram-positive bacteria, whereas biochar mineralisation was significantly associated with actinomycetes and Gram-positive bacteria. NPK addition increased the mineralization of exogenous C and native SOC by increasing the proportion of bacterial community. Limited by the bioavailable C fractions in soil, biochar is more effective than root at buffering drastic changes caused by NPK, promoting a balanced state of microbial communities, and minimizing disturbance to native SOC. This promises the soil C pool to increase steadily over time. Future studies ought to incorporate both 13C-DNA-SIP and 13C-RNA-SIP methodologies in extended field trials to pinpoint microbial species that exploit plant residues or biochar. The relationship between changes in native SOC and microbial community dynamics needs to be further clarified. Additionally, it is crucial to analyze biochar’s substitution impact on its precursors, with a focus on C capture and emission reduction.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol Fertil Soils 42:119–128. https://doi.org/10.1007/s00374-005-0006-0
Acosta-Martínez V, Dowd SE, Sun Y, Allen VGJSB (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770. https://doi.org/10.1016/J.SOILBIO.2008.07.022
Andresen LC, Dungait JAJ, Bol R, Selsted MB, Ambus P, Michelsen A (2014) Bacteria and fungi respond differently to multifactorial climate change in a temperate heathland, traced with 13C-glycine and FACE CO2. PLoS One 9:e85070. https://doi.org/10.1371/journal.pone.0085070
Arcand MM, Helgason BL, Lemke RL (2016) Microbial crop residue decomposition dynamics in organic and conventionally managed soils. Appl Soil Ecol 107:347–359. https://doi.org/10.1016/j.apsoil.2016.07.001
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. https://doi.org/10.1007/s11104-010-0464-5
Awad YM, Ok YS, Abrigata J, Beiyuan JZ, Beckers F, Tsang DCW, Rinklebe J (2018) Pine sawdust biomass and biochars at different pyrolysis temperatures change soil redox processes. Sci Total Environ 625:147–154. https://doi.org/10.1016/j.scitotenv.2017.12.194
Azeem M, Sun D, Crowley DE, Hayat R, Hussain Q, Ali A, Tahir MI, Jeyasundar PGSA, Rinklebe J, Zhang Z (2020) Crop types have stronger effects on soil microbial communities and functionalities than biochar or fertilizer during two cycles of legume-cereal rotations of dry land. Sci Total Environ 715:136958. https://doi.org/10.1016/j.scitotenv.2020.136958
Bai Z, Liang C, Bode S, Huygens D, Boeckx P (2016) Phospholipid 13C stable isotopic probing during decomposition of wheat residues. Appl Soil Ecol 98:65–74. https://doi.org/10.1016/j.apsoil.2015.09.009
Bei Q, Liu G, Tang H, Cadisch G, Rasche F, Xie Z (2013) Heterotrophic and phototrophic 15N2 fixation and distribution of fixed 15N in a flooded rice–soil system. Soil Biol Biochem 59:25–31. https://doi.org/10.1016/j.soilbio.2013.01.008
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211. https://doi.org/10.1016/j.soilbio.2013.08.024
Bossio DA, Cook-Patton SC, Ellis PW, Fargione JE, Sanderman J, Smith P, Wood SU, Zomer RJ, Von Unger M, Emmer IM, Griscom BW (2020) The role of soil carbon in natural climate solutions. Nat Sustain 3:391–398. https://doi.org/10.1038/s41893-020-0491-z
Bowen SR (2006) Biologically relevant characteristics of dissolved organic carbon (DOC) from soil. Ph.D. thesis, Univ. of Stirling, Stirling, U. K
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232. https://doi.org/10.1016/j.soilbio.2006.01.022
Carrara JE, Walter CA, Freedman ZB, Hostetler AN, Hawkins JS, Fernandez IJ, Brzostek ER (2021) Differences in microbial community response to nitrogen fertilization result in unique enzyme shifts between arbuscular and ectomycorrhizal-dominated soils. Global Change Biol 27:2049–2060. https://doi.org/10.1111/gcb.15523
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33. https://doi.org/10.1016/j.scitotenv.2016.08.190
Chen W, Meng J, Han X, Lan Y, Zhang W (2019) Past, present, and future of biochar. Biochar 1:75–87. https://doi.org/10.1007/s42773-019-00008-3
Chen Q, Niu B, Hu Y, Wang J, Lei T, Xu-Ri ZJ, Xi C, Zhang G (2020) Multilevel nitrogen additions alter chemical composition and turnover of the labile fraction soil organic matter via effects on vegetation and microorganisms. J Geophys Res-Biogeo 125:e2019JG005316. https://doi.org/10.1029/2019JG005316
Dai Z, Xiong X, Zhu H, Xu H, Leng P, Li J, Tang C, Xu J (2021) Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3:239–254. https://doi.org/10.1007/s42773-021-00099-x
