Abstract
Influenza (flu) infection increases the risk for disability, falls, and broken bones in older adults. We have employed a preclinical model to examine the impact of flu on muscle function, which has a direct impact on fall risk. In mice, flu causes mobility and strength impairments with induction of inflammatory and muscle degradation genes that are increased and prolonged with aging. To determine if vaccination could reduce flu-induced muscle decrements, mice were vaccinated with flu nucleoprotein, infected, and muscle parameters were measured. Vaccination of aged mice resulted in significant protection from functional decrements, muscle gene expressions alterations, and morphological damage. Vaccination also improved protection from lung localized and systemic inflammation in aged mice. Despite documented decreased vaccine efficacy with aging, vaccination still provided partial protection to aged mice and represents a potential strategy to prevent flu-induced disability. These findings provide translational insight on ways to reduce flu-induced disability with aging.

.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza (flu) and pneumonia are the seventh leading cause of death among older adults in the USA with 90% of all flu-related deaths occurring in late life (Heron 2013). Equally compelling from a clinical and public health perspective, but less well understood, is the relationship between flu infection and disability. Older adults have a greatly increased risk of both progressive and catastrophic disability following flu infection (McElhaney et al. 2012; Ferrucci et al. 1997), including a greater risk of falls and broken bones (McConeghy et al. 2017). Nevertheless, mechanisms by which flu infection contributes to this risk of disability remain unknown and unexplored, resulting in missed opportunities for the discovery of interventions designed to prolong function and independence in late life. Flu infections, even in uncomplicated cases, have some degree of muscle involvement with myalgia being a common symptom (Kuiken and Taubenberger 2008). We previously reported that flu infection, which by its very nature is limited to pulmonary epithelial cells, results in mobility and strength impairments and increased markers of muscle atrophy in a well-established murine model of infection. Moreover, these effects are more pronounced and prolonged with aging, providing a molecular link between flu infection and disability in older adults (Bartley et al. 2016). The pathogenesis of this interaction is unknown and little information exists detailing the systemic impact of flu infection and how it changes with aging. Here, we investigate if prior immunity can prevent the muscular ailment. Our goal is to provide translationally relevant insight on potential ways to protect older adults from flu-induced functional decrements and muscle atrophy.
The average flu-related mortality is between 20,000 and 40,000 people annually (White 2019). Flu vaccination substantially reduces mortality in humans (White 2019). Indeed, a 1% increase in total vaccination rate would result in an estimated 800 fewer deaths (White 2019). Through decades of research, it is clear that vaccination improves the humoral and cellular functions of the immune system in response to flu. Inactivated and recombinant flu vaccines induce robust antibody and CD4+ T cell responses. Neutralizing antibodies work to prevent infection, while non-neutralizing antibodies facilitate viral clearance via mechanisms such as antibody-dependent cell-mediated cytotoxicity (Kaur et al. 2011). This results in faster clearance of flu and less severe illness. Previous vaccination studies from our lab (Lefebvre et al. 2016) emphasized the two-pronged protection of flu nucleoprotein (NP) vaccination with antibody and lung-homing T cell effector generation to provide non-neutralizing protection. Though general vaccination efficacy is reduced with aging, inflammation and lung viral copies were reduced in both young and aged mice with NP vaccination.
While many benefits of vaccination have been previously reported, the effects of vaccination on muscle function and overall muscle quality, specifically in the more vulnerable aged population has not been explored. Indeed, muscle integrity and quality are key components when considering recovery, resilience, and prevention of physical disability in the aging population. Here, we investigated how non-neutralizing immunity induced by vaccination with NP impacts the muscular decrements observed during flu infection. NP vaccination induces a protective heterosubtypic antibody response in young mice (LaMere et al. 2011a; b), and reduces lung inflammation and susceptibility to secondary bacterial infection following primary flu infection (Haynes et al. 2012). Previously, we showed that NP vaccination of aged mice protected them from death following flu infection, but did not protect from flu-induced weight loss (Lefebvre et al. 2016). Thus, we hypothesized that NP vaccination would mediate protection in muscle through reduction in flu-induced inflammation.
Skeletal muscle is a complex tissue consisting of many different cell types, as well as different muscle fibers. The four main fiber types in the murine skeletal muscle are based on different muscle myosin heavy chains (MyHC). MyHC I fibers are considered slow twitch fibers, while MyHC IIA, IIX, and IIB fibers are fast twitch fibers (Sartorius et al. 1998; Augusto et al. 2004). Fiber areas and muscle cross-sectional areas in the skeletal muscles of mice differ with fiber sizes increasing in the order type I < IIA < IIX < IIB (Sartorius et al. 1998). The typical mouse skeletal muscle is 77% MyHC IIB (Augusto et al. 2004). With aging, type IIB and IIX fibers have impaired regeneration capacities in response to immobilization atrophy (Pattison et al. 2003). Translationally, fast twitch fibers control balance, strength, and numerous other necessary functions in humans and rodents. In human aging, there is marked muscle atrophy, along with a decrease in total number of fibers with preferential loss of type II muscle fibers (Thompson 1994). Indeed, the change in composition to greater slower-contracting isoforms leads to symptoms consistent with denervation (Suzuki et al. 2002). In addition to the quantitative loss, there is also a qualitative decline in muscle in terms of specific force (force generation normalized for muscle cross-sectional area), dysfunctional proteins, and other age-associated deficiencies (Brooks and Faulkner 1994; Thompson 1999; Thompson 2002; Thompson 2009). Typically, fast twitch muscle fibers are more vulnerable to degeneration and more sensitive to inflammation (Wright et al. 2017; Ciciliot et al. 2013; Nilwik et al. 2013). Importantly, the decline in muscle strength from type II fiber atrophy and loss can lead to sarcopenia, which increases risk of loss of activities of daily living. Since clinically there is an increase in loss of activities of daily living following flu infection and type II fibers are more sensitive to age-related loss and inflammatory stimuli, we hypothesize that flu will most severely affect type II fibers.
In this report, we examine the impact of NP vaccination on muscular function and quality in a preclinical model of flu infection with aging. As we have previously shown, NP vaccination leads to reduced inflammatory mediators in the bronchoalveolar lavage (BAL), high titers of NP-specific antibody, and improved lung viral clearance (Lefebvre et al. 2016). Here, we show that vaccination was able to preserve the function of young and aged mouse grip strength, gait kinematics, and voluntary mobility parameters, as well as reduce inflammation within the skeletal muscle, and prevent morphological disruption and type II muscle atrophy. Thus, our results point to a novel finding that despite reduced vaccine efficacy with aging, vaccination provides protection from flu-induced muscular impairments highlighting the clinical necessity for flu vaccination with aging.
