Abstract
Purpose
There is a growing interest in the use of soil enzymes as early indicators of soil quality change under contrasting agricultural management practices. In recent years, there has been increasing interest in the use of biochar to improve soil properties and thus soil quality. In addition, earthworms can also be used to ameliorate soil properties. However, there is no literature available on how biochar and earthworms interact and affect soil enzymes.
The general objective of the present study was to test the suitability of adding biochar and earthworms in two tropical soils with low fertility status in order to improve their characteristics and productivity.
Materials and methods
Biochars were prepared from four different materials [sewage sludge (B1), deinking sewage sludge (B2), Miscanthus (B3) and pine wood (B4)] on two tropical soils (an Acrisol and a Ferralsol) planted with proso millet (Panicum milliaceum L.). In addition, in order to investigate the interaction between earthworms and biochar, earthworm Pontoscolex corethrurus was added to half of the mesocosms, while excluded in the remaining half. The activities of invertase, β-glucosidase, β-glucosaminidase, urease, phosphomonoesterase and arylsulphatase were determined. The geometric mean of the assayed enzymes (GMea) was used as an integrative soil quality index.
Results and discussion
Overall, earthworms and especially biochar had a positive effect on soil quality. GMea showed B1, B2 and B3 performing better than B4; however, results were soil specific. Plant productivity increased under both biochar and earthworm addition. Fruit productivity and plant growth was enhanced by B1 and B2 but not by B3 or B4.
Conclusions
Enhancements of productivity and soil enzymatic activities are possible in the presence of earthworms and the combination of the practices earthworm and biochar addition can be suggested in low fertility tropical soils. However, scientists should proceed carefully in the selection of biochars as the results of this study show a high specificity in the biochar–soil interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is a well-established fact that soil management can alter soil quality, defined not only in terms of its productive function but also taking soil as an integral part of the ecosystem. Current agricultural systems should thus aim at maximizing soil productivity while minimizing undesirable environmental effects. Simultaneously, an intensification of agricultural production on a global scale is vital in order to provide food security for an increasing world population.
In most tropical areas, sustainable agriculture is constrained as the soils are prone to nutrient deficiency and have low organic matter content and cation exchange capacity. In these circumstances, the efficiency of applied fertilizers, when farmers are able to purchase them, is very low. As a result, fallow periods are often reduced leading to a loss of soil quality. In tropical agroecosystems, both biochar and earthworm addition have shown an appealing potential to increase soil fertility and thus productivity. Earthworms have been acknowledged to play an important role in the structure and fertility of soils as they create organic structures (biopores, biogenic aggregates) which stimulate nutrient cycling, alter organic matter chemistry and affect the microbial mineralization of organic matter (Lavelle and Spain 2001; Curry and Schmidt 2007). These effects can have a beneficial influence in plant growth. In particular, earthworm species such as Pontoscolex corethrurus and Drawida willsi have shown a potential to increase plant growth by as much as 40% (Brown et al. 1999) at both, field and mesocosm scale. The existence of Terra Preta do Indio (anthropogenic charcoal-enriched dark soils) and the fact they remain highly fertile after hundreds of years suggest a potential of biochar to improve soil fertility in the tropics (Glaser et al. 2002). Related to this, recent meta-analysis have shown the potential of biochar to improve farmland yield being greater for low pH soils (Jeffery et al. 2011; Liu et al. 2013), which prevail in the tropical area.
In recent years, it has been established a direct interaction between earthworms and biochar as the former can ingest biochar particles and reject them in their casts (Ponge et al. 2006; van Zwieten et al. 2010). This process is likely to affect biochar distribution in the soil profile, but also biochar characteristics. Previous work has demonstrated the preference of P. corethrurus to ingest soil with biochar over soil alone (Topoliantz and Ponge 2005; Ponge et al. 2006). However, some other species such as Eisenia fetida could exhibit a stronger preference for soils amended with biochar or for unamended soils, depending of soil characteristics (van Zwieten et al. 2010). The effects of the earthworm–biochar interactions have recently been explored in tropical soils with respect to resource allocation (Noguera et al. 2010) and protein turnover (Noguera et al. 2012). Further studies have been done in temperate soils with respect to greenhouse gas emissions (Augustenborg et al. 2012).
Changes in many soil properties occur slowly and are only apparent when the soil has undergone drastic changes. Hence, it is important to select indicators of soil quality that respond quickly to environmental stress (Paz-Ferreiro et al. 2010, 2011). In particular, soil biological and biochemical properties have been shown in multiple studies to be quickly responsive to changes induced under contrasting management practices or by land use changes. Examples of these properties would include parameters that are directly related to the number and activity of the soil microbiota and also properties associated with the cycling of nutrients and the decomposition of organic compounds present in soils as is the case of hydrolytic soil enzymes.
Numerous studies have assessed soil enzymes involved in the biogeochemical cycles of C, N, P and S (Paz-Ferreiro et al. 2009). This interest is not only a consequence of the sensitivity of these properties to external agents but also due to logistics, as it is relatively inexpensive and easy to determine soil enzymes (Nannipieri et al. 2002). However, in spite of the importance of soil hydrolytic enzymes, the number of reports about the effects of biochar addition on soil enzyme activities are limited (Bailey et al. 2011; Lammirato et al. 2011; Paz-Ferreiro et al. 2012). In addition, when available, most studies have been carried out using bare soils, in the absence of any plant material, although it is a well-known fact that vegetation can alter the microbial–soil interaction (Medina-Roldán et al. 2012), or these studies do not correct for potential biochar adsorption of the product of the enzymatic reaction (Wu et al. 2013). More information is available with respect to earthworm addition to soil, which has been found to increase soil enzyme activity, either indirectly due to an improvement of soil physical properties or directly as earthworm casts exhibit higher enzyme activities than the surrounding soil (Tao et al. 2009).
