Abstract
Marine pollution by trace elements is a global concern due to potential toxicity to species and ecosystems. Copper is a fundamental trace element for many organisms; however, it becomes toxic at certain concentrations. The green turtle (Chelonia mydas) is a good sentinel species, due to its circumglobal distribution, long life cycle, coastal habits when juvenile, and is subject to environmental pollution. Quantifying and comparing copper levels makes it possible to understand the availability of this trace element in nature. During this research, comparisons were made between the levels of copper found in the liver, kidneys, and muscles of 35 turtles, from the United States (Hawaii and Texas), Brazil, and Japan. Copper was found in all specimens. In the liver, animals from Hawaii (91.08 µg g−1), Texas (46.11 µg g−1), and Japan (65.18 µg g−1) had statistically equal means, while those from Brazil (16. 79 µg g−1) had the lowest means. For the kidney, copper means were statistically equal for all Hawaii (3.71 µg g−1), Texas (4.83 µg g−1), Japan (2.47 µg g−1), and Brazil (1.89 µg g−1). In muscle, the means between Texas (0.75 µg g−1) and Japan (0.75 µg g−1) were the same, and the mean for Brazil (0.13 µg g−1) was the lowest. Among the organs, the highest levels of copper were found in the liver (28.33 µg g−1) followed by the kidney (2.25 µg g−1) and with the lowest levels in the muscle (0.33 µg g−1). This is the first study of copper levels among marine vertebrates in distant parts of the globe using similar comparative filters between different locations. Similar levels in turtles from such distant locations may indicate that there is a pantropical pattern of copper distribution in the biota, and that these animals are subject to the process of bioavailability of this metal in the environment and metabolic regulation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace elements may become environmental pollutants, especially in areas that suffer great anthropic pressure, due to their toxicity, human, and ecological health relevance (Nagajyoti et al. 2010; Prashanth et al. 2015). Estuaries and coastal regions are areas highly impacted by these pollutants being used for industrial and urban effluents, generating water pollution and negative impacts on marine biota (Maia et al. 2006).
Two main sources of trace elements are of natural origin, i.e., river input, leaching processes and sediment transport, volcanic eruptions, and atmospheric transport, and anthropic origin, i.e., industrialization, boat paints, mining residues, dust, production fertilizers, and domestic and industrial sewage (ATSDR 2022), making these trace elements including copper (Cu) present at the continent-ocean interface.
Of the trace elements that occur in the oceans, copper is of great relevance, as it is a trace element that brings benefits to biota from the cellular, organismal, and ecological levels. According to Niencheski (2015), Cu in trace concentrations is indispensable as a constituent of hemocyanin in marine invertebrates, enzymes, and chloroplasts; however, it becomes toxic at high concentrations.
Copper inhibits the photosynthetic activities of phytoplankton (USEPA), which trophic webs are based on these activities, in addition to playing an important role in iron metabolism by limiting oceanic primary production (Roshan et al. 2020; Moore et al. 2013). According to Millero (2006a, b), elements such as copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) act to limit the growth of these organisms, in addition to being necessary for the functioning of proteins (Fe) and enzymes (Mn, Mo, Cu, Zn, and Co). The copper is also toxic in polychaetes and in some species of algae, whose toxicity is similar to mercury (USEPA 1977).
Many regional and global studies have already been carried out quantifying Cu in marine organisms, both invertebrates (Rivera-Duarte et al. 2005; Marrugo-Negreti et al. 2021) and vertebrates, such as cetaceans (Marcovecchio, et al. 1990), pinnipeds (Gerpe et al. 2009), seabirds (Espejo et al. 2017), and sea turtles (Lam et al. 2004; Gardner et al. 2006; Barbieri 2009; Shaw et al. 2021). However, the studies do not present uniformity of data (such as species, size, age, sex, chemical analyses, and organs) and when they present this compatibility, they do not involve other distant points on the planet for analysis. This makes them inconclusive for visualizing Cu distribution patterns at geographically broader levels.
Green turtles are “endangered” according to the IUCN (2023) and are cosmopolitan, long-lived species inhabiting neritic habitats as a juvenile. These characteristics make green turtles subject to local and global anthropic impacts and a good bioindicator that can reflect bioaccumulation for years. Two studies were recently published on the global distribution of trace elements, specifically cadmium and zinc, using green turtles as sentinel species (Fraga et al. 2018, 2023).
