Abstract
Pmt1p is an important member of the protein O-mannosyltransferase (PMT) family of enzymes, which participates in the endoplasmic reticulum (ER) unfolded protein response (UPR), an important pathway for alleviating ER stress. ER stress and the UPR have been implicated in aging and age-related diseases in several organisms; however, a possible role for PMT1 in determining lifespan has not been previously described. In this study, we report that deletion of PMT1 increases replicative lifespan (RLS) in the budding yeast Saccharomyces cerevisiae, while overexpression of PMT1 (PMT1-OX) reduces RLS. Relative to wild-type and PMT1-OX strains, the pmt1Δ strain had enhanced HAC1 mRNA splicing and elevated expression levels of UPR target genes. Furthermore, the increased RLS of the pmt1Δ strain could be completely abolished by deletion of either IRE1 or HAC1, two upstream modulators of the UPR. The double deletion strains pmt1Δhac1Δ and pmt1Δire1Δ also displayed generally reduced transcription of UPR target genes. Collectively, our results suggest that PMT1 deficiency enhances basal activity of the ER UPR and extends the RLS of yeast mother cells through a mechanism that requires both IRE1 and HAC1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoplasmic reticulum (ER) stress can result from the accumulation of unfolded proteins caused by impairments in oxidative folding components, chaperones, and other systems involved in protein posttranslational control in the ER. Such ER stress activates a cytoprotective signaling cascade termed the ER unfolded protein response (UPR) (Welihinda and Kaufman 1996; Taylor and Dillin 2013). While activation of the response can resolve the damaged proteins in the ER, persistent high-level activation can also lead to apoptosis. Pertinent to this study, the ability of the UPR pathway to maintain ER homeostasis is closely associated with cellular and organismal aging and exerts a critical role in the development of age-related diseases (Salminen and Kaarniranta 2010).
Protein O-mannosyltransferase (PMT) family enzymes attach mannose to substrate proteins in the ER and are evolutionarily conserved from yeast to mammals. Defects in PMT function result in reduced cell wall integrity and perturb ER homeostasis in yeast (Goto 2007; Lengeler et al. 2008; Loibl and Strahl 2013). In humans, O-mannosylation deficiencies cause a type of severe recessive congenital muscular dystrophy, accompanied by defects in neuronal migration that result in complex brain and eye abnormalities (Boisson-Dernier et al. 2013).
In yeast, seven PMT family members have been identified, which with the exception of Pmt7p, share an overall protein sequence identity of 57 %. PMTs are classified into PMT1/PMT2 and PMT4 subfamily members, and they are functionally redundant. Thus, strains lacking any single PMT gene are viable, and only the simultaneous deletion of PMT1/PMT2 and PMT4 subfamily members results in lethality (Gentzsch and Tanner 1996). O-Mannosylation by PMTs not only keeps proteins soluble (required for ER exit) but also prevents futile protein folding cycles and may in some cases promote protein degradation (Harty et al. 2001; Xu et al. 2013). Genome-wide analysis has indicated that inhibition of O-mannosylation specifically interferes with ER homeostasis and induces the UPR pathway (Arroyo et al. 2011). In addition, there is evidence that the UPR pathway can influence the expression of PMT1, PMT2, PMT3, and PMT5 (Travers et al. 2000; Kimata et al. 2006).
The budding yeast Saccharomyces cerevisiae has been extensively used as a model for studying cellular and organismal lifespan (Kaeberlein 2010; Longo et al. 2012). Yeast replicative lifespan (RLS) can be defined as the total number of daughter cells produced by a mother cell before senescence (Mortimer and Johnston 1959). As part of an ongoing, unbiased screen of the yeast ORF deletion collection for strains with altered RLS (Kaeberlein and Kennedy 2005; Kaeberlein et al. 2005; Steffen et al. 2008, 2012; Delaney et al. 2013; McCormick et al. 2014), we identified the pmt1Δ strain as potentially long-lived. Here, we report the impact of PMT1-deficiency and overexpression on yeast RLS and provide evidence that Pmt1p modulates lifespan by altering expression of UPR target genes.
