Abstract
Purpose
The aim of the study was to examine the remediation effect of biochar derived from three feedstocks on soil acidification.
Materials and methods
The effects of biochar derived from peanut hull, rice straw and rape straw on soil acidity, chemical and microbial properties, nutrients absorption, and growth of orange seedlings planted in an acidic soil were studied in a greenhouse experiment.
Results and discussion
Soil pH was increased 0.70, 0.92, and 0.63 by peanut hull, rice straw, and rape straw biochars. However, only peanut hull biochar significantly increased plant growth and the biomass of trifoliate orange seedlings. Soil microbial biomass C and basal respiration were increased by peanut hull and rice straw biochar, and the geometric mean of enzyme activities (GMea) were increased by the three biochars, peanut hull biochar result in the highest increase. Rice straw and rape straw biochars had more abundant mineral nutrient, led to greater influence on soil and plant nutrient contents than peanut hull biochar. However, peanut hull biochar resulted in higher plant nutrients accumulation due to the improvement of plant biomass.
Conclusions
Although the three biochars effectively neutralized soil acidity, only peanut hull biochar raised plant growth and the biomass of orange seedlings significantly, and the increase of soil microbial properties and enzyme activity would be the key factors for the improvement of plant growth.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Large areas of acidic Ultisols are found in the citrus orchards of subtropical regions in southern China. Acidification directly influences the development of the microbial biodiversity and activity, the enzyme activity and the nutrient availability of soils, and inhibits the root growth and the water and nutrients absorption of plants, usually causing a reduction in crop yields (Bååth et al. 1995; Lalande et al. 2009; Tang et al. 2003).
Most of the biochar produced from the pyrolysis of crop straw has an alkaline pH (Novak et al. 2009). In less fertile soils, the application of biochar can positively affect both the quality and productivity of the soil (Jeffery et al. 2011) due to its ability to neutralize soil acidity directly (Paz-Ferreiro et al. 2014; Yuan and Xu 2011), contain abundant mineral nutrients (Luo et al. 2014), and improve the textural structure (Atkinson et al. 2010). However, the positive effects depend on the feedstocks used and the pyrolysis temperature (Lehmann 2007; Lehmann and Joseph 2009).
The biochemical properties of soil can be used as indicators of its quality because they are more sensitive to alterations in management than its physical or chemical properties (Paz-Ferreiro et al. 2007; 2009). Biochar can be considered as a valuable soil adjunct that has occasionally been reported to increase the degree of the microbial and enzymatic activity of the soil (Kolb et al. 2009; Paz-Ferreiro et al. 2012; Steinbeiss et al. 2009). The effect of biochar on soil microbial biomass has been shown to depend on biochar and soil type and also affected by amendment rate (Chan et al. 2008b; Durenkamp et al. 2010).
Biochars can have very different properties depending on the feedstock used (Chan and Xu 2009), and feedstock composition has the greatest effect on the characteristics of biochar that affect agricultural productivity, such as cation exchange capacity, nutrient content, bulk density, and surface properties (Gaskin et al. 2008; Novak et al. 2009; Rajkovich et al. 2012). Because of the large variation in the properties of biochars, their effects on the acidity and biochemical properties of the soil and plant growth also vary. In previous studies, the biochars used to improve soil acidity were mostly produced from forest wood, greenwaste, poultry litter or pecan shells (Chan et al. 2008a, b; Novak et al. 2009; Van Zwieten et al. 2010). However, the effects of biochar produced from crop residues on soil acidification have been poorly studied.
The Yiling District of Yichang, Hubei Province in China, is a predominant region of Citrus reticulata production. Most of the soil in the Satsuma orange orchards is acidic, yellowish-brown clay (pH < 5), and large amounts of crop residues result annually from the agricultural practices in Hubei Province. We therefore prepared three common crop residues (peanut hull, rice straw, and rape straw) using a pyrolysis system. The aim of this study was to compare the effects of three biochars on the acidity, chemical and microbial properties of the acidic soil, and to evaluate their effects on the nutrient absorption and growth of trifoliate orange seedlings.