Dangi S, Gao S, Duan Y, Wang D (2020) Soil microbial community structure affected by biochar and fertilizer sources. Appl Soil Ecol 150:103452. https://doi.org/10.1016/j.apsoil.2019.103452
De Deyn GB, Quirk H, Oakley S, Ostle N, Bardgett RD (2011) Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed species-rich grasslands. Biogeosciences 8:1131–1139. https://doi.org/10.5194/bg-8-1131-2011
Dungait JAJ, Kemmitt SJ, Michallon L, Guo S, Wen Q, Brookes PC, Evershed RP (2010) Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur J Soil Sci 62:117–126. https://doi.org/10.1111/j.1365-2389.2010.01321.x
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biol 18:1781–1796. https://doi.org/10.1111/j.1365-2486.2012.02665.x
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–297. https://doi.org/10.1016/j.scitotenv.2013.03.090
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.1890/05-1839
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Ge T, Li B, Zhu Z, Hu Y, Yuan H, Dorodnikov M, Jones DL, Wu J, Kuzyakov Y (2016) Rice rhizodeposition and its utilization by microbial groups depends on N fertilization. Biol Fertil Soils 53:37–48. https://doi.org/10.1007/s00374-016-1155-z
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649-660. https://doi.org/10.1038/s41579-020-0402-3
Gomez JD, Denef K, Stewart CE, Zheng J, Cotrufo MF (2014) Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur J Soil Sci 65:28–39. https://doi.org/10.1111/ejss.12097
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agr Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Hamer U, Unger M, Makeschin F (2007) Impact of air-drying and rewetting on PLFA profiles of soil microbial communities. J Plant Nutr Soil Sci 170:259–264. https://doi.org/10.1002/jpln.200625001
Han L, Sun K, Jin J, Xing B (2016) Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol Biochem 94:107–121. https://doi.org/10.1016/j.soilbio.2015.11.023
Helfrich M, Ludwig B, Thoms C, Gleixner G, Flessa H (2015) The role of soil fungi and bacteria in plant litter decomposition and macroaggregate formation determined using phospholipid fatty acids. Appl Soil Ecol 96:261–264. https://doi.org/10.1016/j.apsoil.2015.08.023
Huang Y, Kudo S, Masek O, Norinaga K, Hayashi J-I (2013) Simultaneous maximization of the char yield and volatility of oil from biomass pyrolysis. Energ Fuel 27:247–254. https://doi.org/10.1021/ef301366x
Ibrahim MM, Tong CX, Hu K, Zhou BQ, Xing SH, Mao YL (2020) Biochar-fertilizer interaction modifies N-sorption, enzyme activities and microbial functional abundance regulating nitrogen retention in rhizosphere soil. Sci Total Environ 739:140065. https://doi.org/10.1016/j.scitotenv.2020.140065
Ippolito JA, Stromberger ME, Lentz RD, Dungan RS (2014) Hardwood biochar influences calcareous soil physicochemical and microbiological status. J Environ Qual 43:681–689. https://doi.org/10.2134/jeq2013.08.0324
Jones DL, Rousk J, Edwards-Jones G, Deluca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124. https://doi.org/10.1016/j.soilbio.2011.10.012
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Khan S, Waqas M, Ding F, Shamshad I, Arp HPH, Li G (2015) The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J Hazard Mater 300:243–253. https://doi.org/10.1016/j.jhazmat.2015.06.050
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 40:425–433. https://doi.org/10.1016/j.soilbio.2007.09.016
Kramer S, Dibbern D, Moll J, Huenninghaus M, Koller R, Krueger D, Marhan S, Urich T, Wubet T, Bonkowski M, Buscot F, Lueders T, Kandeler E (2016) Resource partitioning between bacteria, fungi, and protists in the detritusphere of an agricultural soil. Front Microbiol 7:1524. https://doi.org/10.3389/fmicb.2016.01524
Kuzyakov Y (2010) Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Li F, Chen L, Zhang J, Yin J, Huang S (2017) Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front Microbiol 8:187. https://doi.org/10.3389/fmicb.2017.00187
Li D, Li Z, Zhao B, Zhang J (2019a) Relationship between the chemical structure of straw and composition of main microbial groups during the decomposition of wheat and maize straws as affected by soil texture. Biol Fertil Soils 56:11–24. https://doi.org/10.1007/s00374-019-01397-0
Li Z, Song M, Li D, Ma L, Zhao B, Zhang J (2019b) Effect of long-term fertilization on decomposition of crop residues and their incorporation into microbial communities of 6-year stored soils. Biol Fertil Soils 56:25–37. https://doi.org/10.1007/s00374-019-01398-z