Results
Prior vaccination prevents body mass loss and accelerates viral clearance during flu infection
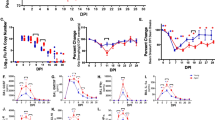
Utilizing established methods from our lab (Lefebvre et al. 2016) and others (LaMere et al. 2011a; Haynes et al. 2012), young (2.5–4 months) and aged (19–22 months) mice were vaccinated with NP/alum (Vax) or PBS control (NoVax), and infected with a sublethal dose of PR8 influenza virus (Online Resource 1). In unvaccinated mice, infection led to greater weight loss (Fig. 1a) and higher flu copy number in the lung (Fig. 1b) in both young and aged mice when compared with vaccinated groups. Young vaccinated mice were protected from weight loss and had accelerated viral clearance, while aged vaccinated mice only had partial protection. At 9 days postinfection (DPI), the vaccinated aged mice follow similar trends as unvaccinated young mice. We have previously shown that aged mice suffer greater and prolonged weight loss compared with young mice (Bartley et al. 2016). Similarly, young mice are more resilient than aged mice with vaccination. Despite similar peak virus levels in the lung at 7 DPI, vaccinated young and aged mice clear the virus faster than their unvaccinated controls (Fig. 1b), though differences between young and aged mice are still evident. Importantly, vaccination induced increased serum anti-NP IgG antibodies (Fig. 1c) supporting the efficacy of the vaccination. Overall, the NP/alum vaccination hastens viral clearance while reducing body mass losses in both young and aged mice.
The NP/alum vaccination hastens viral clearance while reducing body mass loss. Young and aged C57BL/6 mice were vaccinated with NP/alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. a The percent body mass loss graphed over the course of the experiment and bar graph of day 9 postinfection differences (n = 10–15/group). b Influenza acid polymerase (PA) copy number determined by RT-qPCR of mouse lungs at given time points (n = 5–6/group). c IgG titer for influenza nucleoprotein assayed via ELISA of mouse serum (n = 5–6/group). Data analyzed via two-way ANOVA with Bonferroni post hoc corrections comparing with NoVax/NoFlu age-matched controls (* = p < 0.05), comparing with NoVax/Flu age-matched controls (# = p < 0.05), and comparing between day postinfection/ages (brackets = p < 0.05)
Prior vaccination protects muscular function during flu infection
As we have shown previously (Bartley et al. 2016), flu infection without prior vaccination leads to functional decrements in voluntary locomotor activity, grip strength, and gait kinematics. Importantly, prior vaccination prevents these decrements in young mice and either prevents or reduces these decrements in aged mice. Postural gait parameters were assessed utilizing DigiGait at 7 DPI. Hind midline distance decreased with infection in both young and aged unvaccinated mice, while this decrease was not significantly different with vaccination only in young mice (Fig. 2A). Conversely, fore limb midline distance was only decreased in unvaccinated aged mice and vaccination prevented this decrease (Online Resource 2A). Thus, mice have a narrower hind stance during flu infection and vaccination was not able to prevent this narrowing in aged mice, however, the narrowing of the fore limb stance was protected with vaccination in aged mice. Hind and fore stride length increased with infection in aged unvaccinated mice, while this increase was prevented with vaccination in young and aged mice (Fig. 2B and Online Resource 2B). Kinematic gait parameters in the hind and fore limbs such as stride frequency (Fig. 2C and Online Resource 2C), time propelling (Fig. 2D and Online Resource 2D), stance time (Fig. 2E and Online Resource 2E), time in stride (Fig. 2F and Online Resource 2F), and time in swing (Fig. 2G and Online Resource 2G) also followed similar trends in aged mice. In contrast, young mice experienced no kinematic gait changes in their hind (Fig. 2C–G) or fore (Online Resource 2C-G) limbs during infection. Importantly, aged mice had increased time in the propel, stance, stride, and swing phases during infection, which was only partially protected with vaccination as some decrements were still evident in aged vaccinated mice (Fig. 2C–G). Overall, these results suggest that flu infection leads to a narrower stance and slower gait movements in aged mice and that vaccination preserved baseline gait parameters and protected aged mice from many flu-induced changes.
Vaccination against influenza reduced flu-induced functional decrements in voluntary locomotor activity, grip strength, and gait kinematics. Young and aged C57BL/6 mice were vaccinated with NP/alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. Mice were acclimated to testing prior to infection and tested for functional performance at designated time points. (A–G) Gait parameters were assessed utilizing DigiGait 7 days postinfection (n = 8–15/group). (H) Spontaneous voluntary activity was assessed via the open field test at 8 days postinfection (n = 10–15/group). (I) Grip strength was determined by using a grip strength meter at day 8 postinfection (n = 10–15/group). Data analyzed via two-way ANOVA with Bonferroni post hoc corrections comparing with NoVax/NoFlu age-matched controls (* = p < 0.05), comparing with NoVax/Flu age-matched controls (# = p < 0.05), and comparing between ages within a condition (brackets = p < 0.05)
Similarly, vaccination significantly protected both young and aged mice from decreased voluntary activity at 8 DPI, assessed by open field, compared with their unvaccinated counterparts (Fig. 2H). Infection without prior immunity led to an approximate 50% decrease of their initial voluntary activity by 8 DPI. Vaccinated mice were protected from this decrease, however aged vaccinated mice did not perform as well as their young vaccinated counterparts. Additionally, grip strength, as measured by a grip strength meter and normalized to pre-infection levels, was preserved in vaccinated mice, while unvaccinated mice had decreased strength (Fig. 2I) in both young and aged groups.
Vaccination mitigated alterations in cytokine/chemokine inflammatory milieu
Proinflammatory cytokine and chemokines (IFNγ, IL-6, and CXCL10) were assessed in the BAL from young and aged mice postinfection, and they followed similar trends (Fig. 3 A–C and F–H) with increased levels by 7 DPI in unvaccinated infected mice. Young vaccinated mice only showed modestly increased levels of proinflammatory signals during infection and returned to baseline levels by 9 DPI. Vaccination did not mitigate the peak levels of inflammation in aged cohorts but did hasten the resolution of inflammation by 9 DPI. In sum, vaccination prevented prolonged inflammation in aged BAL, but did not reduce peak inflammation at 7 DPI. Type 2 cytokines (IL-4 and IL-10) are generally considered anti-inflammatory and counteract inflammatory cytokines (Fig. 3 D–E and I–J ) (Wynn 2015; Gardner and Murasko 2017). IL-4 increased with infection, peaking at 7 DPI, and was higher in vaccinated mice compared with their unvaccinated age-matched counterparts (Fig. 3 D and I). Interestingly, only aged vaccinated mice still had elevated IL-4 at 9 DPI (Fig. 3D). IL-10 peaked in all groups at 7 DPI and returned to close to baseline levels by 9 DPI (Fig. 3 E and J). Vaccination of aged mice led to significantly increased in IL-10 at 7 DPI, compared with the unvaccinated aged mice (Fig. 3E), while vaccinated young mice had lower IL-10 levels at 7 DPI compared with unvaccinated young mice (Fig. 3E). It is likely the reduced inflammation in the young vaccinated mice did not require strong anti-inflammatory responses at that time point. In totality, vaccination reduced peak inflammation and limited prolonged inflammation in the BAL of young and aged mice while also promoting IL-4 and IL-10 production in aged mice.