Methods and materials
Yeast strains and plasmids
All yeast strains used in this study were derived from BY4742 wild-type strain (Table S1). The PMT1, HAC1, and IRE1 single-gene deletion strains were produced through polymerase chain reaction (PCR)-mediated one-step gene disruption (Baudin et al. 1993; Zhao et al. 2014). The open reading frames (ORFs) of the target genes were replaced by URA3 using PCR-mediated homologous recombination in BY4742. The URA3 cassette was amplified from pRS306 (Sikorski and Hieter 1989) using primers consisting of 40 nucleotides identical to the target genes’ flanking regions at the 5′ end and 20 nucleotides for the amplification of the URA3 gene at the 3′ end. Primers used for the disruptions are listed in Table S2. The PCR product was transformed into the BY4742 MATα haploid strain.
The PMT1-overexpression plasmid (pRS306-PMT1-OX) was constructed by inserting a 1900-bp BamHІ–EcoRІ fragment and a 1417-bp EcoRІ–ClaІ fragment amplified from yeast genomic DNA into the BamHІ and ClaІ sites of pRS306 (Table S3). In addition to the ORF of PMT1, ~533 nucleotides of upstream sequence and ~300 nucleotides of downstream sequence were amplified (Stearns et al. 1990; Kaeberlein et al. 1999; Zhao et al. 2014). Thus, expression of PMT1 would be driven by its natural promoter. The overexpressing PMT1 strain was constructed by transforming wild-type yeast cells with HpaІ-digested plasmid pRS306-PMT1-OX (Fig. S2).
The pmt1Δhac1Δ and pmt1Δire1Δ double-deletion strains were constructed through homologous recombination (Sikorski and Hieter 1989; Zhou et al. 2009). First, the plasmid pRS305-pmt1-ko was constructed for deletion of PMT1. Briefly, PCR-amplified MluІ–HindIII fragment (nucleotides −805~0 bp) and BamHI–MluІ fragment (nucleotides 2454~2849 bp) were cloned into HindIII–BamHI sites of pRS305 (Table S3 and Fig. S6). Three micrograms DNA of pRS305-pmt1-ko was linearized and transformed into the HAC1 and IRE1 deletion strains. In each case, the entire ORFs of the target genes were removed.
Transformation of yeast cells was accomplished using the modified lithium acetate method. Transformants were selected on SD/-Ura agar or SD/-Leu/-Ura agar media (Clontech). The PMT1-overexpression strain and all of the gene-deletion strains were verified by PCR or quantitative PCR (Table S4, Figs. S1, S3–S5, S7–S8, and Fig. 2a).
Replicative lifespan
RLS analysis was performed as described (Steffen et al. 2009; Delaney et al. 2013). Each individual replicate experiment involved 20 to 40 cells per genotype and was carried out at least twice independently. All lifespan experiments were carried out on standard YPD plates (1 % yeast extract, 2 % peptone, 2 % glucose, 2 % agar). In order to prevent possible bias, strains were coded and the dissectors were blinded to the identity of the strains. For statistical analysis, the RLS datasets were analyzed using a Wilcoxon Rank-Sum test (MATLAB “rank-sum” function). Strains were stated to have a significant difference for p < 0.01.
Quantitative RT-PCR
Total RNA was extracted from 1 ml cells with OD600nm at 2.0 following the instructions of the Yeast RNAiso Kit (TaKaRa). RNA quantity was measured with a nanodrop ND-1000 spectrophotometer (Thermo Scientific), and RNA integrity was verified by electrophoresis on 1 % (w/v) agarose gel. First-strand complementary DNAs (cDNAs) were synthesized using the PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa) according to the recommendations.