Li SL, Wang S, Fan MC, Wu Y, Shangguan ZP (2020) Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Till Res 196:104437. https://doi.org/10.1016/j.still.2019.104437
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Liu Z, Zhu M, Wang J, Liu X, Guo W, Zheng J, Bian R, Wang G, Zhang X, Cheng K, Liu X, Li L, Pan G (2019) The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. GCB Bioenergy 11:1408–1420. https://doi.org/10.1111/gcbb.12644
Liu Q, Wu C, Wei L, Wang S, Deng Y, Ling W, Xiang W, Kuzyakov Y, Zhu Z, Ge T (2024) Microbial mechanisms of organic matter mineralization induced by straw in biochar-amended paddy soil. Biochar 6:18. https://doi.org/10.1007/s42773-024-00312-7
Mitchell PJ, Simpson AJ, Soong R, Simpson MJ (2015) Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem 81:244–254. https://doi.org/10.1016/j.soilbio.2014.11.017
Moore-Kucera J, Dick RP (2008) Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biol Biochem 40:2485–2493. https://doi.org/10.1016/J.SOILBIO.2008.06.002
Müller K, Marhan S, Kandeler E, Poll C (2017) Carbon flow from litter through soil microorganisms: from incorporation rates to mean residence times in bacteria and fungi. Soil Biol Biochem 115:187–196. https://doi.org/10.1016/j.soilbio.2017.08.017
Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L (2018) Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol 17:95–109. https://doi.org/10.1038/s41579-018-0116-y
Olsson P A (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310. https://doi.org/10.1111/j.1574-6941.1999.tb00621.x
Oneill B, Grossman JM, Grossman JM, Tsai M-T, Gomes J, Lehmann J, Peterson J, Neves EG, Thies JE (2009) Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58:23–35. https://doi.org/10.1007/s00248-009-9515-y
Pan F, Li Y, Chapman SJ, Yao H (2016a) Effect of rice straw application on microbial community and activity in paddy soil under different water status. Environ Sci Pollut R 23:5941–5948. https://doi.org/10.1007/s11356-015-5832-5
Pan FX, Li YY, Chapman SJ, Khan S, Yao HY (2016b) Microbial utilization of rice straw and its derived biochar in a paddy soil. Sci Total Environ 559:15–23. https://doi.org/10.1016/j.scitotenv.2016.03.122
Pang ZQ, Huang JW, Fallah N, Lin WX, Yuan ZN, Hu CH (2022) Combining N fertilization with biochar affects root-shoot growth, rhizosphere soil properties and bacterial communities under sugarcane monocropping. Ind Crop Prod 182:114899. https://doi.org/10.1016/j.indcrop.2022.114899
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Global Change Biol 19:1562–1571. https://doi.org/10.1111/gcb.12140
Potthast K, Hamer U, Makeschin F (2010) Impact of litter quality on mineralization processes in managed and abandoned pasture soils in Southern Ecuador. Soil Biol Biochem 42:56–64. https://doi.org/10.1016/J.SOILBIO.2009.09.025
Powlson DS, Whitmore AP, Goulding KWT (2011) Soil carbon sequestration to mitigate climate change: a critical re-examination to identify the true and the false. Eur J Soil Sci 62:42–55. https://doi.org/10.1111/j.1365-2389.2010.01342.x
Puget P, Drinkwater LE (2001) Short-term dynamics of root- and shoot-derived carbon from a leguminous green manure. Soil Sci Soc Am J 65:771–779. https://doi.org/10.2136/sssaj2001.653771x
Santos F, Torn MS, Bird JA (2012) Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol Biochem 51:115–124. https://doi.org/10.1016/j.soilbio.2012.04.005
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. https://doi.org/10.1890/06-0219
Schmatz R, Recous S, Aita C, Tahir MM, Schu AL, Chaves B, Giacomini SJ (2016) Crop residue quality and soil type influence the priming effect but not the fate of crop residue C. Plant Soil 414:229–245. https://doi.org/10.1007/s11104-016-3120-x
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Singh H, Northup BK, Rice CW, Prasad PVV (2022) Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4:8. https://doi.org/10.1007/s42773-022-00138-1
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347. https://doi.org/10.1016/j.soilbio.2010.09.013
Song DL, Chen L, Zhang S, Zheng Q, Ullah SM, Zhou W, Wang XB (2020) Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2-year field trial. Eur J Soil Biol 99:103212. https://doi.org/10.1016/j.ejsobi.2020.103212
Stewart CE, Zheng JY, Botte J, Cotrufo MF (2013) Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. GCB Bioenergy 5:153–164. https://doi.org/10.1111/gcbb.12001
Strickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils—methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. https://doi.org/10.1016/J.SOILBIO.2010.05.007