It is thus evident that changes in soil quality caused by different types of biochar should be assessed in order to combine the most sustainable types of crop and biochar. The general objective of the present study was to provide information about the activity of several hydrolytic enzymes in tropical soils with contrasting fertility and amended with several types of biochar in the presence or absence of earthworms. More specifically, this study aims to address the following concerns: First, if biochar and earthworms influence plant productivity, they should impact soil biogeochemical cycles and, consequently, soil enzymatic activities. Analysing biochars with contrasting characteristics is necessary to understand if there is a consistent effect of biochar on soil enzyme activities. Secondly, investigate the relative impact of biochar and earthworm on plant growth. Thirdly, ascertain whether biochar and earthworms interact (if there are synergistic or antagonistic effects) with respect to plant growth or soil enzymatic activity. Finally, we hypothesized that biochar addition would increase its effectiveness on less fertile soils.
2 Materials and methods
2.1 Soil sampling
Soils were sampled at Heshan Hilly Land Interdisciplinary Experimental Station, Chinese Academy of Sciences in Guangdong Province, China, located at 22°41′N and 112°54′E in March of 2012. The climate of the region is subtropical monsoon with a mean annual precipitation of 1,700 mm and a mean annual evapotranspiration of 1,600 mm. Precipitation mainly occurs in the rainy season from April to September. The mean annual temperature is 21.7 °C with a mean maximum monthly temperature of 29.2 °C in July and a mean monthly minimum of 12.6 °C in January.
Two soils differing in their fertility status were sampled, although the two of them were relatively infertile. Soil was sampled from the top 10 cm of the profile in several points of a 20 × 20 m plot. Soils (approximately 20 kg each) were sampled using a spade and taken to the laboratory in plastic bags where they were sieved (<5 mm) and stored in a fridge at 4 °C. The more fertile soil (Ferralsol, FAO 2006) had a 1.34% content of organic matter, a pH of 5.00, an available P content of 35.8 mg kg−1 and a sandy–loam–clay texture (Table 1). The more infertile soil (Acrisol, FAO 2006) had an organic matter content of 3.79%, pH of 3.35 and an available P content of 3.6 mg kg−1 (see Table 1).
2.2 Biochars
The feedstock used for producing B1 biochar in this study was sewage sludge collected from a wastewater treatment plant in Madrid (Spain), while the feedstock used for producing B2 biochar was a deinking sewage sludge from a recycled paper plant. These two feedstocks were first air-dried and then pyrolysed as described by Gascó et al. (2012). Feedstock (20 g) was placed in a covered ceramic cup. The ceramic cup was introduced in a covered nickel recipient. The cavity between these two recipients (nickel and ceramic cups) was filled up with fuel coke particles (<1 mm). As the temperature increases, O2 from air is consumed by fuel coke particles and samples pyrolysed in the inert atmosphere generated. Pyrolysis were performed in a tubular furnace Carbolite, where the temperature was increased to 600 °C at a rate of 10 °C min−1 and the final temperature was maintained for 2 h.
B3 was produced from Miscanthus using an auger pyrolyser by BTG Biomass Technology Group B.V. (Enschede, the Netherlands). The Miscanthus biomass (average moisture content of 11%) was manually fed into an initial auger reactor and heated to temperatures around 130 °C to reduce moisture content. After passing through the initial auger reactor, the biomass was pyrolysed in a second auger reactor at a pyrolysis temperature of 450 °C. Both reactors consisted of two concentric tubes. The inner tube holds a conveyor while the outer shell acts as the heating jacket. Both reactors are heated by combusting pyrolysis process residual vapors and gases with air at 850 °C and passing the hot combustion gases through the outer reactor shell. Residence times in the second auger reactor at final pyrolysis temperatures averaged 14.7 (±0.4) min.
B4 was produced from wood using pyrolysis gasification at a temperature of 800 °C during seconds by O-Gen UK Ltd. The wood used as feedstock in B4 is a by-product of house demolishing and restructuring.
In all cases, the particle sizes of the biochars were smaller than 5 mm. Table 2 shows the general properties of the biochars used in this experiment.
2.3 Mesocosms
The experiment was conducted at a greenhouse in the South China Botanical Garden, Guangzhou. Pots (diameter 10 cm and 15 cm height) were filled with 400 g of soils (sieved to 5 mm). Biochars were added to the soil to a rate of 3% (w/w) and afterwards soils were planted with eight seeds of proso millet (Panicum milliaceum L.). Two weeks after planting, three adult individuals of Pontoscolex corethrurus (Annelida: Oligocheata: Glossoscolecidae) were introduced at each mesocosm. The geophagous earthworm P. corethrurus is an endogeic species feeding on soils with a low content of organic matter (Lavelle et al. 1987). Earthworms were collected at the same location where soils were taken (Heshan station). Drains at the bottom of pots were covered with 1 mm plastic mesh to prevent earthworms from escaping. Soils were maintained at 60% soil water holding capacity, which was checked through regular weighting of the pots. Mesocosms were arranged in a completely randomized design inside the greenhouse and their position was changed every few weeks. After 3 months, the mesocosms were sampled for soil properties and plant productivity. Survival of earthworms was higher than 90% at the end of the experiment and no differences were found between treatments (data not shown).