For quantitative PCR, three replicates per sample were prepared by adding 1 μl of cDNA, oligonucleotides, and SYBR® Premix Ex Taq™ II (TaKaRa). Quantitative PCR assays were carried out in an ABI 7500 Real-Time PCR System (Applied Biosystems). Specific primers were designed based on the ORF sequences in Saccharomyces Genome Database (Lussier et al. 1995; Hamann and Denness 2011; Wang and Cheng 2012). The abundance of each gene was determined relative to the standard transcript of PRP8 with the comparative Ct method. Comparative Ct values were tested using Student’s t test, and p < 0.05 indicated a significant difference (qPCR primers are listed in Table S5).
HAC1 transcript splicing analysis
Total RNA was derived from yeast cells treated with or without 2 μg/ml tunicamycin (BBI, TF1129) for 1 h. For the analysis of HAC1 messenger RNA (mRNA) splicing, 0.5 μl of cDNA was used as a template for amplification of HAC1 cDNA by PCR. The PCR conditions were 94 °C for 5 min followed by 31 cycles at 94 °C for 30 s, 56 °C for 30 s, 72 °C for 30 s, and finally 72 °C for 5 min. The primers used in these reactions were HAC1-F (CCGTAGACAACAACAATTTG) and HAC1-R (CATGAAGTGATGAAGA AATC). PRP8 was used as an internal RNA control. The PCR product was detected at 433 bp when the HAC1 transcript was not spliced and at 181 bp when spliced (Mori et al. 2010). PCR fragments were run on 2 % (w/v) agarose gels, stained with Goldview, and quantified by densitometry.
Western blotting
Whole-cell lysates were derived from yeast cells cultured for 12 h. Total protein was first separated by SDS-PAGE and then transferred to PVDF membranes by semidry blotting. Immunoreactive bands were visualized with the Pierce ECL Western Blotting Substrate, and the signals were detected and analyzed with the visible fluorescent Western imaging system (Azure c400). Antibodies directed against Kar2p (Santa Cruz, sc33630) and GAPDH (Sigma, G0763) were used to evaluate the relative expression levels of Kar2p in wild-type, pmt1Δ, and PMT1-OX strains.
Results
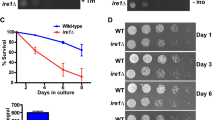
PMT1 deletion extends the lifespan of S. cerevisiae
Based on our unpublished observation that the pmt1Δ strain from the ORF deletion collection is long-lived, we constructed a new PMT1 single-gene deletion (pmt1Δ) strain by homologous recombination and verified gene disruption by PCR to validate the longevity effect (Fig. S1). The RLS of this mutant strain was determined, and a significant increase in mean lifespan of 20 % was observed for pmt1Δ mother cells (Fig. 1, p < 0.0001), relative to BY4742 wild-type mother cells.
Overexpression of PMT1 shortens the lifespan of yeast
To further confirm the effect of PMT1 on RLS, a PMT1 overexpression (PMT1-OX) strain was constructed and verified by PCR (Fig. S3). The PMT1-OX strain displayed a roughly threefold higher expression level of PMT1 mRNA relative to wild type (Fig. 2a), and had a mean RLS of 17 % less than wild type (Fig. 2b, p < 0.001).