Terrer C, Phillips RP, Hungate BA, Rosende J, Pett-Ridge J, Craig ME, Van Groenigen KJ, Keenan TF, Sulman BN, Stocker BD, Reich PB, Pellegrini AFA, Pendall E, Zhang H, Evans RD, Carrillo Y, Fisher JB, Van Sundert K, Vicca S, Jackson RB (2021) A trade-off between plant and soil carbon storage under elevated CO2. Nature 591:599. https://doi.org/10.1038/s41586-021-03306-8
Theuerl S, Buscot F (2010) Laccases: toward disentangling their diversity and functions in relation to soil organic matter cycling. Biol Fertil Soils 46:215–225. https://doi.org/10.1007/s00374-010-0440-5
Tian J, Wang J, Dippold M, Gao Y, Blagodatskaya E, Kuzyakov Y (2016) Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci Total Environ 556:89–97. https://doi.org/10.1016/j.scitotenv.2016.03.010
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284. https://doi.org/10.1007/s00442-003-1419-9
Wang J, Xiong Z, Kuzyakov Y (2015a) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523. https://doi.org/10.1111/gcbb.12266
Wang X, Song D, Liang G, Zhang Q, Ai C, Zhou W (2015b) Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl Soil Ecol 96:265–272. https://doi.org/10.1016/j.apsoil.2015.08.018
Wang Y, Zhao X, Guo Z, Jia Z, Wang S, Ding K (2018) Response of soil microbes to a reduction in phosphorus fertilizer in rice-wheat rotation paddy soils with varying soil P levels. Soil Till Res 181:127–135. https://doi.org/10.1016/j.still.2018.04.005
Waqas M, Khan S, Qing H, Reid BJ, Chao C (2014) The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 105:53–61. https://doi.org/10.1016/j.chemosphere.2013.11.064
Watzinger A, Feichtmair S, Kitzler B, Zehetner F, Kloss S, Wimmer B, Zechmeister-Boltenstern S, Soja G (2013) Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: results from a short-term incubation and pot experiment. Eur J Soil Sci 65:40–51. https://doi.org/10.1111/ejss.12100
Xu Y, Sun L, Lal R, Bol R, Wang Y, Gao X, Ding F, Liang S, Li S, Wang J (2020) Microbial assimilation dynamics differs but total mineralization from added root and shoot residues is similar in agricultural Alfisols. Soil Biol Biochem 148:107901. https://doi.org/10.1016/j.soilbio.2020.107901
Yang CD, Liu JJ, Ying HC, Lu SG (2022) Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil Till Res 224:105505. https://doi.org/10.1016/j.still.2022.105505
Yuan MS, Zhu XZ, Sun HR, Song JR, Li C, Shen YF, Li SQ (2023) The addition of biochar and nitrogen alters the microbial community and their cooccurrence network by affecting soil properties. Chemosphere 312:137101. https://doi.org/10.1016/j.chemosphere.2022.137101
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129. https://doi.org/10.1007/s003740050533
Zhang P, Scheu S, Li B, Lin GH, Zhao JY, Wu JH (2020) Litter C transformations of invasive Spartina alterniflora affected by litter type and soil source. Biol Fertil Soils 56:369–379. https://doi.org/10.1007/s00374-019-01429-9
Zhang MY, Zhang L, Riaz M, Xia H, Jiang CC (2021) Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J Clean Prod 292:126062. https://doi.org/10.1016/j.jclepro.2021.126062
Zheng JF, Chen JH, Pan GX, Liu XY, Zhang XH, Li LQ, Sian RJ, Cheng K, Jinweizheng Z (2016) Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci Total Environ 571:206–217. https://doi.org/10.1016/j.scitotenv.2016.07.135
Zornoza R, Moreno-Barriga F, Acosta JA, Muñoz MA, Faz A (2016) Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 144:122–130. https://doi.org/10.1016/j.chemosphere.2015.08.046
Acknowledgements
We are very grateful to Peiyu Luo, Xue Li and Zhiyu Sun for their insightful suggestions and comments on this paper.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No.31972511).
Author information
Authors and Affiliations
Contributions
Xiaori Han conceived, designed and financially supported the study; Zonglin Lu analyzed the data and wrote the paper, and conducted the analytical work with Tong Lu, Junmei Shi and Hangming Guo; Na Li guided and supervised the trial; Kun Chen reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, this manuscript.
Additional information
Handling editor: Jörg Rinklebe
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Z., Lu, T., Shi, J. et al. Short-term microbial community dynamics induced by 13C-labeled maize root, its derived biochar and NPK in long-term amended soil. Biochar 6, 72 (2024). https://doi.org/10.1007/s42773-024-00363-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00363-w