The experimental design was a full-factorial experiment with three factors and four replicates, resulting in an overall number of 80 mesocosms. One factor was determined by the presence/absence respectively of earthworms, while the second one was determined by the absence of biochar or the presence of one of the four biochars utilised in this experiments. The third factor was soil type (Ferralsol vs. Acrisol).
When the experiment was ended, a part of the soil was air-dried for general analyses and sieved to 2 mm.
2.4 Plant analyses
At the end of the mesocosm experiment, the fruits and plants were harvested and oven-dried at 70 °C to constant weight. Plants were weighted to determine dry matter. The number of fruits per plant was counted and the total number of fruits per pot was calculated.
2.5 Soil general properties
Soil bulk density was determined from three oven-dried undisturbed cores as mass per volume of oven-dried soil.
A subsample of soil was air-dried, crushed and sieved (<2 mm) prior to the determination of soil properties. Initial and final pH levels were determined with a soil/water or KCl ratio of 1:2.5 using a Crison micro-pH 2000. Initial and final total C and total N analysed using a Carlo Erba EA-1108 elemental analyser. Available phosphorus was determined as in Bray and Kurtz (1945). Soil texture was determined as reported by Day (1965).
2.6 Biochar properties
Electrical conductivity and pH were determined in a ratio biochar/water 1:2.5 g mL−1 using a Crison 222 conductivimeter in the case of EC and a Crison micro-pH 2000 for pH. Total C and total N were analysed using a Carlo Erba EA-1108 elemental analyser. Biochar nitrogen adsorption analysis (BET surface) was carried out at 77 K in a Micromeritics Tristar 3000.
Proximate analysis was calculated by thermogravimetry using a Labsys Setaram equipment. Samples were heated up to 900 °C under an N2 atmosphere at a flux of 40 mL min−1 using a heat rate of 20 °C min−1.Volatile matter was determined as the weight loss from 150 to 900 °C. At 900 °C, air flux was introduced until a constant weight was reached and ashes were determined as the final weight of the samples. Fixed carbon was calculated as the weight produced when the final sample was burnt under air atmosphere.
Biochar metal content was determined using a PerkinElmer 2280 atomic absorption spectrophotometer after sample extraction by digestion with concentrated HCl/HNO3 following the 3051a method (USEPA 1997).
2.7 Soil enzyme activities
Acid phosphomonoesterase, β-glucosidase and arylsulphatase activities were determined following modifications of the original methods (Saá et al. 1993; Eivazi and Tabatabai 1988; Tabatabai and Bremner 1970), as described by Paz-Ferreiro et al. (2009). Briefly, these enzyme activities were determined after incubating soils at 37 °C and then measuring, by spectrophotometry, the amount of p-nitrophenol released during enzymatic hydrolysis. Acid phosphomonoesterase was estimated following the method of Saá et al. (1993), using 16 mM p-nitrophenyl phosphate as substrate. β-Glucosidase activity was determined similarly as described for phosphomonoesterase activity, but using 25 mM p-nitrophenyl-β-d-glucopyranoside as substrate (Eivazi and Tabatabai 1988). Arylsulphatase activity was assessed after incubating the samples with 5 mM p-nitrophenyl sulphate (Tabatabai and Bremner 1970). The p-nitrophenol released during enzymatic hydrolysis was determined using a spectrophotometer at a wavelength of 400 nm. The activity of each of these three enzymes was expressed as micromole p-nitrophenol per gram per hour.
β-Glucosaminidase activity was assayed as described by Parham and Deng (2000), but stopping the reaction with 2 M CaCl2 instead of 0.5 M CaCl2. Essentially, soil (1 g) was incubated in the presence of 4 mL of 0.1 M acetate buffer (pH 5.5) and 1 mL of 10 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide solution at 37 °C. After stopping the reaction with CaCl2, the activity was measured at a wavelength of 400 nm and expressed as micromole p-nitrophenol per gram per hour.
Invertase activity was determined after incubating the samples with 35.06 mM saccharose in 2 M acetate buffer (pH 5.5) at 50 °C during 3 h and assessing the released reducing sugars following the method of Schinner and von Mersi (1990). Invertase activity was measured at 690 nm and expressed as micromole glucose per gram per hour.
Urease activity was determined as in Kandeler and Gerber (1988). A modified Berthelot reaction is used in this essay to obtain an NH4 + coloured complex after incubation of soil with urea for 2 h. The coloured complex was measured at 610 nm. The activity was expressed as micromole NH4 + per gram dry soil per hour.
All enzyme activities were determined in triplicate. Different standard curves were prepared for every combination of biochar × soil × earthworm to account for both, the adsorption that some of the biochars had on the product of the reaction (Paz-Ferreiro et al. 2012) and adsorption of the product of the enzymatic reaction by soil organic matter.
For each soil sample, the geometric mean of the assayed enzyme activities (GMea) was calculated as:
where Pm, Glu, Ary, Ure, Inv and Gsm are phosphomonoesterase, β-glucosidase, arylsulphatase, urease, invertase and β-glucosaminidase, respectively. This algorithm has been shown to indicate soil quality in plots under different type of management.