Overexpression of PMT1 decreases yeast RLS. a Relative transcription level of PMT1 mRNA in wild type and PMT1 overexpression (PMT1-OX) strains. The data are expressed as mean ± SD (n = 3). **p < 0.01 vs. wild type. b RLS is decreased in the PMT1-OX strain (p < 0.01). Mean RLS is shown in parentheses, and n is the number of mother cells scored
PMT1 deletion elevates basal UPR activity
The ER stress sensor Ire1p acts through the ER-stress responsive transcription factor Hac1p to regulate transcription of UPR target genes (Cox and Walter 1996; Labunskyy et al. 2014). HAC1 mRNA splicing by Ire1p is a key step in the activation of the UPR pathway and is commonly used to evaluate UPR activity (Cox and Walter 1996; Labunskyy et al. 2014). We quantified the levels of spliced and un-spliced HAC1 mRNA in wild-type, pmt1Δ, and PMT1-OX strains with or without tunicamycin treatment. In the absence of tunicamycin, the pmt1Δ strain displayed increased expression of spliced HAC1 mRNA (29.5 ± 14.9 %), relative to wild-type (5.0 ± 2.6 %) and PMT1-OX (6.0 ± 3.1 %) strains (Fig. 3a). While tunicamycin treatment increased HAC1 mRNA splicing in all three strains, the pmt1Δ strain still displayed a slightly higher level of spliced HAC1 mRNA (68.3 ± 16.0 %) than the wild-type (50.5 ± 15.6 %) or PMT1-OX (50.8 ± 11.9 %) strain.
Deletion of PMT1 enhances basal UPR activity. Basal UPR activity was evaluated based on the splicing of HAC1 mRNA and the expression levels of the UPR target genes. a HAC1 mRNA splicing in the pmt1Δ, PMT1-OX, and wild-type strains treated with or without 2 μg/ml tunicamycin (Tm) for 1 h. HACu indicates the unspliced HAC1 mRNA, and HACi indicates the spliced mRNA. PRP8 is an internal RNA control. b The relative expression levels of the canonical UPR target genes, including EUG1, ERO1, KAR2, LHS1, FKB2, and PDI1, in wild-type, pmt1Δ and PMT1-OX strains. c Relative expression of Kar2p levels by Western blotting with GAPDH as an internal control. All experiments were repeated at least three times and showed similar results. The data are expressed as mean ± SD (n = 4). *p < 0.05; **p < 0.01 vs. wild type
To further examine UPR activity in the pmt1Δ and PMT1-OX strains, the expression levels of several canonical UPR target genes were measured by quantitative RT-PCR. The results showed that most of the UPR target genes, including genes involved in chaperone function (KAR2 and LHS1), oxidative folding (EUG1 and ERO1), and protein trafficking (FKB2), showed increased expression levels in pmt1Δ strain relative to wild type. Of the genes examined, only PDI1, involved in oxidative folding, was unaltered. PMT1 overexpression did not significantly influence the expression of most UPR target genes, but reduced the expression levels of LHS1 and PDI1 (Fig. 3b). Consistent with the quantitative PCR data, the protein levels of Kar2p were obviously increased in the pmt1Δ strain relative to wild type (Fig. 3c). It has been reported that Kar2p acts as a chaperone in the ER to mediate protein folding and regulate the UPR, which improves the ER folding capacity (Jonikas et al. 2009; Xu et al. 2013). Thus, the above results support the idea that the UPR activity is upregulated by deletion of PMT1.
Deletion of IRE1 or HAC1 prevents lifespan extension in the pmt1Δ strain
To test the possibility that induction of the UPR underlies the increased lifespan of pmt1Δ mother cells, we constructed the HAC1 and IRE1 single-deletion (hac1Δ and ire1Δ) strains, as well as double-deletion strains lacking either HAC1 or IRE1 in addition to PMT1 (pmt1Δhac1Δ and pmt1Δire1Δ). Deletion of either IRE1 or HAC1 alone had no significant effect on lifespan, as previously reported (Labunskyy et al. 2014). Although the mean RLS of the pmt1Δhac1Δ strain was 1.8 generations longer than the hac1Δ strain, the difference was not significant (p > 0.05). Deletion of PMT1 was not able to extend lifespan in the absence of IRE1 or HAC1 (Fig. 4a).