Vaccination reduces flu-induced cytokines and chemokines in the BAL and serum. Young and aged C57BL/6 mice were vaccinated with NP/alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. (A–E) Bronchoalveolar lavage (BAL) was collected from mice at time of sacrifice as described in methods. The BAL was then analyzed via Millipore chemokine/cytokine multiplex (n = 16–21). (F–J) Group mean area under the curve (AUC) between 5 and 9 DPI for each cytokine. (K–O) Serum was collected from mice at the time of sacrifice as described in the “Methods” section and analyzed via Millipore chemokine/cytokine multiplex (n = 16–21). (P–T) Group mean AUC between 5 and 9 DPI for each cytokine. Data analyzed via three-way ANOVA (treatment × age × time) with Bonferroni post hoc corrections comparing with NoVax/NoFlu age-matched controls (* = p < 0.05) and comparing with NoVax/Flu age-matched controls (# = p < 0.05)
Systemic inflammation, as measured by serum cytokines and chemokines, followed a similar trend as in the BAL. Flu infection led to increased serum IFNγ, IL-6, and CXCL10 at 7 DPI in all groups (Fig. 3 K–M and P–R). Vaccination reduced serum IFNγ levels at 7 DPI in both young and aged compared with unvaccinated controls. Conversely, serum IL-6 was elevated by 5 DPI in all groups (Fig. 3 L and Q). Vaccinated mice began to decrease levels of IL-6 by 7 DPI, while unvaccinated young mice continued to increase at 7 DPI before returning to baseline levels (Fig. 3L) and unvaccinated aged mice levels remained elevated at 9 DPI (Fig. 3L). Serum CXCL10 increased with infection in all groups (Fig. 3 M and R), with only minor increases in the young vaccinated mice. Conversely, aged vaccinated mice followed similar trends to the unvaccinated young mice with increases at 7 DPI that returned to near baseline levels by 9 DPI (Fig. 3 M and R). Similar to IL-6, serum CXCL10 continued to rise in unvaccinated aged mice with peak levels seen at 9 DPI. Thus, the unvaccinated aged group had prolonged IL-6 and CXCL10 elevation at 9 DPI compared with other groups. Prior vaccination resulted in increased serum IL-4 at 7 and 9 DPI in young mice only (Fig. 3N). Serum IL-10 was increased at 7 and 9 DPI in young vaccinated mice (Fig. 3 O and T), but only at 9 DPI in aged vaccinated mice (Fig. 3 O and T). Importantly, serum IL-4 and IL-10 did not change in both young (Fig. 3N–O) and aged (Fig. 3S–T) unvaccinated mice, suggesting vaccination speeds up systemic anti-inflammatory signals. In sum, vaccination reduced prolonged systemic inflammation and provided modest increases in anti-inflammatory signals in aged mice. It is known that muscle atrophy can be triggered by external stress signals and overall inflammation status of an individual. Thus, we hypothesized that by limiting systemic flu-induced inflammation with vaccination, we could reduce the negative impact of flu on muscle.
Vaccination mitigates negative skeletal muscle gene and protein expression changes
To determine if vaccination-related reduction in BAL and serum inflammation would be associated with reduced negative changes in skeletal muscle, we first examined muscle gene and protein expression. We have previously shown that flu infection results in dramatic increases in muscle atrophy genes (Bartley et al. 2016). Here, we found that vaccination mitigates flu-induced changes in skeletal muscle gene expression (Fig. 4A–J) and protein expression in gastrocnemius (Fig. 4K–T). Vaccination in both young and aged (Fig. 4 A and F) mice prevents MuRF-1 expression (considered a major regulator of skeletal muscle atrophy). Similarly, ubiquitin C (UBC) upregulation is reduced with vaccination in young mice (Fig. 4B), with minor upregulation evident in aged vaccinated mice at 9 DPI (Fig. 4 B and G). Conversely, unvaccinated aged mice show dramatic upregulation at 9 DPI (Fig. 4B). This suggests that vaccination completely protects young mice from muscle atrophy gene expression changes, while only partially protecting aged mice. Insulin growth factor-1 (IGF-1), a positive regulator of the mTOR pathway that promotes muscle hypertrophy and suppresses atrophy gene expression (White 2016; Zou et al. 2018), was surprisingly not statistically significantly decreased, but was trending towards significance in unvaccinated infected groups 7 DPI (young NoVax/Flu p = 0.08 and aged NoVax/Flu p = 0.09) (Fig. 4 D and I). The aged vaccinated mice had an increase at 9 DPI (Fig. 4 D and I). It is possible that a decrease in IGF-1 was missed in the unvaccinated groups during the experimental time course. It is also possible that an increase in IGF-1 was not observed in the young vaccinated mice due to decreased damage and need for any muscle repair. Myoblast determination protein 1 (MyoD), a critical factor regulating satellite cell proliferation and muscle repair, was downregulated in young and aged unvaccinated mice at 7 DPI (Fig. 4 E and J), but remained downregulated at 9 DPI only in unvaccinated aged mice compared with their aged vaccinated counterparts (Fig. 4 E and J). Conversely, vaccination protected both young and aged mice from downregulation of MyoD (Fig. 4 E and J). Thus, vaccination promoted positive muscle regulators of both young and aged mice at later time points, suggesting accelerated healing from flu-induced muscle damage.