2.8 Statistical analyses
All statistical analyses were done using SPSS 15.0. A three-way ANOVA was carried out using the different soil parameters measured as variables and type of biochar (derived from sewage sludge, deinking sewage sludge, Miscanthus or pine wood), soil type (Acrisol or Ferralsol) and earthworm (presence or absence) as factors. Post hoc analyses using the Tukey test were conducted for the factor “biochar”. Unless otherwise stated, the differences were significant at P < 0.05 level.
3 Results
3.1 Plant characteristics
Both, dry matter and number of fruits produced were higher in the Ferralsol than in the Acrisol, implying a higher fertility of the former compared to the latter (see Fig. 1a, b). The most prominent factor explaining the differences among the number of fruits produced per plant was the type of biochar used. As a result, mesocosms amended with biochars B1 and B2 exhibited larger number of fruits per plant, compared to the control soil. The average number of fruits in the mesocosms amended with B1 was 139 and in the case of B2 was 78. Those values were much higher than in the control soils (4). Soil type exerted the second largest influence on plant productivity. The number of fruits was higher in the Ferralsol (56 ± 60) compared to the Acrisol (39 ± 61). The presence of earthworms increased the number of fruits produced per plant (P < 0.05); however, this effect was uneven. Thus, the number of fruits per plant was 53 ± 70 in the case of mesocosms with presence of earthworms and 42 ± 51 in mesocosms without earthworms. A significant biochar × earthworm interaction revealed that the effect of earthworms on plant productivity was only significant in soils amended with B1. Plants grown with presence of earthworms had 47% more fruits than plants cultivated in the absence of earthworms for the B1 treatments. In addition, a significant biochar × soil interaction showed that B2 exerted a significant effect in the number of fruits produced in the Ferralsol, but not in the Acrisol. B3 and B4 addition did not result in a significant increase in fruit production.
The dry matter weight of proso millet shoots varied significantly among the different treatments, depending on biochar addition (F = 140.17, P < 0.001) and on the interaction biochar × soil (F = 7.17, P < 0.001). Plant mass was almost multiplied by 11 in the treatment B1 compared to the control (Fig. 1b), although B1 worked better for the Ferralsol than for the Acrisol. Also, plants mass increased five times in soils where B2 was added compared to the control soils.
3.2 Soil properties
As expected, there was an increase on soil pH and organic matter content after the addition of different biochars (Table 3). Earthworm addition did not result in changes in soil pH or in soil organic matter (data not shown).
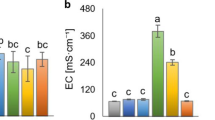
The activity of β-glucosidase (Fig. 2a; Table 4) was influenced mainly by biochar (F = 38.6, P < 0.001) and earthworms (F = 27.3, P < 0.001). Thus, β-glucosidase activity followed the order B3 > B4 > B1 ~ B2 ~ Control. On the other hand, β-glucosidase values had an average value of 1.54 ± 0.57 μmol p-nitrophenol g−1 h−1 in the mesocosms containing earthworms vs. 1.30 ± 0.44 μmol p-nitrophenol g−1 h−1 in the treatments without earthworms. β-Glucosidase values also were affected by soil type (1.48 ± 0.67 μmol p-nitrophenol g−1 h−1 in the Ferralsol vs. 1.36 ± 0.31 μmol p-nitrophenol g−1 h−1 in the Acrisol, F = 5.8, P < 0.05). The significant value of the biochar × soil interaction (F = 31.3, P < 0.001) revealed that the increases in β-glucosidase activity after biochar addition were larger for the Acrisol than for the Ferralsol. In the case of the Acrisol, biochar addition resulted in β-glucosidase values that were 161–181% of those of the control soil, whilst in the Ferralsol biochar resulted in higher, same or lower β-glucosidase values than in the control soil, depending on the type of biochar added. As an example, β-glucosidase in B3 had 169% of the value in the control Ferralsol, while in B2 this activity was 59% of the control Ferralsol. There was a marginally significant interaction earthworm × soil (F = 4.4, P < 0.05).
Invertase activity (Fig. 2b) was affected by soil type (F = 112.0, P < 0.001), with greater values in the Ferralsol (11.55 ± 4.49 μmol glucose g−1 h−1) than in the Acrisol (4.35 ± 2.91 μmol glucose g−1 h−1). Values of invertase activity were higher in the mesocosms amended with B1 and B2 (F = 27.3, P < 0.001). Values of this enzyme were also higher (F = 5.8, P < 0.05) in mesocosms with earthworms (8.62 ± 5.55 μmol glucose g−1 h−1) than in treatments without earthworms (7.28 ± 4.65 μmol glucose g−1 h−1). There was also a significant interaction between biochar and soil (F = 31.3, P < 0.001).
The activity of β-glucosaminidase (Fig. 2c) was influenced by biochar addition (F = 19.2, P < 0.001) and by soil type (F = 8.1, P < 0.010). There was a strongly significant interaction soil × biochar (F = 35.7, P < 0.001). Thus, biochar amendment resulted in an improved β-glucosaminidase activity for the Acrisol in all cases, but only B4 resulted in an increase of β-glucosaminidase activity in the Ferralsol.
Urease activity (Fig. 3a) was affected by soil type (F = 192.3, P < 0.001) and by the presence of biochar (F = 87.7, P < 0.001). In addition, although all biochars had a positive effect on this activity, the strength of the biochar effect depended on soil type, as there was a statistical significant soil × biochar interaction (F = 45.6, P < 0.001). Thus, urease activity in the Acrisol was highest after amendment with B2, whilst it was highest in the Ferralsol after addition of B1 and B3.