To the best of our knowledge, there is only one global model about the distribution of Cu in the water column (Richon & Tagliabue 2019a, b), and there is a gap in studies at a broader level spatially about the bioaccumulation of Cu in vertebrate tissues, relevant due to its toxicological action at an individual and ecological level. Therefore, it is important to understand the distribution of Cu in marine biota and compare patterns of global distribution that can provide information for the long-term conservation of the species and determination of comparative patterns for future studies.
The objective of this was to compare copper concentrations in green turtles from four different locations around the world: Rio Grande do Sul, Brazil; Texas, USA; Japan; and Hawaii, USA. These locations were chosen to cover both the Pacific and Atlantic regions. This study is innovative in that it performs analyses with a standardized approach to data collection at geographically distant sites in both oceans, providing comprehensive information on copper distribution in green turtles on a pantropical scale.
Materials and methods
Summary of the methodology used in the work
This work was carried out through a compilation of data and comparative analysis of Cu concentration in 103 specimens of green turtles from which liver, kidney, and pectoral muscle tissues were collected. These data were provided by da Silva et al. (2014) from Brazil; Aguirre et al. (1994) from Hawaii; Faust et al. (2014) from the Texas; and Sakai et al. (2000) from Japan and in addition to providing biometric data for each animal with measurements performed using standards such as curvilinear carapace length (CCL) measured in centimeters (cm).
The previous studies that gave rise to the data of the present study for the determination of Cu have the relevant methodology for the analyses of this present work; therefore, these methodologies were described and simplified with their respective citations at each locality.
Only raw data such as biometric data of animals and Cu levels were used in this work for comparative analyses, not using the interpretations and results that the authors performed in their study, so this study consists of a new use of data, a new approach and results found.
This study was separated into two stages of comparisons to determine levels of Cu contamination: direct and additional analyses (Fig. 1). The first analysis was performed for the direct analyses, which compared the levels of samples from the Texas, Japan, and Brazil; for additional analyses samples from Hawaii were used. This separation was carried out using Hawaii as additional data due to the absence of certified reference material in the chemical analyses of samples from this location.
In addition, for both analyses, an initial filtration was performed on the animals, where specimens of green turtles of CCL less than 60 cm were selected, forming a sample N of 72 individuals. This selection was due to alterations in the pattern of bioaccumulation of trace elements among sea turtles that are in the juvenile stage and as adults at reproductive age.
According to Godley et al. (1999), reproductive activities such as oviposition by females may be an important metal excretion route. And levels of metals such as cadmium were found with differences between adults and juveniles by Storelli et al. (1998) that might be associated with sexual maturity. In sea turtles, increased hormonal activity may interfere with metabolic processes associated with both the absorption and distribution of metals in these animals. Due to these issues, turtles of reproductive age were not used in the present study.
Furthermore, after selecting the 72 individuals for the analysis, the sizes of the specimens from each site were statistically different. With CCL size averaging in 45.75 cm Japan, 46.77 cm in Texas, and 49.69 cm in Hawaii. Brazil had the smallest specimens at 38.62 cm. Due to the reduced sample size of some locations and the differences in CCL size, it was necessary to use a range of equal size to all locations.
Therefore, a sample N of seven individuals from each location (Hawaii, Texas, Brazil, and Japan) was used in the present study for direct and additional comparisons. These specimens were randomly selected where only the number that represented the turtle and its size was used, hiding information such as individual levels of Cu (Fraga et al. 2018).
Of the total number of individuals that were available within the previously mentioned filters, 21 animals were selected for direct analyses from Texas, Japan, Brazil (Fig. 2), and Hawaii (for additional analyses), with statistically equal sizes, and belonging to the same size range between locations, allowing comparisons without the influence of size as a variable.
Curvilinear carapace length and copper concentrations of all turtles used in the present study such as Brazil (square), USA—Texas (triangle), and Japan (circle) before the size selection process (symbols with white filling) and selected individuals (symbols painted in gray) in direct analyses for comparison tests (n = 21) in liver (A) and kidney (B)
Additional comparisons were made using the sample N of 21 individuals from the direct analyses adding data from Hawaii as complementary data, reaching a total sample N of the present study of 28 individuals.
For the direct analyses (Brazil, Texas, and Japan) and Hawaii (additional analyses), the methodologies for collecting samples and chemical analyses to obtain the data used in the present study from each location were briefly described below.
The summarized methodology utilized to acquire data from Brazil, Texas, and Japan is outlined below, and it was used in direct comparisons to create the work’s results. Table 1 summarizes the approach utilized in the direct analysis.