Vaccination reduces flu-induced skeletal muscle gene expression and protein expression in the gastrocnemius. Young and aged C57BL/6 mice were vaccinated with NP/alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. (A–E) Vaccination reduced changes in gastrocnemius muscle gene expression (n = 10–19). (F–J) Group mean AUC between 5 and 9 DPI for each gene. (K–O) Vaccination altered the kinetics of muscle cytokine, chemokine, and myokine levels (n = 4–6). (P–T) Group mean AUC between 5 and 9 DPI for each cytokine. Data analyzed via three-way ANOVA (treatment × age × time) with Bonferroni post hoc corrections comparing with NoVax/NoFlu age-matched controls (* = p < 0.05) and comparing to NoVax/Flu age-matched controls (# = p < 0.05)
Since it is known that the inflammatory milieu of the skeletal muscle affects muscle atrophy and muscle repair pathways (Bartley et al. 2016), we next examined how vaccination impacts the flu-induced cytokine and chemokine protein expression in the gastrocnemius muscle. Not surprisingly, baseline differences existed in multiple cytokines and chemokines (Gomez et al. 2005; Bruunsgaard et al. 2001; Morley and Baumgartner 2004; Hawkley and Cacioppo 2004). IFNγ only significantly increased in unvaccinated aged mice at 7 DPI and unvaccinated young mice at 9 DPI, while vaccinated groups are protected from this change (Fig. 4 K and P). Conversely, IL-6 changes are not vaccination dependent. Young mice have increased IL-6 at 9 DPI (Fig. 4 L and Q), and aged mice have increased IL-6 at 7 DPI irrespective of vaccination status (Fig. 4 L and Q). CXCL10, a chemokine for recruiting type 1 inflammatory cells, remained unchanged in young unvaccinated and vaccinated mice (Fig. 4 M and R), while it is elevated at 7 DPI in both unvaccinated and vaccinated aged mice but to a greater degree in the unvaccinated mice (Fig. 4 M and R). By 9 DPI, only unvaccinated aged mice have elevated CXCL10, suggesting that vaccination prevented prolonged expression of this chemokine (Fig. 4 M and R). In totality, since IL-6 actions are pleiotropic in the skeletal muscle, it is hard to distinguish the pathology of the discordant increases with age. Importantly, however, IL-6 in the aged muscle is increased at the same time as IFNγ and CXCL10. This suggests a more inflammatory environment. In contrast, in vaccinated young mice, there is no simultaneous IFNγ or CXCL10 increase, possibly suggesting an appropriate signal for myogenesis. On the anti-inflammatory side, IL-4 and IL-10 were both increased in aged mice at baseline (NoVax/NoFlu) compared with young mice (NoVax/NoFlu) (Fig. 4N, O). IL-4 increased steadily in both unvaccinated and vaccinated aged mice, and was elevated at 7 DPI and 9 DPI with greater levels in the vaccinated aged group (Fig. 4 N and S), while young mice did not exhibit any changes in IL-4 irrespective of vaccination (Fig. 4 N and S). IL-10 was increased in all groups except the unvaccinated aged mice at 9 DPI (Fig. 4 O and T). It is likely that the lower levels of inflammatory signals in young mice did not require a robust anti-inflammatory response as evident by the low levels of IL-4 (Fig. 4N). Furthermore, this is corroborated by the gene expression changes at this time showing that young mice have decreased muscle atrophy gene expression when compared with aged mice with or without vaccination. In totality, vaccination prevents muscle atrophy gene expression changes in young mice and reduces muscle atrophy gene expression in aged mice, which is accompanied by similar changes in the inflammatory milieu including reduced proinflammatory signals and increased anti-inflammatory signals in the aged vaccinated mice.
Vaccination reduces muscle morphological changes and fast twitch fiber atrophy
To determine if the changes in gene expression and inflammatory milieu resulted in detectable changes in muscle morphology and architecture, we investigated gastrocnemius histology. Cross-sections of the gastrocnemius muscle of unvaccinated and vaccinated infected mice at 9 DPI were stained with hematoxylin and eosin (H&E), and blindly scored on multiple histological parameters adapted from the literature (Meador et al. 2008; Mikhak et al. 2006). Flu infection increased architectural damage and cellular infiltration in young and aged unvaccinated mice (Fig. 5a, b). Importantly, vaccination mitigated these changes in both age groups (Fig. 5a. b and Online Resource 3A-B). Similarly, total H&E scores showed pronounced protection from morphological changes with vaccination (Fig. 5b). Further immunohistochemistry revealed that flu infection induced fiber-specific atrophy, where type IIB fibers were the only ones detrimentally affected during infection (Fig. 5c, d and Online Resource 3C). Type IIB fibers lose one-third of their cross-sectional area by 9 DPI in both unvaccinated groups (Fig. 5 c and d), while vaccination prevented these losses. Type I, IIA, and IIX muscle fibers were not severely impacted during flu infection. This agrees with the general consensus that MyHC IIB is more sensitive to inflammation and other stressors (Wright et al. 2017; Ciciliot et al. 2013; Nilwik et al. 2013).
Vaccination reduces muscle morphological changes and fast twitch fiber atrophy. Young and aged C57BL/6 mice were vaccinated with NP/Alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. Mice were sacrificed at 9 days postinfection and the gastrocnemius muscle was harvested for histological analyses. a Represented hematoxylin and eosin (H&E) staining of the gastrocnemius skeletal muscle from uninfected (left), unvaccinated/infected (middle), and vaccinated/infected (right) 9 DPI aged mice. Scale bars are 100 μm. b Quantification (detailed in methods) of muscle morphological changes (n = 3–5). c Muscle cross-sectional area via immunofluorescent staining from uninfected (left), unvaccinated/infected (middle), and vaccinated/infected (right) 9 DPI aged mice. Colors: myosin heavy chain I (red), myosin heavy chain IIA (green), myosin heavy chain IIB (yellow), myosin heavy chain IIX (no stain), nuclei (cyan). Scale bars are 100 μm. d Quantification of fiber type cross-sectional area (n = 3–5). Data analyzed via two-way ANOVA with Bonferroni post hoc corrections comparing age-matched counterparts (* = p < 0.05)
Discussion
With age, the immune response to flu infection diminishes, resulting in slower viral clearance and increased lung inflammation (Lefebvre et al. 2016). Indeed, unvaccinated aged mice follow this pattern in our study. Aged mice are typically slower to clear virus and exhibit higher and prolonged inflammatory cytokines in the BAL and serum when compared with young. This lingering inflammation in response to flu is associated with dramatic changes in mouse mobility, gait kinematics, strength, and other functional parameters. Interestingly, vaccination in aged mice reduced weight loss, but did not prevent it as it did in young mice. Vaccination also resulted in faster viral clearance and improved anti-IgG antibody titers in both young and aged mice. In older adults, flu infection increases the susceptibility to secondary infection and other flu complications (Keilich et al. 2019). Thus, ways to better control viral infection, especially in older adults, are a clinically important goal. Here, we show that vaccination not only controls viral loads in the lung, but also prevents flu-induced muscle dysfunction, both on a functional and molecular level. We believe that the impact of flu infection on muscle may directly predispose older adults for catastrophic disability and sarcopenia, as well as increase risk of falls and other musculoskeletal injuries. Thus, we have identified vaccination as a proactive protective preventative measure with the potential to increase resilience in older adults.
We previously demonstrated that flu infection leads to prolonged muscular deficits, both functionally and molecularly, with more pronounced changes in aged mice compared with young mice (Bartley et al. 2016). This provided a molecular link for the increased risk of disability in older adults following a flu infection seen clinically. Here, we investigated the clinical significance of prior immunity to protect against flu-induced muscle decrements. Indeed, we have shown that vaccination mitigates functional losses. Importantly, vaccination reduces the decrements in voluntary mobility, grip strength, and gait kinetics observed with flu infection in aged mice.