IRE1 and HAC1 are required for increased lifespan of pmt1Δ strain. a RLS of ire1Δ, hac1Δ, pmt1Δire1Δ, pmt1Δhac1Δ, and wild-type strains. Mean RLS is shown in parentheses, and n is the number of mother cells scored. Deletion of IRE1 or HAC1 had no effect on wild-type RLS, and either prevented increased RLS upon deletion of PMT1. b The relative expression levels of UPR target genes in wild-type, pmt1Δ, pmt1Δire1Δ, and pmt1Δhac1Δ strains. The data are expressed as mean ± SD (n = 3). *p < 0.05; **p < 0.01 vs. wild type
We next asked whether the observed upregulation of UPR target genes upon deletion of PMT1 depended on Hac1p or Ire1p. In both pmt1Δhac1Δ and pmt1Δire1Δ strains, the expression of most UPR target genes was significantly reduced (Fig. 4b).
Discussion
Accumulating evidence has demonstrated that ER stress and the UPR pathway play critical roles in the development of numerous diseases, as well as normal aging processes. Previous studies have suggested that the UPR can influence lifespan in yeast, and here, we extend these findings by demonstrating that reduced activity of the O-mannosyltransferase enzyme Pmt1p extends RLS in a manner that requires activation of the UPR. A prior study revealed that UPR target gene deletion, including ALG12 (N-linked glycosylation) and BST1 (protein trafficking), can extend RLS in yeast and that UPR activity was increased in these long-lived mutants (Labunskyy et al. 2014). Consistent with our data, lifespan extension in these cases required both HAC1 and IRE1. Thus, we propose that increased UPR activity plays a causal role in the extended lifespan of pmt1Δ mutants, as well as the previously described lifespan extensions associated with deletion of ALG12 or BST1.
Our observation that deletion of either HAC1 or IRE1 has no significant effect on the lifespan of otherwise wild-type cells, which was also reported by Labunskyy et al. (Labunskyy et al. 2014), strongly suggests that basal UPR signaling does not limit the RLS of wild-type cells under standard conditions. This is consistent with evidence that UPR signaling is not necessary for the normal coupling of metabolism with cell division (Henry et al. 2010).
It is interesting that overexpression of PMT1 shortens RLS, but does not significantly impact basal expression of most UPR target genes. One possibility is that overexpression of PMT1 shortens lifespan by a mechanism distinct from the UPR. Another alternative is that ER stress becomes important specifically in aged cells, and that PMT1 overexpression antagonizes this response in aged cells but has no detectable effect in young cells. Although speculative, such a model could also explain why some mutants with constitutive induction of UPR genes are long-lived, as these cells would already be primed to deal with ER stress that occurs normally during aging. However, as discussed in the preceding paragraph, this model would also require that the basal UPR respond to age-associated ER stress in a HAC1- or IRE1-independent manner.
The possibility should also be considered that Pmt1p could affect lifespan by altering the cell wall integrity pathway, given that PMT function has been previously shown to affect this pathway (Lengeler et al. 2008; Arroyo et al. 2011; Loibl and Strahl 2013). Prior studies have shown that mutations in other genes that modulate cell wall integrity, such as SSD1, MPT5, and SLT2, can have significant effects on RLS, both positive and negative (Kennedy et al. 1997; Kaeberlein and Guarente 2002; Ray et al. 2003; Kaeberlein et al. 2004). It will be important to test this pathway in the context of long-lived ER-related mutants in future studies.
At this time, it remains unclear whether deletion of PMT family member genes other than PMT1 can extend RLS and whether deletions of the other PMT genes induce the UPR in a similar manner. There is precedent for differential functions of PMT family members in yeast, which are required for not only fungal growth but also differentiation processes including cell fusion, cell polarization, and sensing of external cues (Lussier et al. 1995; Henis-Korenblit et al. 2010; Burtner et al. 2011; Gonzalez et al. 2013). For example, the endochitinase Cts1p, a key protein involved in the separation of mother and daughter cells, can be glycosylated by several Pmt isoforms, including Pmt1p, Pmt2p, Pmt3p, Pmt4p, and Pmt6p (Immervoll et al. 1995; Lussier et al. 1995; Gentzsch and Tanner 1996; Girrbach and Strahl 2003; Nishida et al. 2014). Likewise, Fus1p, a protein involved in cell fusion during yeast mating, can only be glycosylated by Pmt4p (Chen et al. 2009). Based on the specific substrate proteins, deletion of different PMT members may result in different phenotypes.