Direct comparisons (n = 21)
Brazil
In Brazil, sampling was conducted in Rio Grande do Sul, along a 130 km stretch of the sandy beach extending from “Molhe Oeste da Barra do Rio Grande” (32° 09′ 42″ S, 52° 05′ 53″ W) to “Farol do Abraão” (33° 25′ 30″ S, 52° 57′ 06″ W), located at Praia do Cassino in southern Brazil (Fig. 3A). The main economic activities in this region include agriculture, cattle raising, and the processing industry (SEPLAN 2022).
Beach monitoring for stranded green turtles was conducted on a weekly basis between August 2008 and April 2009. The turtles were identified and their straight carapace length (SCL) was measured. Tissue samples, including liver, kidney, and muscle, were thawed and weighed using an electronic microscale with a precision of 0.1 mg. Subsequently, they were dried in an oven at 40 °C for 48 h until a constant weight was attained. The samples were then reweighed (dry weight) and completely digested in 2 ml of HNO3 (Suprapur, Merck, Germany). The tissue digestion process was carried out in sealed plastic tubes using an oven set at 40 °C. Prior to the analysis, this digestion procedure was validated using standard reference material, as described below. The digested samples were diluted with 10 ml of MilliQ water. The concentrations of Cu were determined using flame atomic absorption spectrophotometry (model AAS-932 Plus; GBC; New Hampshire, IL, USA). The measurement accuracy was assessed by constructing standard curves using standard solutions of Cu (Standard Reference Material 3114; National Institute of Standards & Technology, Gaithersburg, MD, USA) (da Silva et al. 2014).
To ensure quality assurance, metal recovery analysis was performed using a reference material. The percentages of metal recovery based on the standard reference material (European Reference Material ERM-CE278, Geel, Belgium), prepared following the same procedures as the tissue samples, were found to be 94.2%, Cu (da Silva et al. 2014).
United States (Texas)
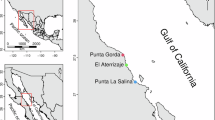
In the United States, specimen collections were carried out in Laguna Madre, located in southern Texas, within the Padre Island National Seashore (Fig. 3B), located between latitudes 26° and 28° 40′ and longitudes − 98° 40′ and − 95° 20′ (Faust et al. 2014). Laguna Madre is an estuarine region characterized by its hypersaline waters. In the state of Texas, the main economic activities include the oil industry, mining, agriculture, and services.
Samples were frozen and shipped to Texas Tech University on March 29, 2011, where they were stored at − 80 °C until samples were digested. Tissues (0.5 ± 0.01 g wet weight) were placed in 50-ml polypropylene centrifuge tubes. Briefly, 3 ml of 1:1 nitric acid:hydrochloric acid was added to each tube. The tubes were placed in a water bath at 90 °C and digested for approximately 1 h, until the solution became clear. Digested samples were removed from the water bath and cooled for 15 min (Faust et al. 2014).
After adding 1.5 ml of 30% hydrogen peroxide to each tube, the digested materials were returned to the water bath for about 45 min. Then, the digested material was removed from the water bath and centrifuged at 3000 g for 30 min in a Beckman Allegra 6R centrifuge, in order to remove undigested fats. Supernatants were adjusted to a volume of 50 ml with 18 MΩ water and transferred to new 50-ml polypropylene centrifuge tubes for analysis (Faust et al. 2014).
For Cu determination, an Agilent 7500cs series inductively coupled plasma mass spectrometer (ICP-MS) was used. In order to guarantee the quality of the instrument, duplicates of the instrument and verification standards were performed every 10 samples (Faust et al. 2014).
For quality assurance and control, the following samples were digested and analyzed using the same method alongside each set of tissue samples: one duplicate of a randomly selected sample for each tissue, 0.25 ± 0.01 g of the National Research Council Canada DORM-2 certified reference material for trace metals from dogfish muscle, 0.25 ± 0.01 g of the National Research Council Canada DOLT-3 certified reference material for trace metals from dogfish liver, and reagent blanks (Faust et al. 2014).
Japan
In Japan, green turtles were incidentally caught in Yaeyama Islands, located in the Ryukyu archipelago, Okinawa prefecture. The islands are situated between 24° to 25° N and 123° 20″ to 125° W (Fig. 3C) and are characterized by subtropical environments and abundant coral reefs (Sakai et al. 2000). The archipelago comprises both inhabited and uninhabited islands, some of which are covered by tropical forests. The predominant economic activity in the region is tourism (Embassy of Japan in Brazil 2012).