With respect to phosphomonoesterase values (Fig. 3b), soil had a strong effect (F = 53.4, P < 0.001) with values being higher in the Acrisol (10.2 ± 3.2 μmol p-nitrophenol g−1 h−1) than in the Ferralsol (7.7 ± 3.1 μmol p-nitrophenol g−1 h−1). Biochar addition (F = 37.4, P < 0.001) and the presence of earthworms (F = 24.8, P < 0.001) also affected the activity of this enzyme. There was a significant interaction between biochar and soil (F = 20.7, P < 0.001). Thus, for the Acrisoll, B1, B3 and B4 but not B2 resulted in a significant increase of phosphomonoesterase activity; however for the Ferralsol, B1 and B2 resulted in lesser phosphomonoesterase activity, while B3 and B4 showed the same values as the control.
Arylsulphatase (Fig. 3c) was influenced by both, biochar addition (F = 190.6, P < 0.001) and soil type (F = 26.2, P < 0.001). In addition, there was a significant biochar × soil interaction (F = 81.2, P < 0.001) and biochar × earthworm interaction (P = 4.0, P < 0.010). All biochars increased arylsulphatase with respect to the control with the only exception of B4 in the Ferralsol.
The geometric mean of the five assayed enzymes (Fig. 4) increased with biochar (F = 38.3, P < 0.001) and earthworm addition (F = 22.2, P < 0.001). GMea ranked in the order B1 ~ B2 ~ B3 > B4 > control and had larger values in the Ferralsol compared to the Acrisol (F = 56.9, P < 0.001). There was also a strongly significant biochar × soil interaction (F = 40.2, P < 0.001) and, while every biochar increased significantly the values of GMea with respect to the control in the Acrisol, this was untrue in the Ferralsol, where only B3 resulted in a significant increase of GMea.
4 Discussion
4.1 Biochar effect on soil properties
The soils in South China are usually acidic due to the climate, which presents values of rainfall higher than those of evapotranspiration. Moreover, soil organic matter contents are low due to the rapid mineralization of organic matter as a consequence of the high average annual temperatures. As a result of this depletion in soil organic matter, nutrients are lost from the system through leaching and runoff.
The results obtained in this greenhouse experiment showed the potential to apply biochar to low fertility tropical soils in order to improve proso millet productivity. It was observed that the average yield produced was greater for the treatments B1 and B2. Concomitant to an increment in productivity, an increase in most of the enzymes studied was observed. There are several mechanisms that can explain a build up in soil enzyme activity after biochar addition. Firstly, bacteria may sorb to biochar surfaces via different processes including hydrophobic attraction or electrostatic forces. This process rends bacteria less susceptible to leaching in soil (Pietikäinen et al. 2000). Secondly, although there is no quantitative evidence, both bacteria and fungi are hypothesized to be protected against grazers or competitors into the pore habitats of the biochars (Thies and Rillig 2009). Thirdly, some fractions of biochar are labile and can provide a ready-to-use substrate for microbial populations. Besides, C supply for heterotrophic microorganisms could be increased by exudation or root turnover in the rhizosphere. Finally, there is a positive liming effect of biochar, particularly when added to acidic soil, which not only increases soil pH but also improves nutrient cycling (Acosta-Martínez and Tabatabai 2000). It should also be taken into account that enzyme activity is generally well correlated to organic matter stocks. However, in this study other factors were more important, as the biochars with a higher organic matter content (B3 and B4) did not result in greater crop productivity or higher enzymatic activity.
In spite of the vast number of articles dealing either the effect of soil management on soil enzymes, most of these focus on the effects of fertilizers or a combination of management practices. However, not so many studies have been performed to look into the effect of pH changes on soil enzymes (but see Acosta-Martínez and Tabatabai 2000; Mijangos et al. 2010). It is generally accepted that moderate soil pH contributes to an enhancement of soil microbial populations and thus enzyme activity. However, even though pH generally correlated positively to soil enzyme activity, this was not always the case in this study. In fact, for the Ferralsol, β-glucosidase activity was negatively correlated to pH (r = −0.67, P < 0.001) as was the case of β-glucosaminidase (r = −0.40, P < 0.010). Both activities were positively correlated with pH for the Acrisol (r = 0.41, P < 0.010 and r = 0.86, P < 0.001, respectively, for β-glucosidase and β-glucosaminidase). In the case of urease, the correlation with pH was positive for the Acrisol (r = 0.80, P < 0.001) but non-existent for the Ferralsol. This suggests that the positive impact of biochar on soil enzyme activities is linked to pH increases in strongly acidic soils and other mechanisms, described in the previous paragraph, in other soils.
Glycosidases play a key role in degrading organic compounds such as crop residues or animal manure in soils. In this respect, β-glucosidase is sensitive to soil pH increasing after liming (Acosta-Martínez and Tabatabai 2000). In addition, β-glucosidase has been used to monitor quick changes in soil organic matter caused by soil management practices (Bandick and Dick 1999) as this enzyme is strongly related to soil organic matter content. The activity of β-glucosidase has been also utilised as a component in multiparametric indices estimating soil quality (Puglisi et al. 2006). However, even biochar is generally an alkaline material that increases soil pH; the response of β-glucosidase and the mechanisms involved in this response after biochar addition are not well established. Lammirato et al. (2011) and Paz-Ferreiro et al. (2012) have reported a diminished activity in biochar amended soils, while Bailey et al. (2011) found this effect to be dependent on the type of soil analysed. A possible explanation given by the decrease of β-glucosidase is an improved microbial efficiency as a consequence of the co-location of microorganisms and C on biochar surfaces.