Vaccination also protected young and aged mice from increased and prolonged systemic inflammation. Some level of inflammation is necessary to clear flu virus from the lungs and, in fact, without IL-6, mice die from flu infection (Yang et al. 2017; Lauder et al. 2013). Similarly, without adaptive immune cell trafficking to the lung via ligands, such as CXCL10, there is no proper viral clearance (Oslund et al. 2014). We emphasize here that prolonged inflammatory responses exacerbate the damage to the skeletal muscle. IFNγ is primarily produced by immune cells and has been known to trigger CXCL10 expression (Oslund et al. 2014; Groom and Luster 2011; Mikhak et al. 2006). The production of these inflammatory cytokines and chemokines were not completely prevented in our vaccinated aged mice, but their prolonged elevation was mitigated. Vaccinated groups also had higher levels of IL-4 and IL-10 in the BAL, serum, and skeletal muscle. While serum IL-4 and IL-10 play a role in IgG antibody responses (Mathers and Cuff 2004), they also act to suppress the systemic inflammation seen during peak infection. In fact, we see increases in IL-4 and IL-10 in the BAL and serum at 7 and 9 DPI, as viral titers are diminishing and the lung is polarized towards type-2 regenerative response. These factors are also crucial to skeletal muscle regeneration. While IL-6, IL-1β, and TNFα have all been associated with skeletal muscle atrophy Frost and Lang 2005; Guttridge 2004; Roth et al. 2006; Kumar et al. 2002) and contribute to a catabolic environment which over time reduces muscle function in humans and rodents, IL-4 and IL-10 have crucial roles in muscle repair and regeneration. IL-4 is a molecular signal that controls myoblast fusion with myotubes. Indeed, muscle cells lacking IL-4 signaling/receptors form normally but are reduced in size and myonuclear number (Horsley et al. 2003). Absence of IL-10 is associated with elevated IL-6, IL-1β, and TNFα expression in response to lipopolysaccharide (LPS) in the skeletal muscle, with aging further exacerbating these responses (Meador et al. 2008). Similarly, both IL-4 and IL-10 trigger changes in the macrophage phenotype that promotes muscle growth and regeneration (Makita et al. 2014; Deng et al. 2012). In fact, muscle growth and regeneration are greatly slowed by loss of IL-10 (Deng et al. 2012). Our research confirms that flu infection induces a proinflammatory environment in the muscle and that vaccination can increase the anti-inflammatory milieu with IL-4 and IL-10.
This more anti-inflammatory environment generated upon vaccination corresponded with both improved muscle gene expression and preserved muscle morphology. Fast twitch muscle fibers (mainly MyHC IIB) are most sensitive to inflammation (Wright et al. 2017; Ciciliot et al. 2013; Nilwik et al. 2013) and we found that they were significantly decreased in size by 9 DPI in unvaccinated young and aged mice. Similarly, histology scores showed poor architecture and increased cellularity at this time point in unvaccinated mice. Importantly, these same mice had increased expression of the atrophy genes Murf-1 and UBC, as well as pronounced functional deficits. Vaccination reduced or prevented all of these declines with preserved type IIB fiber cross-sectional area and morphology, as well as reduced expression of atrophy genes and less impairment of muscle function. Indeed, there was no visible difference between naïve controls and vaccinated mice of both age groups. Additionally, we observed upregulated skeletal muscle IGF1 in vaccinated aged mice at 9 DPI. In vitro, IGF-1 stimulation of C2C12 myotubes increased mouse type IIB MyHC mRNA, suggesting IGF-1 has a role in MyHC IIB gene expression (Shanely et al. 2009). Further, overexpression of IGF-1 for 9 months in the extensor digitorum longus (EDL) muscles (primarily composed of type IIB fibers) prevented muscle atrophy and loss of type IIB fibers in aged mice (Barton-Davis et al. 1998). This suggests that vaccination is also promoting positive muscle signals to prevent loss of function. Our research clearly highlights the association between inflammatory milieu, muscle gene expression, muscle atrophy, and muscle function.
As previously mentioned, we anticipated the fiber-specific cross-sectional area changes due to the increased sensitivity to stressors in type II fibers. Translationally, fast twitch fibers control balance, strength, and numerous other necessary functions in humans and rodents. It is possible that the type II fiber atrophy explains the narrowing of stance in infected mice and potentially the increased fall risk in humans due to both weakness and lack of balance. In long-stay nursing home residents, influenza-like illnesses are associated with a 13% average increase in hip fracture hospitalization risk (McConeghy et al. 2017). The corresponding observations during acute illness focused on unsteady gait and dizziness, which also included a greater risk of falls and broken bones (McConeghy et al. 2017). The loss of fast twitch fibers in our mice might suggest a potential mechanism connecting flu-induced fast twitch fiber loss with fall risk and frailty. Importantly, the decrease in mouse fast twitch fiber cross-sectional area was prevented by vaccination in both young and aged mice. Correspondingly, function was preserved in vaccinated mice as well. We also observed increased cellularity in the H&E of unvaccinated mice. In other animal models, cachexia was caused by CD8+ T cells during anti-viral responses (Baazim et al. 2019) and some suggest the possibility of autoimmune reactivity (Nagaraju et al. 2000; Prieto and Grau 2010; Pummerer et al. 1996; Rose et al. 1988; Suzuki et al. 2009). Future research is necessary to confirm the identity and function of these infiltrating cells during flu infection as it is possible that these cells could be a potential target for therapeutics.
In summary, this manuscript is the first to identify in a controlled experimental setting flu-induced muscle inflammation and atrophy mitigation by vaccination/prior immunity. We demonstrated that prior immunity, induced by vaccination, prevents muscle fast twitch fiber atrophy and consequently protects muscle functionality. We also determined that vaccine-induced protection is not NP antibody mediated (data not shown), and more research is necessary to determine if protection is solely mediated via a T cell–dependent mechanism. As disability is one of the major complications of flu infection in older adults, the goal of this study was to determine if prior non-neutralizing immunity could provide protection to skeletal muscle. We determined that vaccination indeed could prevent or reduce muscle decrements due to flu infection despite reduced vaccine efficacy with aging. Thus, flu vaccination is still an essential part of protecting older adults from flu-induced disability. Future research may be able to identify specific pathways and therapeutics to fully prevent flu-induced atrophy and potential loss of quality of life in older adults.
Methods
Mice
Young (2.5–4-month old) C57BL/6 male mice were purchased from Jackson Laboratories or obtained from the National Institute on Aging. Aged (19–22-month old) C57BL/6 male mice were obtained from the National Institute on Aging rodent colony. All mice were housed in a climate controlled environment with 12:12 light:dark cycle and fed standard rodent chow and water ad libitum. All mice were cared for in accordance with the recommendations in the Guide for the Care and use of Laboratory Animals of the National Institutes of Health. All procedures were approved by the University of Connecticut Medical School IACUC, protocol number 100705. Recumbent mice and mice that lost more than 30% body weight were considered moribund and euthanized. All mice underwent gross pathological examination at time of sacrifice and animals with obvious pathology were excluded from the study.