2 Materials and methods
2.1 Materials
Soils were collected from the surface (0–20 cm) of a citrus orchard in the Yiling District of Yichang City, Hubei Province, China (111°33′ N, 30°37′ E; 146 m above sea level). The climate of the region is subtropical monsoon. The soil was highly acidic yellowish-brown clay (Haplic Luvisols) produced by granite weathering. Three types of plant material (peanut hulls, rice straw, and rape straw) were collected locally, dried at 70 °C, and ground to pass through a 1.0-mm sieve. The powder was placed in ceramic crucibles, covered with a fitted lid, and pyrolyzed under oxygen-limited conditions in a muffle furnace; the temperature was increased to 550 °C at a rate of 15 °C/min, and the final temperature was maintained for 4 h. The chemical properties of the soil and biochars are given in Table 1.
2.2 Determination of biochar chemical properties
The pH of biochar was measured in deionized water using a 1:5 (biochar:water) ratio (Gaskin et al. 2008). Total organic carbon was determined using wet dichromate digestion method. The biochar was digested with sulfuric acid/hydrogen peroxide to determine total N, P, and K; digestion with nitric acid/perchloric acid (v/v, 4:1) and an atomic absorption spectrophotometer (Z 2000, HITACHI, Japan) were used to measure total Ca, Mg, Fe, Mn, Cu, and Zn concentrations. The alkaline hydrolysis diffusion method was used to determine the available N in the biochar; the available P was determined using Olsen’s method; and the available K was extracted with 1 mol/L of ammonium acetate and determined using a flame photometer (FP6410, INESA, China). Exchangeable Ca and Mg were extracted with 1 mol/L of ammonium acetate and determined using an atomic absorption spectrophotometer (Z 2000, HITACHI, Japan).
2.3 Experimental design
Biochars derived from three different feedstocks were added to the soil at a rate of 1.5 % (w/w) to obtain the following treatment groups: (i) control, (ii) peanut hull biochar, (iii) rice straw biochar, and (iv) rape straw biochar. Biochar was mixed with 6 kg of soil, and the mixture was then placed into black pots; four replicates per treatment group were incubated. Trifoliate orange (Poncirus trifoliata (L.) Raf.) is the most common citrus root stock in China and were used as the plant material. At budding time, the seedlings were 1-year old and were selected depending on a uniform stem diameter. All of the plants were washed in tap water to remove surface contaminants, followed by transplantation into the black pots (two plants per pot). Thereafter, the plants were placed in a greenhouse and treated with uniform nitrogen, phosphorous, potassium, and microelement fertilizers.
The treatments started at the beginning of April 2013 and were terminated after 206 days when the plants had completed 1 year of vegetative growth. Urea, KH2PO4, KNO3, and MgSO4 7H2O were applied in each treatment at the rates of 300 mg N, 150 mg P2O5, 250 mg K2O, and 25 mg Mg per kg soil, respectively, before seedling transplantation. Microelements were supplemented to each pot, including Fe-EDTA 0.025, MnCl2 H2O 1.81, ZnSO4 7H2O 0.22, CuSO4 5H2O 0.08, and H3BO3 2.86 mg per kg soil. No pesticides or herbicides were applied, because no weeds were expected and no disease or pest symptoms were visible. At the end of the experiment, both soil and plant samples were collected. The soils in the pots were sampled using a small core drill (four cores unified to one composite sample per pot). Soil samples were removed and split into two subsamples for chemical and microbial analysis. One was stored at 4 °C for microbial analysis, and the other was air-dried and stored for chemical and enzymatic activity analysis. The trifoliate orange plants were divided into roots, stems, and leaves. The above-ground biomass (stems and leaves) was collected by severing at the base of the plant stalk. The below-ground biomass was manually separated from the potting soil, and the soil and biochar particles were washed off. All root, stem, and leaf biomasses were weighed separately, dried to a constant weight at 60 °C, weighed, and finely ground with a ball mill for chemical analyses.
2.4 Measurements and observations
2.4.1 Chemical analyses of the soil
Soil samples were air-dried, triturated with a wooden roller to pass through a 1-mm sieve, and stored at room temperature after homogenization. Soil organic carbon was determined using wet dichromate digestion method (Walkley and Black 1934). The soil pH was determined potentiometrically in 1:2.5 soil/distilled water suspensions after shaking. The alkaline hydrolysis diffusion method was used to determine the available N; the available P was determined using Olsen’s method; and the available K was extracted with 1 mol/L of ammonium acetate and determined using a flame photometer (FP6410, INESA, China). Exchangeable Ca and Mg were extracted with 1 mol/L of ammonium acetate and available Fe, Mn, Cu, and Zn were extracted with a diethylene triamine pentaacetic acid (DTPA) extracting agent. Exchangeable Ca and Mg, and available Fe, Mn, Cu, and Zn were determined using an atomic absorption spectrophotometer (Z 2000, HITACHI, Japan).