Invertase activity is involved in the degradation of fructofuranosides and can be used as an indicator of the release of low molecular weight sugars, which can be used as energy sources for microorganisms. Invertase activity is strongly dependent on plant cover (Ross 1976). Increases in plant productivity could explain the higher values of invertase activity for the mesocosms amended with B1 and B2. In fact, there was a statistical significant correlation between the number of fruits per plant and invertase activity (r = 0.48, P < 0.001).
β-Glucosaminidase catalyses the hydrolysis of N-acetyl-β-d-glucosamine residues from the terminal non-reducing ends of chitooligosaccharides and has been associated with the N acquisition process of microorganisms (Parham and Deng 2000) while urease catalyses the hydrolysis of urea. Urease and β-glucosaminidase are sensitive to land use and agricultural management. Previous research suggests that organic C and N associated to soil microbial biomass (Ekenler and Tabatabai 2003) might be important N substrates for soil microorganisms to produce soil enzymes related to N cycling (β-glucosaminidase and urease in this study). It is likely that biochar has increased total organic C and maybe N associated to the microbial biomass and subsequently promoting the activity of enzymes involved in the N cycle as this effect has been observed before in previous works (Paz-Ferreiro et al. 2012).
Phosphatases catalyse the hydrolysis of both esters and anhydrides of phosphoric acid. Phosphomonoesterase activity is an inducible enzyme (Acosta-Martínez and Tabatabai 2000) and, therefore, excretion by plant roots and microorganisms are regulated by their requirement for orthophosphate. As expected, phosphomonoesterase was lower in the Acrisol than in the Ferralsol due to a shortage of phosphorus in the first one. Phosphomonoesterase is an inducible enzyme, which explains the enhanced activity observed in low ash content biochars (B3 and B4).
Arysulphatase regulates S availability in soils. As far as we know, the influence of biochar on sulphur transformations has attracted little attention. Previous work found no biochar effect on this enzyme (Paz-Ferreiro et al. 2012). However, in the present work, biochar addition to soil was found to have an effect on S cycling, although the mechanism for explaining the role of biochar addition on sulphur transformations remains elusive.
4.2 Earthworm effect
The stimulatory effect of earthworms on soil enzymes could be explained by mucus and casts production. The first one provides easily assimilable C for microorganisms (Doube and Brown 1998). Casts (both in the surface and underground) effect on soil enzymes is possibly due to the provision of a rich nutrient substrate for microbial and microfaunal growth, as casts are enriched in available forms of C, N and P and the earthworms' own enzymes (Zhang et al. 2000). Tiwari et al. (1989) suggested that higher enzyme activity in earthworm casts can be due to an increase in soil bacterial and fungal biomass.
An increase in β-glucosidase activity in the treatments with earthworms might have been caused by the higher cellulolytic activity characteristic in the casts of many earthworms (Zhang et al. 2000). The presence of earthworms increased invertase activity significantly as has been shown by other researchers (Tao et al. 2009). As found in the present study, the existence of earthworms has been associated with higher phosphatase activities in previous works for several earthworm species with different feeding habits (Aira et al. 2003). The mechanism to explain this observation is that earthworms could lead to reduced amounts of available phosphorus (Li et al. 2012).
In general, biochar and earthworms did not interact with respect to soil enzyme activity in this work. A scarce interaction between earthworms and biochar has been found before (Noguera et al. 2010). Apparently, except in the case of arylsulphatase, soil enzymes were affected in a consistent way by earthworms. However, in the case of arysulphatase, we noticed a significant biochar × earthworm interaction, showing that the response of the sulphur cycle to biochar addition could be more complex than that of other biogeochemical cycles. However, this study does not allow drawing conclusions on the underlying mechanism of the interactions between earthworms and biochar.
In general, there was no significant interaction between soil type and the presence of earthworms, being the only exception β-glucosidase activity. Generally, it is assumed that earthworms' effect are more positive in poorer soils (Brown et al. 1999), as earthworms increase the mineralization of soil organic matter. Thus, in soils rich in mineral nutrients, this effect could disappear. However, we found earthworms to have a more positive effect in β-glucosidase activity in the Ferralsol, rather than in the Acrisol.
4.3 Soil quality
Soil processes are determined by a large number of diverse physical, chemical and biochemical properties. Changes in these properties can alter soil quality; however, individual soil properties are not adequate to estimate soil quality and there is an increasing tendency to use linear expressions or other type of relationships as an index of soil quality (Puglisi et al. 2006; Paz-Ferreiro et al. 2007, 2009, 2012; Li et al. 2013). GMea has been proved to be responsive to organic management (García-Ruiz et al. 2008), to biochar or sewage sludge addition (Paz-Ferreiro et al. 2012) and to other by-products that can be used as soil amendments such as straw, chaff and slag (Li et al. 2013). In this work, GMea values in soils amended with biochar or with presence of earthworms were significantly higher than in the controls. On average, GMea increased by 53, 53, 53 and 36% in soils amended with B1, B2, B3 and B4, respectively and by 12% in the samples with earthworms. Our data point out that, overall, biochar addition had a stronger effect than earthworm addition, although exceptions were observed for some individual soil enzymes. Nevertheless, the magnitude of the differences depended on soil type and a high specificity of the soil–biochar interaction. Overall, the less fertile soil (Acrisol) responded better to biochar addition. For the Ferralsol, B3 and B1 increased GMea values by 37 and 20%, respectively. Samples amended with B2 and B4 had the same value as the control in the Ferralsol. In the case of the Acrisol, B1, B2, B3 and B4 increased GMea values by 118, 164, 81 and 73%, respectively. These figures are higher than the 18% GMea increment found previously in an Umbrisol (Paz-Ferreiro et al. 2012) and ratifies our hypotheses that, after biochar addition, the lower soil fertility is, the higher increases in soil enzymatic activity are to be expected. GMea values showed lower variability than those of the individual enzyme activities (data not shown), suggesting that GMea is a more suitable index for assessing soil quality than individual soil enzymatic activities.