Viral infection
Mice were anesthetized with isoflurane and intranasally inoculated with 50 μL of 500 EID50 of influenza virus A/PR/8/34 (PR8). Mice were weighed daily to monitor infection progression. At time points indicated, whole lung tissue was homogenized and RNA was isolated via TRIzol/chloroform extraction per manufacturer’s protocol (Ambion by Life Technologies, Naugatuck, CT, and Sigma Aldrich, Natick, MA, respectively). RNA was reverse transcribed with iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA) and flu viral copies were detected via reverse transcription quantification PCR of flu acid polymerase (PA) as previously described (Jelley-Gibbs et al. 2007).
Vaccination
Recombinant A/PR/8/ influenza nucleoprotein (NP) was generated by the Protein Expression Core at UConn Health. Immunizations were prepared using NP protein and Imject® Alum (Thermo Scientific) at 1:1. Injections were administered intraperitoneally at a concentration of 30 μg NP in 100 μL per mouse. Control mice were given 100 μL PBS. Previous studies in our laboratory showed no differences between PBS control and PBS/alum control (Lefebvre et al. 2016). Mice received one dose of vaccination or control at 30 days and a second dose at 20 days prior to influenza infection (LaMere 2011).
Antibody titers
96-well plates were coated with recombinant A/PR/8/34 influenza NP (generated by UConn Health Protein Expression Core). Serum samples were serial diluted (1:10 to 1:1 × 108). Anti-NP IgG titers in serum were then determined using anti-mouse IgG-HRP (Southern Biotech, Birmingham, AL) and o-phenylenediamine (Sigma, Natick, MA) buffered in hydrogen peroxide. Samples were read in duplicate (490 nm, Bio-Rad iMark microplate reader, Hercules, CA). Antibody titers were determined by the last serum dilution with an optical density above background.
Gastrocnemius reverse transcription quantitative PCR
At the time points indicated for gastrocnemius gene expression, mice were fasted with the exception of water for 4–6 h prior to sacrifice to minimize potential confounding results due to postprandial muscle protein synthesis. The gastrocnemius muscle was dissected and placed in RNAlater (Qiagen Inc., Germantown, MD) overnight at 4 °C. RNAlater was removed and gastrocnemius was frozen at − 80 °C until RNA extraction. The muscle was homogenized and RNA was extracted via TRIzol/chloroform extraction per manufacturer’s instructions (Ambion by Life Technologies and Sigma Aldrich, respectively). RNA quantity and quality was assessed with Nanodrop 2000c (Thermo Scientific, Waltham, MA) and was reverse transcribed via iScript Advanced cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). Reverse transcription quantitative PCR (RT-qPCR) was performed using predesigned commercially available primers (Bio-Rad Laboratories, Inc.). Gene expression was calculated via a modified Pfaffl method utilizing multiple reference genes (RPS18 and TBP, which showed the least variability between conditions and thus suitable reference genes as previously shown (Bartley et al. 2016)) and normalized to the unvaccinated uninfected young mice to give comparable changes.
Multiplex protein analysis
BAL fluid was collected by flushing lungs with 1 mL PBS. Supernatant was collected after centrifugation and assayed for cytokine and chemokine content. Similarly, the blood was collected via cardiac puncture, allowed to clot at room temperature, and the resultant serum was assayed for cytokine and chemokine content. Gastrocnemius muscle was dissected from each mouse and homogenized in Tissue Protein Extraction Reagent (Thermo Scientific, Bedford, MA) supplemented with 5 mM EDTA (Invitrogen, Carlsbad, CA) and Protease/Phosphatase Inhibitor Cocktail (Thermo Scientific)). Debris was removed via centrifugation and total protein content was determined using a Pierce™ BCA Protein Assay Kit (Thermo Scientific).
Voluntary locomotor activity
Spontaneous voluntary locomotor activity was measured via open field test at time points indicated. All tests were performed between 7 and 9 am to control for diurnal variations. Following acclimation to the dim-lit testing room (at least 1 h), mice were placed in the center of the photobeam activity system-open field (PAS-OF, 16”×16”×15” acrylic animal enclosure, San Diego Instruments, San Diego, CA) and their activity was recorded for 20 min. The first 5 min was excluded as this is generally considered to be exploratory behavior rather than general voluntary locomotor activity. The number of beam breaks per minute during the last 15 min was then used to assess voluntary locomotor activity.
Grip strength
Grip strength was determined by using a grip strength meter BIOSEB In Vivo Research Instruments, Pinellas Park, FL. Briefly, mice were permitted to grab onto a T-shaped bar and pulled horizontally by the tail until they released their grip. The force (grams) read by the force meter at the release of the mouse’s grasp was averaged by their mass (grams) on that day and compared with their pre-infection results. The same researcher performed all testing to minimize variability.
Gait analysis
Gait analysis was performed using the DigiGait instrument (Mouse Specifics, Inc. Quincy, MA) and software (DigiGait Imager 4.0.0 and DigiGait Analysis 11.5, Mouse Specifics, Inc.). The DigiGait instrument consists of a clear treadmill with a high-speed camera mounted underneath that collects images at 147 frames per second for high resolution of postural temporal gait parameters. Mice run within a 2-in.-wide acrylic running chamber at set speeds. The ventral plane videos are analyzed with the DigiGait software which identifies portions of the paw that are in contact with the treadmill belt to produce both postural and kinematic gait parameters. Mice were introduced to the DigiGait system at a low speed (10 cm/s) briefly (30 s) prior to the initial testing. Mice were allowed to acclimate to the dim-lit room for 1 h prior to each testing period and all tests were performed between 8 and 10 am. Mice ran at the testing speed (16 cm/s, as previously optimized (Bartley et al. 2016)) until approximately 5 s of consecutive walking was recorded, and this video segment was analyzed via DigiGait software.
Histology
The gastrocnemius muscle was carefully dissected and blotted on a Kim Wipe before being placed in a cryomold, embedded in optimal cutting temperature (OCT, ThermoScientific Inc., Waltham, MA) and frozen in liquid nitrogen cooled isopentane. Samples were stored at − 80° C freezer until sectioning. A total of 10 μm thick muscle cross-sections were mounted on charged slides (Superfrost Plus Glass Slides, ThermoScientific Inc., Waltham, MA) for histological analyses. Standard H&E staining was performed and imaged at × 20. H&E images were blindly scored by multiple evaluators utilizing a rubric adapted from the literature (Jin et al. 2017; Erben et al. 2014). The rubric was based on two criteria, i.e., (1) infiltration of nuclei and (2) architecture disruption and tissue damage. Each criterion was independently scored from 0 to 3 and combined for a total score that ranged 0–6.
Fiber type staining
Primary antibodies for muscle fiber types were acquired from the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa, i.e., myosin heavy chain type I (DSHB, BA-D5), myosin heavy chain type IIA (DSHB, SC-71), and myosin heavy chain type IIB (DSHB, BF-F3). Secondary antibodies Alexa Fluor 647 (Invitrogen Carlsbad, CA), Alexa Fluor 488 (Life Technologies St. Petersburg, FL), and Alexa Fluor 555 (Life Technologies St. Petersburg, FL) were utilized, respectively. Nuclei were stained with Hoechst 33342 (Life Technologies St. Petersburg, FL). Stained slides were imaged via confocal microscopy (Zeiss LSM 880, Peabody, MA). Cross-sectional area was quantified using ImageJ (NIH, Bethesda, MD).