2.4.2 Microbial analyses of the soil
Microbial biomass C and N of the soil were determined by the chloroform fumigation–extraction method. The difference in the C and N contents of the fumigated and unfumigated extracts was converted to microbial biomass C and N by applying a KC factor of 0.38 (Vance et al. 1987) and a KN factor of 0.54 (Brookes et al. 1985). Soluble C in the potassium sulfate extracts was analyzed using a Multi 2100 soluble C/N analyzer. The basal respiration of the soil was measured using the alkali (1 M NaOH) absorption of the CO2 developed over 24 h, followed by titration of the residual hydroxide using a standardized acid (Isermeyer 1952). The microbial metabolic quotient (qCO2) was calculated as the ratio between the basal respiration rate of the soil and the amount of microbial biomass C (Anderson and Domsch 1993).
2.4.3 Analyses of the enzymatic activity of the soil
The enzyme activities of the soil were assayed in triplicate air-dried samples as described by Guan (1986). Briefly, soil urease activity was determined using urea as the substrate, and the soil mixture was incubated at 37 °C for 24 h; the ammoniacal nitrogen produced was determined by indophenols blue colorimetric method. Invertase activity was determined using sucrose as a substrate and incubation at 37 °C for 24 h, and the produced glucose was measured using the colorimetric method. Catalase activity was determined by measuring the O2 absorbed by KMnO4 after addition of H2O2 to the samples (Rodriguez-Kabana and Truelove 1982). Cellulose activity was assayed according to the modified method of Deng and Tabatabai (1994). The soil sample was incubated for 4 h at 50 °C with 50 mM acetate buffer (pH 5.5) and 2 % carboxymethyl cellulose; the mixture was centrifuged and the supernatant was treated with the Somogyi–Nelson reagent. The solution was centrifuged once as described above before the color measurement of reducing sugars. Soil β-glucosidase activity was assayed according to Eivazi and Tabatabai (1988). The soil sample was incubated for 1 h at 37 °C with modified universal buffer (pH 6.0) and 5 mM p-nitrophenyl β-D-glucoside, and the reaction was stopped by adding 0.5 M calcium chloride and 0.1 M tris-hydroxymethyl aminomethane (pH 12); the amount of p-nitrophenol released from the p-nitrophenyl β-D-glucoside was measured in the filtrate at 412 nm. The geometric mean (a general index to integrate information from variables that possess different units and ranges of variation) of the assayed enzyme activities was calculated for each sample as:
2.4.4 Analysis of plant growth parameters and nutrient content
Plant growth parameters (plant height, stem diameter, number of leaves, and root length) were measured manually using a meter ruler and vernier caliper. The biomass of roots, stems, and leaves were weighed using an analytical balance. The samples were digested with sulfuric acid/hydrogen peroxide to determine total N, P, and K or digested with nitric acid/perchloric acid (v/v, 4:1) and placed in an atomic absorption spectrophotometer (Z 2000, HITACHI, Japan) to measure total Ca, Mg, Fe, Mn, Cu, and Zn concentrations.
2.4.5 Statistical analysis
Unless otherwise noted, the results are given as the means ± standard error (SE). The data were analyzed using an analysis of variance (ANOVA), and the differences between the means were determined by the least significant difference (LSD) test at p ≤ 0.05. The SPSS PASW Statistics 18.0 analytical software package was used for all statistical analyses.
3 Results
3.1 Characteristics of biochar
Chemical analysis showed that peanut hull biochar, rice straw biochar, and rape straw biochar had high pH (10.09, 10.16, and 10.61). Rice straw and rape straw biochar had higher total organic carbon (TOC) and carbon/nitrogen ratio (C/N) than peanut hull biochar. Moreover, rice straw and rape straw biochar had more abundant mineral nutrient contents than peanut hull biochar, except for Cu content. Rice straw and rape straw biochar showed relatively similar chemical properties, while rape straw biochar had higher TOC and C/N and rice straw biochar had higher nutrient contents (Table 1).