Our results suggest that deletion of PMT1 might lead to accumulation of unglycosylated proteins, thus triggering the UPR. Several studies have suggested that the UPR plays a central role in longevity in Caenorhabditis elegans. Neuronal activation of the UPR through transgenic expression of a constitutively active form of the Hac1p ortholog XBP-1 has recently been reported to be sufficient to extend lifespan in worms (Taylor and Dillin 2013). This follows prior studies indicating that enhanced ER protein homeostasis is associated with lifespan extension through SIR-2.1 (Viswanathan et al. 2005), insulin-like signaling (Henis-Korenblit et al. 2010), and downstream of dietary restriction (Chen et al. 2009).
In conclusion, the current study demonstrates that PMT1 deletion induces the UPR and extends the replicative lifespan of yeast mother cells. This induction of the UPR is required for lifespan extension of the PMT1 deletion strain. It will be informative in future studies to further explore the causal role of the ER UPR in yeast lifespan and the relationship between PMT1 and other known longevity pathways.
References
Arroyo J, Hutzler J, Bermejo C et al (2011) Functional and genomic analyses of blocked protein O-mannosylation in baker's yeast. Mol Microbiol 79(6):1529–1546. doi:10.1111/j.1365-2958.2011.07537.x
Baudin A, Ozier-Kalogeropoulos O, Denouel A et al (1993) A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res 21(14):3329–3330
Boisson-Dernier A, Lituiev DS, Nestorova A et al (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11(11):e1001719. doi:10.1371/journal.pbio.1001719
Burtner CR, Murakami CJ, Olsen B et al (2011) A genomic analysis of chronological longevity factors in budding yeast. Cell Cycle 10(9):1385–1396
Chen D, Thomas EL, Kapahi P (2009) HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5(5):e1000486. doi:10.1371/journal.pgen.1000486
Cox JS, Walter P (1996) A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87(3):391–404
Delaney JR, Ahmed U, Chou A et al (2013) Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell 12(1):156–166. doi:10.1111/acel.12032
Gentzsch M, Tanner W (1996) The PMT gene family: protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J 15(21):5752–5759
Girrbach V, Strahl S (2003) Members of the evolutionarily conserved PMT family of protein O-mannosyltransferases form distinct protein complexes among themselves. J Biol Chem 278(14):12554–12562. doi:10.1074/jbc.M212582200 M212582200
Gonzalez M, Brito N, Frias M et al (2013) Botrytis cinerea protein O-mannosyltransferases play critical roles in morphogenesis, growth, and virulence. PLoS One 8(6):e65924. doi:10.1371/journal.pone.0065924
Goto M (2007) Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci Biotechnol Biochem 71(6):1415–1427
Hamann T, Denness L (2011) Cell wall integrity maintenance in plants: lessons to be learned from yeast? Plant Signal Behav 6(11):1706–1709. doi:10.4161/psb.6.11.17782 17782
Harty C, Strahl S, Romisch K (2001) O-mannosylation protects mutant alpha-factor precursor from endoplasmic reticulum-associated degradation. Mol Biol Cell 12(4):1093–1101
Henis-Korenblit S, Zhang P, Hansen M et al (2010) Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A 107(21):9730–9735. doi:10.1073/pnas.1002575107
Henry KA, Blank HM, Hoose SA et al (2010) The unfolded protein response is not necessary for the G1/S transition, but it is required for chromosome maintenance in Saccharomyces cerevisiae. PLoS One 5(9):e12732. doi:10.1371/journal.pone.0012732