Statistical analysis
All data were analyzed via two-way (treatment × age) or three-way (treatment × age × time) ANOVA with Bonferroni post hoc corrections. Significance was set at p < 0.05. Area under the curve (AUC) was calculated with group means (as study design did not allow for repeated measures in the same mice over time) via standard trapezoidal method.
References
Augusto V, Padovani CR, Campos GR. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphol Sci. 2004;21(2):89–94. https://doi.org/10.1055/s-00040444.
Baazim H, Schweiger M, Moschinger M, Xu H, Scherer T, Popa A, et al. CD8+ T cells induce cachexia during chronic viral infection. Nat Immunol. 2019;20(6):701–10. https://doi.org/10.1038/s41590-019-0397-y.
Bartley JM, Pan SJ, Keilich SR, Hopkins JW, Al-Naggar IM, Kuchel GA, et al. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY). 2016;8(4):620. https://doi.org/10.18632/aging.100882.
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci. 1998;95(26):15603–7. https://doi.org/10.1073/pnas.95.26.15603.
Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26(4):432–9.
Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–6. https://doi.org/10.1097/00062752-200105000-00001.
Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45(10):2191–9. https://doi.org/10.1016/j.biocel.2013.05.016.
Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189(7):3669–80. https://doi.org/10.4049/jimmunol.1103180.
Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557–76.
Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277(9):728–34. https://doi.org/10.1001/jama.1997.03540330050034.
Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8(3):255–63. https://doi.org/10.1097/01.mco.0000165003.16578.2d.
Gardner EM, Murasko DM. Age-related changes in type 1 and type 2 cytokine production in humans. In: Handbook of Immunosenescence: Basic Understanding and Clinical Implications; 2017. p. 1–34. https://doi.org/10.1023/a:1020151401826.
Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17(5):457–62. https://doi.org/10.1016/j.coi.2005.07.013.
Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–31. https://doi.org/10.1016/j.yexcr.2010.12.017.
Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(4):443–50. https://doi.org/10.1097/01.mco.0000134364.61406.26.
Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav Immun. 2004;18(2):114–9. https://doi.org/10.1016/j.bbi.2003.09.005.
Haynes L, Szaba FM, Eaton SM, Kummer LW, Lanthier PA, Petell AH, et al. Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections. J Immunol. 2012;189(10):4921–9. https://doi.org/10.4049/jimmunol.1201916.
Heron M. Deaths: leading causes for 2010. Natl Vital Statistics Rep. 2013;62(6):1–96 Hyattsville, MD: National Center for Health Statistics.
Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113(4):483–94. https://doi.org/10.1016/s0092-8674(03)00319-2.
Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178(12):7563–70. https://doi.org/10.4049/jimmunol.178.12.7563.
Jin RM, Blair SJ, Warunek J, Heffner RR, Blader IJ, Wohlfert EA. Regulatory T cells promote myositis and muscle damage in Toxoplasma gondii infection. J Immunol (Baltimore, Md. : 1950). 2017;198(1):352–62. https://doi.org/10.4049/jimmunol.1600914.
Kaur K, Sullivan M, Wilson PC. Targeting B cell responses in universal influenza vaccine design. Trends Immunol. 2011;32(11):524–31. https://doi.org/10.1016/j.it.2011.08.007.
Keilich SR, Bartley JM, Haynes L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell Immunol. 2019:103992. https://doi.org/10.1016/j.cellimm.2019.103992.
Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26:D59–66. https://doi.org/10.1016/j.vaccine.2008.07.025.
Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002;6(6):500–8. https://doi.org/10.1186/cc1822.
LaMere MW, Lam H-T, Moquin A, Haynes L, Lund FE, Randall TD, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011a;186(7):4331–9. https://doi.org/10.4049/jimmunol.1003057.
LaMere MW, Moquin A, Lee FE-H, Misra RS, Blair PJ, Haynes L, et al. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol. 2011b;85(10):5027–35. https://doi.org/10.1128/JVI.00150-11.
Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43(10):2613–25. https://doi.org/10.1002/eji.201243018.
Lefebvre JS, Lorenzo EC, Masters AR, Hopkins JW, Eaton SM, Smiley ST, et al. Vaccine efficacy and T helper cell differentiation change with aging. Oncotarget. 2016;7(23):33581. https://doi.org/10.18632/oncotarget.9254.
Makita N, Hizukuri Y, Yamashiro K, Murakawa M, Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. 2014;27(3):131–41. https://doi.org/10.1093/intimm/dxu090.
Mathers AR, Cuff CF. Role of interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic reovirus infection. J Virol. 2004;78(7):3352–60. https://doi.org/10.1128/JVI.78.7.3352-3360.2004.
McConeghy KW, Lee Y, Zullo AR, Banerjee G, Daiello L, Dosa D, et al. Influenza illness and hip fracture hospitalizations in nursing home residents: are they related? J Gerontol Ser A. 2017;73(12):1638–42. https://doi.org/10.1093/gerona/glx200.
McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30(12):2060–7. https://doi.org/10.1016/j.vaccine.2012.01.015.
Meador BM, Krzyszton CP, Johnson RW, Huey KA. Effects of IL-10 and age on IL-6, IL-1β, and TNF-α responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J Appl Physiol. 2008;104(4):991–7. https://doi.org/10.1152/japplphysiol.01079.2007.
Mikhak Z, Fleming CM, Medoff BD, Thomas SY, Tager AM, Campanella GS, et al. STAT1 in peripheral tissue differentially regulates homing of antigen-specific Th1 and Th2 cells. J Immunol. 2006;176(8):4959–67. https://doi.org/10.4049/jimmunol.176.8.4959.
Morley JE, Baumgartner RN. Cytokine-related aging process, vol. 59: Oxford University Press; 2004. p. M924–9. https://doi.org/10.1093/gerona/59.9.M924.
Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci. 2000;97(16):9209–14. https://doi.org/10.1073/pnas.97.16.9209.
Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–8. https://doi.org/10.1016/j.exger.2013.02.012.
Oslund KL, Zhou X, Lee B, Zhu L, Duong T, Shih R, et al. Synergistic up-regulation of CXCL10 by virus and IFN γ in human airway epithelial cells. PLoS One. 2014;9(7):e100978. https://doi.org/10.1371/journal.pone.0100978.
Pattison JS, Folk LC, Madsen RW, Childs TE, Spangenburg EE, Booth FW. Expression profiling identifies dysregulation of myosin heavy chains IIb and IIx during limb immobilization in the soleus muscles of old rats. J Physiol. 2003;553(2):357–68. https://doi.org/10.1113/jphysiol.2003.047233.