3.2 Soil pH and nutrient status
Soil pH was increased 0.70, 0.92, and 0.63 by peanut hull biochar, rice straw biochar, and rape straw biochar, respectively. The soil available N concentration declined after the three biochars application, while the soil available P concentration was increased by rice straw biochar and the soil available K concentration was increased by the three biochars. The soil available Ca and Mg concentrations were not affected by all the three biochars (Table 2), as biochar application decreased the soil available Fe, Mn, Cu, and Zn concentrations with the increased pH (Table 3).
3.3 Soil microbial properties and enzymatic activity
Soil microbial biomass C and basal respiration were increased significantly by using peanut hull and rice straw biochars, particularly peanut hull biochar, but no significant effect was observed for the rape straw biochar utilization, and no significant differences in the microbial biomass N and metabolic quotient (qCO2) were observed (Fig. 1).
Effect of peanut hull biochar, rice straw biochar, and rape straw biochar on microbial biomass C (a), microbial biomass N (b), basal respiration (c), and microbial metabolic quotient (qCO2) (d) of soil. Values represent the means + SE (n = 4). In the bars, the different letter means significant difference (p < 0.05), and the same letter means not significantly different (p > 0.05)
Soil urease activity was significantly increased by the three biochars, particularly peanut hull biochar. In addition, the three biochars markedly increased soil catalase activity. Soil invertase and cellulose activity were also increased by using peanut hull and rice straw biochar compared with the control soils, and peanut hull biochar resulted in more increase than the other two. Soil β-glucosidase activity was markedly decreased by the three biochars. Finally, the geometric mean of enzyme activities (GMea) of the assayed enzyme activities increased 47, 37, and 26 % by peanut hull, rice, and rape straw biochar, respectively (Fig. 2).
Effect of peanut hull biochar, rice straw biochar, and rape straw biochar on urease activity (a), catalase activity (b), invertase activity (c), cellulase activity (d), β-glucosidase activity (e), and the geometric mean of the assayed enzyme activities (GMea) (f) of soil. Values represent the means + SE (n = 4). In the bars, the different letter means significant difference (p < 0.05), and the same letter means not significantly different (p > 0.05)
3.4 Trifoliate orange seedlings growth
The height, stem diameter, leaves number, and root length of the trifoliate orange seedlings were increased significantly by peanut hull biochar, while there were no significant effects of rice and rape straw biochars on the growth of trifoliate orange plants (Table 4). Similarly, the biomass of trifoliate orange roots, stems, and leaves were all increased significantly by peanut hull biochar (Table 5), but the peanut hull biochar did not affect the single leaf weight, which was increased by rice straw and rape straw biochars.
In addition, plant total biomass of trifoliate orange seedlings was positively correlated with soil urease activity, invertase activity, cellulase activity, the geometric mean of the assayed enzyme activities (GMea), and basal respiration. Plant total biomass was negatively correlated with soil β-glucosidase activity (Fig. 3).
3.5 Nutrient concentration and accumulation in tissues of trifoliate orange seedlings
The nutrient concentrations in the plant tissues varied with the biochars derived from different feedstocks. The tissue N or P concentrations were not affected by peanut hull biochar application, while the root P concentration was significantly increased by rice straw and rape straw biochars. The root K concentration was increased by rice straw biochar, as the stem K concentration was increased by the three biochars markedly. The Ca concentrations of root and leaf were significantly increased by rape straw biochar, but the root Mg concentration was decreased by the three biochars (Table 6). The stem Fe concentration was increased, but the leaf Cu concentration was decreased by applications of rice and rape straw biochars. The Mn concentrations of the root, stem, and leaf and the Zn concentration of root were all decreased by three biochars application (Table 7). But Fe and Cu concentrations of Plant tissue were not affected by peanut hull biochar.
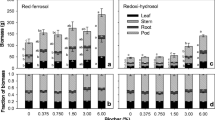
The N, P, K, Ca, and Mg accumulations of whole plant were increased by peanut hull biochar, and the K accumulation was increased by rice straw biochar (Fig. 4). The Mn accumulation of whole plant was significantly declined by all the three biochar, while the Fe, Cu, and Zn accumulation of whole plant were unaffected, and the seedlings treated with peanut hull biochar absorbed more Cu and Zn than the plant treated with rape straw biochar (Fig. 5).