Immervoll T, Gentzsch M, Tanner W (1995) PMT3 and PMT4, two new members of the protein-O-mannosyltransferase gene family of Saccharomyces cerevisiae. Yeast 11(14):1345–1351. doi:10.1002/yea.320111403
Jonikas MC, Collins SR, Denic V et al (2009) Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323(5922):1693–1697. doi:10.1126/science.1167983 323/5922/1693
Kaeberlein M (2010) Lessons on longevity from budding yeast. Nature 464(7288):513–519. doi:10.1038/nature08981 nature08981
Kaeberlein M, Guarente L (2002) Saccharomyces cerevisiae MPT5 and SSD1 function in parallel pathways to promote cell wall integrity. Genetics 160(1):83–95
Kaeberlein M, Kennedy BK (2005) Large-scale identification in yeast of conserved ageing genes. Mech Ageing Dev (1) 17–21. doi:10.1016/j.mad.2004.09.013
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13(19):2570–2580
Kaeberlein M, Andalis AA, Liszt GB et al (2004) Saccharomyces cerevisiae SSD1-V confers longevity by a Sir2p-independent mechanism. Genetics 166(4):1661–1672
Kaeberlein M, Powers RW 3rd, Steffen KK et al (2005) Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310(5751):1193–1196. doi:10.1126/science.1115535
Kennedy BK, Gotta M, Sinclair DA et al (1997) Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89(3):381–391
Kimata Y, Ishiwata-Kimata Y, Yamada S et al (2006) Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 11(1):59–69. doi:10.1111/j.1365-2443.2005.00921.x
Labunskyy VM, Gerashchenko MV, Delaney JR et al (2014) Lifespan extension conferred by endoplasmic reticulum secretory pathway deficiency requires induction of the unfolded protein response. PLoS Genet 10(1):e1004019. doi:10.1371/journal.pgen.1004019
Lengeler KB, Tielker D, Ernst JF (2008) Protein-O-mannosyltransferases in virulence and development. Cell Mol Life Sci 65(4):528–544. doi:10.1007/s00018-007-7409-z
Loibl M, Strahl S (2013) Protein O-mannosylation: what we have learned from baker's yeast. Biochim Biophys Acta 1833(11):2438–2446. doi:10.1016/j.bbamcr.2013.02.008
Longo VD, Shadel GS, Kaeberlein M et al (2012) Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab 16(1):18–31. doi:10.1016/j.cmet.2012.06.002
Lussier M, Gentzsch M, Sdicu AM et al (1995) Protein O-glycosylation in yeast. The PMT2 gene specifies a second protein O-mannosyltransferase that functions in addition to the PMT1-encoded activity. J Biol Chem 270(6):2770–2775
McCormick MA, Mason AG, Guyenet SJ et al (2014) The SAGA histone deubiquitinase module controls yeast replicative lifespan via Sir2 interaction. Cell Rep 8(2):477–486. doi:10.1016/j.celrep.2014.06.037
Mori T, Ogasawara C, Inada T et al (2010) Dual functions of yeast tRNA ligase in the unfolded protein response: unconventional cytoplasmic splicing of HAC1 pre-mRNA is not sufficient to release translational attenuation. Mol Biol Cell 21(21):3722–3734. doi:10.1091/mbc.E10-08-0693
Mortimer RK, Johnston JR (1959) Life span of individual yeast cells. Nature 183(4677):1751–1752
Nishida N, Jing D, Kuroda K et al (2014) Activation of signaling pathways related to cell wall integrity and multidrug resistance by organic solvent in Saccharomyces cerevisiae. Curr Genet 60(3):149–162. doi:10.1007/s00294-013-0419-5
Ray A, Hector RE, Roy N et al (2003) Sir3p phosphorylation by the Slt2p pathway effects redistribution of silencing function and shortened lifespan. Nat Genet 33(4):522–526. doi:10.1038/ng1132