Prieto S, Grau JM. The geoepidemiology of autoimmune muscle disease. Autoimmun Rev. 2010;9(5):A330–4. https://doi.org/10.1016/j.autrev.2009.11.006.
Pummerer CL, Luze K, Grässl G, Bachmaier K, Offner F, Burrell SK, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97(9):2057–62. https://doi.org/10.1172/JCI118642.
Rose NR, Herskowitz A, Neumann DA, Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988;9(4):117–20. https://doi.org/10.1016/0167-5699(88)91282-0.
Roth SM, Metter EJ, Ling S, Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18(6):625–30. https://doi.org/10.1097/01.bor.0000245722.10136.6d.
Sartorius CA, Lu BD, Acakpo-Satchivi L, Jacobsen RP, Byrnes WC, Leinwand LA. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J Cell Biol. 1998;141(4):943–53. https://doi.org/10.1083/jcb.141.4.943.
Shanely RA, Zwetsloot KA, Childs TE, Lees SJ, Tsika RW, Booth FW. IGF-I activates the mouse type IIb myosin heavy chain gene. Am J Phys Cell Phys. 2009;297(4):C1019–27. https://doi.org/10.1152/ajpcell.00169.2009.
Suzuki T, Bless DM, Connor NP, Ford CN, Lee K, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. Ann Otol Rhinol Laryngol. 2002;111(11):962–7. https://doi.org/10.1177/000348940211101102.
Suzuki S, Utsugisawa K, Yoshikawa H, Motomura M, Matsubara S, Yokoyama K, et al. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Arch Neurol. 2009;66(11):1334–8. https://doi.org/10.1001/archneurol.2009.229.
Thompson LV. Effects of age and training on skeletal muscle physiology and performance. Phys Ther. 1994;74(1):71–81. https://doi.org/10.1093/ptj/74.1.71.
Thompson L. Contractile properties and protein isoforms of single skeletal muscle fibers from 12-and 30-month-old Fischer 344 Brown Norway F1 hybrid rats. Aging Clin Exp Res. 1999;11(2):109–18. https://doi.org/10.1007/BF03399649.
Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002;32(2):44–57. https://doi.org/10.2519/jospt.2002.32.2.44.
Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44(1–2):106–11. https://doi.org/10.1016/j.exger.2008.05.003.
White JP. Control of skeletal muscle cell growth and size through adhesion GPCRs: Adhesion G Protein-coupled Receptors, Springer; 2016. p. 299–308. https://doi.org/10.1007/978-3-319-41523-9_13.
White C. Measuring social and externality benefits of influenza vaccination. J Hum Resour: 1118-9893R1112. 2019. https://doi.org/10.3368/jhr.56.3.1118-9893R2.
Wright CR, Allsopp GL, Addinsall AB, McRae NL, Andrikopoulos S, Stupka N. A reduction in selenoprotein S amplifies the inflammatory profile of fast-twitch skeletal muscle in the mdx dystrophic mouse. Mediat Inflamm. 2017;2017:1–12. https://doi.org/10.1155/2017/7043429.
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82. https://doi.org/10.1038/nri3831.
Yang M-L, Wang C-T, Yang S-J, Leu C-H, Chen S-H, Wu C-L, et al. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep. 2017;7:43829. https://doi.org/10.1038/srep43829.
Zou Y, Dong Y, Meng Q, Zhao Y, Li N. Incorporation of a skeletal muscle-specific enhancer in the regulatory region of Igf1 upregulates IGF1 expression and induces skeletal muscle hypertrophy. Sci Rep. 2018;8(1):2781. https://doi.org/10.1038/s41598-018-21122-5.
Acknowledgments
This research was partially conducted while Jenna Bartley was a Glenn/AFAR Postdoctoral Fellow. The primary monoclonal antibodies for myosin heavy chain type I (BA-D5), IIA (SC-71), and IIB (BF-F3), developed by S. Schiaffino at the University of Padova, was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH, and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. The authors would like to thank Sandra Jastrzebski and Darcy Ahern for their assistance with experimental assays and manuscript preparation.
Funding
This work was supported by the National Institutes of Health/National Institute on Aging grants AG021600 and AG060389 (to L.H.)
Author information
Authors and Affiliations
Contributions
SRK, ECL, JMB, and LH conceived and designed the experiments. SRK, ECL, AGH, and BLT carried out experiments. SRK and ECL analyzed data. SRK led data interpretation and manuscript preparation. JMB and LH supervised the project and assisted with data interpretation and acquisition of funding. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
All mice were cared for in accordance with the recommendations in the Guide for the Care and use of Laboratory Animals of the National Institutes of Health. All procedures were approved by the University of Connecticut Medical School IACUC, protocol number 100705.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
Experimental plan for the experiments described. The timeline for our experiments depicting when mice were vaccinated, infected, and sacrificed. Figure made with biorender.com (PNG 439 kb)
Online Resource 2
Vaccination to influenza reduced flu-induced functional decrements in voluntary locomotor activity, grip strength, and gait kinematics. Young and aged C57BL/6 mice were vaccinated with NP/Alum or PBS before being intranasally infected with 500 EID50 of PR8 influenza. Mice were acclimated to testing prior to infection and tested for functional performance at designated time points. A-G) Gait parameters were assessed utilizing DigiGait, a ventral plane videography treadmill system 7 days post infection (n = 8–15). G) Spontaneous voluntary activity was assessed via the open field test at 8 days post infection (n = 10–15/group). I) Grip strength was determined by using a grip strength meter at day 8 post infection (n = 10–15/group). Data analyzed via two-way ANOVA with Bonferroni post hoc corrections comparing to NoVax/NoFlu age-matched controls (* = p < 0.05), comparing to NoVax/Flu age-matched controls (# = p < 0.05), and comparing between ages within a condition (brackets = p < 0.05) (PNG 141 kb)
Online Resource 3
Vaccination preserves architecture and nuclear infiltration of young and aged skeletal muscle H&E’s. A) The H&E’s are representative for each group at each time point analyzed. Scale bars are 100 μm. B) Blindly scored H&E summaries across each timepoint (n = 3–5). C) Muscle cross-sectional area via immunofluorescent staining from uninfected (left), unvaccinated/infected (middle), and vaccinated/infected (right) 9 DPI young mice. Colors: ‘myosin heavy chain I (red), myosin heavy chain IIA (green), myosin heavy chain IIB (yellow), myosin heavy chain IIX (no stain), nuclei (cyan). Scale bars are 100 μm. Data analyzed via two-way ANOVA with Bonferroni post hoc corrections comparing to NoVax/NoFlu age-matched controls (* = p < 0.05), comparing to NoVax/Flu age-matched controls (# = p < 0.05) (PNG 945 kb)
About this article
Cite this article
Keilich, S.R., Lorenzo, E.C., Torrance, B.L. et al. Vaccination mitigates influenza-induced muscular declines in aged mice. GeroScience 42, 1593–1608 (2020). https://doi.org/10.1007/s11357-020-00206-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00206-z