Effect of peanut hull biochar, rice straw biochar, and rape straw biochar on plant N, P, K, Ca, and Mg accumulation in trifoliate orange seedlings. Values represent the means + SE (n = 4). In the bars, the different letter means significant difference (p < 0.05), and the same letter means not significantly different (p > 0.05)
Effect of peanut hull biochar, rice straw biochar, and rape straw biochar on plant Fe, Mn, Cu, and Zn accumulation in trifoliate orange seedlings. Values represent the means + SE (n = 4). In the bars, the different letter means significant difference (p < 0.05), and the same letter means not significantly different (p > 0.05)
4 Discussion
The acid soil pH was increased by all the three biochar materials application (Table 2), which was observed previously (Wang et al. 2009; Yuan and Xu 2011). However, only peanut hull and rice straw biochars increased the soil microbial biomass C and basal respiration which related soil microbial activities, and peanut hull biochar had the greatest influence. The positive effect is in line with the work of Liang et al. (2010) and Paz-Ferreiro et al. (2012). This can be ascribed to that biochar has a porous structure and a large internal surface area and can adsorb soluble organic matter, gasses, and inorganic nutrients, making it a highly suitable habitat for microbial growth and proliferation (Lehmann and Joseph 2009). We therefore summarized that only peanut hull and rice straw biochars improved the soil microbial biomass and activities, which did not resulted from the soil pH increase induced by biochar but should result from other factors, such as biochar porous structure.
Moreover, our study showed that the soil GMea of the assayed enzyme activities was raised by all biochars derived from three crop straws, while the effect of biochar on soil enzyme activity varied with straw of crops (Fig. 2). Our result showed that soil pH was increased by three biochars application; the increase of soil pH would be one factor for the improvement of soil GMea. Acosta-Martinez and Tabatabai (2000) reported that the activities of soil enzymes were positively correlated with soil pH significantly. In addition, soil β-glucosidase activity was decreased by biochar treatment, as Lammirato et al. (2011) reported. The interesting thing was that the soil enzyme activity raised more by the peanut hull biochar than the other two biochars, although the pH increase induced by the rice straw biochar was more than the peanut hull biochar. This can be ascribed to that peanut hull biochar had the greatest influence on soil microbial biomass C and basal respiration. Therefore, we concluded that the effect of biochar on soil enzyme activity may depend on the influence on the pH and microbial properties of soil.
The loss of crop biomass during pyrolysis resulted in high nutrient concentrations in the biochar (Amonette and Joseph 2009). No considerable effect of biochar on soil available N should be attributed to the gaseous nitrogen loss during pyrolysis (Amonette and Joseph 2009). The rice and rape straw biochars resulted in more increase of soil P and K concentrations than peanut hull biochar. This may be ascribed to that the rice and rape straw biochars derived from plant vegetative organs had higher nutrient contents than peanut hull biochar derived from plant reproductive organ (Table 1). In addition, although biochar is rich in microelements, soil available concentrations of Fe, Mn, Cu, and Zn were decreased by three biochars utilization (Table 3), due to the increase of soil pH by the three biochars could potentially decrease the availability of soil Fe, Mn, Cu, and Zn (Bolan et al. 2003). Therefore, we concluded that soil available nutrient concentrations were influenced by biochars addition, which varied with the biochar derived from different feedstocks.
The soil pH was increased by all the three biochar materials application (Table 2). However, only peanut hull biochar raised plant growth and the biomass of trifoliate orange seedlings significantly (Tables 4 and 5). The different effects of three biochar on the plant growth of seedlings may depend on the different influence on the microbial properties, enzyme activity, and nutrient availability of soil. Our result showed that plant biomass of trifoliate orange seedlings was positively correlated with soil urease activity, invertase activity, cellulase activity, the geometric mean of the assayed enzyme activities (GMea), and basal respiration (Fig. 3). Therefore, the increase of soil microbial properties and enzyme activity would be the key factors for the improvement of plant growth because the biochemical properties of rhizospheric environment are more sensitive to alterations in management than its physical or chemical properties (Paz-Ferreiro et al. 2007 and 2009).