When making conclusions about our data, it is imperative to take into account that the interpretation of measurements from enzyme assays is a complex issue as enzyme activity has multiple locations (Nannipieri et al. 2002). However, spectophotometric assays do not separate the contribution of activity linked to soil colloids and those of free enzymes (Nannipieri et al. 2002). It should also be taken into account that different soil enzymes assays can render distinct results and that no standard methods are currently available for measuring enzyme activities. Also, the experimental design in this experiment does not allow to isolate the effect of different factors involved when biochar is added to the soil or deepen into the mechanistic processes involved. Thus, we believe that scientists are at the very beginning of establishing the effect of biochar addition on soil enzyme activity. Our results are valuable, even though this experiment was a short-term mesocosm experiment and results may not be extrapolated to field conditions. It was established for the first time that earthworms and biochar could interact synergistically to improve soil biological properties and soil fertility and a good agreement between the value of soil enzymes and plant productivity was generally detected.
5 Conclusions
Biochars and earthworms had a positive influence in both soil enzymatic activities and plant growth, with high mineral ash biochars (B1 and B2) performing better than low mineral ash biochars derived from plant material (B3 and B4). In general, biochar type was more determinant than the presence of earthworms to explain increases in plant productivity and soil enzyme activity. Biochar effects, but not earthworm effects, were highly dependent on soil type. Also, there was some evidence of a positive further interaction between some of the biochars used and earthworms, at least for plant productivity and for some of the enzymatic properties analysed (arylsulphatase). Hence, further enhancements of productivity are possible in the presence of earthworms and combining the practices of earthworm and biochar addition can be suggested in tropical soils. This should be made with caution as when adding biochar to soil it should be born in mind the high specificity of the soil–biochar interaction. In this aspect, increases in soil enzymatic activity were more pronounced in the less fertile soil (Acrisol) rather than in the Ferralsol. Further research will be needed to predict accurately the biological response of different soil types when diverse biochars are added.
References
Acosta-Martínez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biol Fertil Soils 31:85–91
Aira M, Monroy F, Domínguez J (2003) Effects of two species of earthworms (Allolobophora spp.) on soil systems: a microfaunal and biochemical analysis. Pedobiologia 47:877–881
Augustenborg CA, Hepp S, Kammann C, Hagan D, Schmidt O, Müller C (2012) Biochar and earthworm effects on soil nitrous oxide and carbon dioxide emissions. J Environ Qual 41:1203–1209
Bailey VL, Fansler SJ, Smith JL, Bolton H (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301
Bandick AB, Dick RP (1999) Field management effects on soil enzyme activities. Soil Biol Bioche 31:1471–1479
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–45
Brown G, Pashanasi B, Villenave C, Patron JC, Senapati BK, Giri S, Barois I, Lavelle P, Blanchart E, Blakemore RJ, Spain BJ (1999) Effects of earthworms on plant production in the tropics. In: Lavelle P, Brussaard L, Hendrix P (eds) Earthworm management in tropical agroecosystems. CABI, Wallingford, pp 87–147
Curry JP, Schmidt O (2007) The feeding ecology of earthworms—a review. Pedobiologia 50:463–477
Day PR (1965) Particle fractionation and particle-size analysis. In: Black CA, Evans DD, White JL, Ensminger LE, Clark FE (eds) Methods of soil analysis, part 1, physical and mineralogical properties. Including statistics of measurement and sampling. ASASSSA, Madison, pp 545–567
Doube BM, Brown GG (1998) Life in a complex community: functional interactions between earthworms, organic matter, microorganisms, and plant growth. In: Edwards CA (ed) Earthworm ecology. St. Lucie Press, Boca Raton, pp 179–211
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606
Ekenler M, Tabatabai MA (2003) Tillage and residue management effects on β-glucosaminidase activity in soils. Soil Biol Biochem 35:871–874
FAO (2006) World reference base for soils resources. World Soil No. 103. Rome, Italy
García-Ruiz R, Ochoa V, Hinojosa MB, Carreira JA (2008) Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol Biochem 40:2137–2145
Gascó G, Paz-Ferreiro J, Méndez A (2012) Thermal analysis of soil amended with sewage sludge and biochar from sewage sludge pyrolysis. J Therm Anal Calorim 108:769–775
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agr Ecosyst Environ 144:175–187
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942
Lavelle P, Spain AV (2001) Soil ecology. Kluwer Academic, Dordrecht, 654 pp
Lavelle P, Barois I, Cruz I, Fragoso C, Hernandez A, Pineda A, Rangel P (1987) Adaptive strategies of Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta), a peregrine geophagous earthworm of the humid tropics. Biol Fertil Soils 5:188–194
Li H, Xiang D, Wang C, Li X, Lou Y (2012) Effects of epigeic earthworm (Eisenia fetida) and arbuscular mycorrhizal fungus (Glomus intraradices) on enzyme activities of a sterilized soil–sand mixture and nutrient uptake by maize. Biol Fertil Soils 48:879–887