Salminen A, Kaarniranta K (2010) ER stress and hormetic regulation of the aging process. Ageing Res Rev 9(3):211–217. doi:10.1016/j.arr.2010.04.003
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122(1):19–27
Stearns T, Ma H, Botstein D (1990) Manipulating yeast genome using plasmid vectors. Methods Enzymol 185:280–297
Steffen KK, MacKay VL, Kerr EO et al (2008) Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133(2):292–302. doi:10.1016/j.cell.2008.02.037
Steffen KK, Kennedy BK, Kaeberlein M (2009) Measuring replicative life span in the budding yeast. J Vis Exp (28). doi:10.3791/1209 1209
Steffen KK, McCormick MA, Pham KM et al (2012) Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics 191(1):107–118. doi:10.1534/genetics.111.136549
Taylor RC, Dillin A (2013) XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153(7):1435–1447. doi:10.1016/j.cell.2013.05.042
Travers KJ, Patil CK, Wodicka L et al (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258
Viswanathan M, Kim SK, Berdichevsky A et al (2005) A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell 9(5):605–615
Wang SL, Cheng MY (2012) The defects in cell wall integrity and G2-M transition of the htl1 mutant are interconnected. Yeast 29(1):45–57. doi:10.1002/yea.1916
Welihinda AA, Kaufman RJ (1996) The unfolded protein response pathway in Saccharomyces cerevisiae. Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are required for kinase activation. J Biol Chem 271(30):18181–18187
Xu C, Wang S, Thibault G et al (2013) Futile protein folding cycles in the ER are terminated by the unfolded protein O-mannosylation pathway. Science 340(6135):978–981. doi:10.1126/science.1234055
Zhao W, Fang BX, Niu YJ et al (2014) Nar1 deficiency results in shortened lifespan and sensitivity to paraquat that is rescued by increased expression of mitochondrial superoxide dismutase. Mech Ageing Dev 138:53–58. doi:10.1016/j.mad.2014.01.007
Zhou J, Zhou BO, Lenzmeier BA et al (2009) Histone deacetylase Rpd3 antagonizes Sir2-dependent silent chromatin propagation. Nucleic Acids Res 37(11):3699–3713. doi:10.1093/nar/gkp233
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31101051, 81170327), the Natural Science Foundation of Guangdong Province (9252402301000002), the Science & Technology Innovation Fund of Guangdong Medical College (STIF201102), and the Natural Science on the Surface of Guangdong Medical College (XK1204). This work was also supported by NIH Grant R01AG039390 to MK. MM was supported by NIH training grant T32AG000266. BMW was supported by NIH training grant T32 ES007032.
Conflict of interest
None of the authors has any conflict of interests that could affect the performance of the work or the interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 133 kb)
Fig. S2
(DOC 92 kb)
Fig. S3
(DOC 97 kb)
Fig. S4
(DOC 87 kb)
Fig. S5
(DOC 116 kb)
Fig. S6
(DOC 62 kb)
Fig. S7
(DOC 117 kb)
Fig. S8
(DOC 665 kb)
Table S1
(DOC 63 kb)
Table S2
(DOC 33 kb)
Table S3
(DOC 35 kb)
Table S4
(DOC 52 kb)
Table S5
(DOC 89 kb)
Table S6
(DOC 36 kb)
Table S7
(DOC 31 kb)
Table S8
(DOC 35 kb)
Table S9
(DOC 33 kb)
Table S10
(DOC 33 kb)
Table S11
(DOC 96 kb)
About this article
Cite this article
Cui, HJ., Liu, XG., McCormick, M. et al. PMT1 deficiency enhances basal UPR activity and extends replicative lifespan of Saccharomyces cerevisiae . AGE 37, 46 (2015). https://doi.org/10.1007/s11357-015-9788-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-015-9788-7