Therefore, we concluded that in fertile acidic soils, the three biochars could effectively neutralize soil acidity. However, the effect of biochar on the soil microbial properties, enzyme activity, and nutrient availability varied with different feedstocks. Peanut hull biochar showed more significant effect on soil microbial properties, enzyme activity, and secondarily rice straw biochar. However, rice and rape straw biochars resulted in more increase on soil nutrient availability than peanut hull biochar. Only peanut hull biochar raised plant growth and the biomass of trifoliate orange seedlings significantly, and the increase of soil microbial properties and enzyme activity would be the key factors for the improvement of plant growth.
References
Acosta-Martinez V, Tabatabai M (2000) Enzyme activities in a limed agricultural soil. Biol Fertil Soils 31:85–91
Amonette JE, Joseph S (2009) Characteristics of biochar: microchemical properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 33–52
Anderson T-H, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Bååth E, Frostegård Å, Pennanen T, Fritze H (1995) Microbial community structure and pH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biol Biochem 27:229–240
Bolan NS, Adriano DC, Curtin D (2003) Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv Agron 78:215–272
Brookes P, Landman A, Pruden G, Jenkinson D (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008a) Agronomic values of greenwaste biochar as a soil amendment. Soil Res 45:629–634
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008b) Using poultry litter biochars as soil amendments. Soil Res 46:437–444
Deng S, Tabatabai M (1994) Cellulase activity of soils. Soil Biol Biochem 26:1347–1354
Durenkamp M, Luo Y, Brookes P (2010) Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biol Biochem 42:2026–2029
Eivazi F, Tabatabai M (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606
Gaskin J, Steiner C, Harris K, Das K, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51:2061–2069
Guan SY (1986) Soil enzymes and their methodology. Agricultural Press, Beijing
Isermeyer H (1952) Estimation of soil respiration in closed jars. In: Alef K, Nannipieri P (eds) Method in applied soil microbiology and biochemistry. Academy, London, pp 214–216
Jeffery S, Verheijen F, Van Der Velde M, Bastos A (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73:1173–1181
Lalande R, Gagnon B, Royer I (2009) Impact of natural or industrial liming materials on soil properties and microbial activity. Can J Soil Sci 89:209–222
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from aspergillus niger. Soil Biol Biochem 43:1936–1942
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387
Lehmann J, Joseph S (eds) (2009) Biochar for environmental management: science and technology. Earthscan, London
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213
Luo Y, Jiao YJ, Zhao XR, Li GT, Zhao LX, Meng HB (2014) Improvement to maize growth caused by biochars derived from six feedstocks prepared at three different temperatures. J Integr Agric 13:533–540
Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das K, Ahmedna M, Rehrah D, Watts DW, Busscher WJ (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci 3:195–206
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MC, Seoane S, Gil-Sotres F (2007) Biochemical properties of acid soils under native grassland in a temperate humid zone. New Zeal J Agric Res 50:537–548
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MC, Seoane S, Gil-Sotres F (2009) Biochemical properties in managed grassland soils in a temperate humid zone: modifications of soil quality as a consequence of intensive grassland use. Biol Fertil Soils 45:711–722
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A (2012) Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils 48:511–517
Paz-Ferreiro J, Fu SL, Méndez A, Gascó G (2014) Interactive effect of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activity. J Soils Sediments 14:483–494
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284
Rodriguez-Kabana R, Truelove B (1982) Effects of crop rotation and fertilization on catalase activity in a soil of the south-eastern United States. Plant Soil 69:97–104
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Tang C, Rengel Z, Diatloff E, Gazey C (2003) Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Field Crop Res 80:235–244
Van Zwieten L, Kimber S, Morris S, Chan K, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Wang N, Li JY, Xu RK (2009) Use of agricultural by-products to study the pH effects in an acid tea garden soil. Soil Use Manag 25:128–132
Yuan JH, Xu RK (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag 27:110–115
Acknowledgments
This study was supported financially by the fund for Modern Agroindustry Technology Research System (CARS-27).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Zhang, Y., Tan, Q., Hu, C. et al. Differences in responses of soil microbial properties and trifoliate orange seedling to biochar derived from three feedstocks. J Soils Sediments 15, 541–551 (2015). https://doi.org/10.1007/s11368-014-1032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-1032-z