Li JY, Liu ZD, Zhao AZ, Xu RK (2013) Microbial and enzymatic properties in response to amelioration of an acidic Ultisol by industrial and agricultural by-products. J Soils Sediments. doi:10.1007/s11368-013-0666-6
Liu X, Zhang A, Ji C, Joseph S, Bian R, Li L, Pan G, Paz-Ferreiro J (2013) Biochar's effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil. doi:10.1007/s11104-013-1806-x
Medina-Roldán E, Paz-Ferreiro J, Bardgett RD (2012) Grazing exclusion affects soil and plant communities, but has no impact on soil carbon storage in an upland grassland. Agr Ecosyst Environ 149:118–123
Mijangos I, Albizu I, Epelde L, Amezaga I, Mendarte S, Garbisu C (2010) Effects of liming on soil properties and plant performance of temperate mountainous grasslands. J Environ Manag 91:2066–2074
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soils. In: Burns RG, Dick RP (eds) Enzymes in the Environment: Activity, Ecology and Applications. Marcel Dekker Inc, Nueva York, pp 1–34
Noguera D, Rondón M, Laossi KR, Hoyos V, Lavelle P, de Carvalho MHC, Barot S (2010) Contrasted effect of biochar and earthworms on rice growth and resource allocation in different soils. Soil Biol Biochem 42:1017–1027
Noguera D, Barot S, Laossi KR, Cardoso J, Lavelle P, Cruz de Carvalho MH (2012) Biochar but not earthworms enhances rice growth through increased protein turnover. Soil Biol Biochem 52:13–20
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MC, Seoane S, Gil-Sotres F (2007) Biochemical properties of acid soils under native grassland in a temperate humid zone. N Z J Agr Res 50:537–548
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MC, Seoane S, Gil-Sotres F (2009) Biochemical properties in managed grassland soils in a temperate humid zone: modifications of soil quality as a consequence of intensive grassland use. Biol Fertil Soils 45:711–722
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MC, Seoane S, Gil-Sotres F (2010) Effect of management and climate on biochemical properties of grassland soils from Galicia (NW Spain). Eur J Soil Biol 46:136–143
Paz-Ferreiro J, Trasar-Cepeda C, Leirós MdC, Seoane S, Gil-Sotres F (2011) Intraannual variation in biochemical properties and the biochemical equilibrium of different grassland soils under contrasting management and climate. Biol Fertil Soils 47:633–645
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A (2012) Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils 48:511–517
Pietikäinen J, Kiikkilä O, Fritze H (2000) Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89:231–242
Ponge JF, Ballof S, Rossi JP, Lavelle P, Betsch JM, Gaucher P (2006) Ingestion of charcoal by the Amazonian earthworm Pontoscolux corethrurus: a potential for tropical soil fertility. Soil Biol Biochem 38:2008–2009
Puglisi E, Del Re AAM, Rao MA, Gianfreda L (2006) Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol Biochem 38:1673–1681
Ross DJ (1976) Invertase and amylase activities in ryegrass and white clover plants and their relationships with activities in soils under pasture. Soil Biol Biochem 8:351–356
Saá A, Trasar-Cepeda MC, Gil-Sotres F, Carballas T (1993) Changes in soil phosphorus and acid phosphatase activity immediately following forest fires. Soil Biol Biochem 25:1223–1230
Schinner F, von Mersi W (1990) Xylanase-, CM-cellulase and invertase activity in soil: an improved method. Soil Biol Biochem 22:511–515
Tabatabai MA, Bremner JM (1970) Arylsulfatase activity of soils. Soil Sci Soc Am Proc 34:225–229
Tao J, Griffiths B, Zhang S, Chen X, Liu M, Hu F, Li H (2009) Effects of earthworms on soil enzyme activity in an organic residue amended rice–wheat rotation agro-ecosystem. Appl Soil Ecol 42:221–226
Thies JE, Rillig M (2009) Characteristics of biochar: biological properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 85–105
Tiwari SC, Tiwari BK, Mishra RR (1989) Microbial populations, enzyme activities and nitrogen–phosphorus–potassium enrichment in earthworm casts and in the surrounding soil of a pineapple plantation. Biol Fertil Soils 8:178–182
Topoliantz S, Ponge JF (2005) Charcoal consumption and casting activity by Pontoscolex corethrurus (Glossoscolecidae). Appl Soil Ecol 28:217–224
USEPA (1997) Method 3051a: microwave assisted acid dissolution of sediments, sludges, soils, and oils, 2nd edn. U.S. Gov. Print. Office, Washington
van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Wu F, Jia Z, Wang S, Chang SX, Startsev A (2013) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49:555–565
Zhang B, Li G, Shen T, Wang T, Sun Z (2000) Changes in microbial biomass C, N and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol Biochem 32:2055–2062
Acknowledgments
We are grateful to three anonymous reviewers for their comments, which contributed to improve the quality of the article. J. Paz-Ferreiro thanks the Chinese Academy of Sciences for financial support (fellowship for young international scientists number 2012Y1SA0002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
Rights and permissions
About this article
Cite this article
Paz-Ferreiro, J., Fu, S., Méndez, A. et al. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J Soils Sediments 14, 483–494 (2014). https://doi.org/10.1007/s11368-013-0806-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0806-z