Turtle samples were collected during the period from March to May 1992. The straight carapace length was measured, and the liver was extracted from 50 animals and immediately placed in polyethylene bags after dissection. All tissue samples were promptly frozen and stored at − 20 °C until chemical analysis was conducted (Sakai et al. 2000).
Tissue and organ samples were subjected to analysis to determine the concentrations of Cu. Briefly, samples weighing between 1 and 10 g were digested using a mixture of nitric, perchloric, and sulfuric acids, followed by dilution with deionized water. The cadmium concentrations were directly determined using a flame atomic absorption spectrophotometer (AAS, Shimadzu Model AA-680). The concentrations were expressed in micrograms per gram (µg/g) based on wet weight for the turtle samples (Sakai et al. 2000).
The accuracy of the analyses was verified by employing a standard reference material, NIES No. 1 (Okamoto et al. 1978). The triplicate analysis of these methods exhibited an accuracy greater than 95% for each element (Table 1; Sakai et al. 2000).
Complementary comparisons
For the purposes of the complementing comparisons, the procedures utilized to gather the data from Hawaii were condensed and detailed below. According to the aforementioned methodological considerations, such comparisons were only utilized to supplement the outcomes of the direct analyses.
United States (Hawaii)
Liver, kidney, and muscle were collected from 12 carcasses of C. mydas from the Hawaiian Islands. Digestion with nitric acid and distilled water were performed for chemical analyses. Samples were analyzed by inductively coupled plasma spectrophotometer (ICP) (Aguirre et al. 1994).
Statistical analyses
The same methodological process was used for all data (direct and additional), which involved gathering and choosing 7 individuals from each site. The following statistical analyses were carried out following these procedures.
First, the copper concentrations from each location were compared using the nonparametric Kruskal–Wallis ANOVA test to see whether there was any difference, such as in group sizes between locations. The PAST software was employed.
If a significant difference was found using ANOVA, the Mann–Whitney test was used to compare copper concentrations between two groups (one from each site, such as BRA X USA; BRA X JP; BRA X HW; USA X JP; USA X HW; HW X JP), were made. The Mann–Whitney test was used to compare copper concentrations between different organ tissues.
The Mann–Whitney test was chosen since it is non-parametric and sensitive for small samples like the one in this study. All statistical analyses were conducted using PAST 3.14 software at a significance level of p < 0.05. It is worth noting that the whole approach outlined in this paper was the same as that employed by Fraga et al. (2018).
It is worth mentioning that the entire methodology described in the present work was the same used by Fraga et al. (2018) and Fraga et al. (2023), with few changes in the counts, as it is another metal analyzed.
Results
Direct (N = 21) and additional (N = 28) analyses were performed with data from Brazil (South Atlantic), Texas (North Atlantic), and Japan, and additional analyses with data from Hawaii.
The presence of Cu was found in the organs of all specimens in this study. It is observed the formation of two groups, the first formed by animals from Texas (46.11 µg g−1) (SE = 29.59, range 18.26–103.9 µg g−1) and Japan (65.18 µg g−1) (SE = 27.66, range 23.69–94.36 µg g−1), with the highest average concentrations and without significant differences between them, and the second with organisms from Brazil (16.79 µg g−1) (SE = 5.64, range 11.31–28.33 µg g−1), with the lowest mean concentrations and with a significant difference from the others (p < 0.05).
When we add the data from Hawaii in the additional liver comparisons, the specimen means (91.08 µg g−1) (SE = 58.29, range 1.3–173 µg g−1), these follow the pattern of absence of significant differences to the from the specimens from Texas and Japan (p > 0.05) and maintaining the differences from the specimens from Brazil, which remained with the lowest mean concentrations (16.79 µg g−1) (SE = 58.2, range 11.31–28.33 µg g−1), with a statistically significant difference (p < 0.05). Direct and additional analyses were compiled with median and scatter points represented in Fig. 4.
Direct and additional analyses: median concentrations (µg g−1 w.w.) of copper recorded in the liver of juvenile green turtles of Texas (n = 7), Japan (n = 7), Hawaii (n = 7), and Brazil (n = 7). Boxplot: central line is median, upper lines of the boxes represent the 75th quartile, lower lines of the boxes represent the 25th quartile, end of whiskers are minimum and maximum. Dots represent the individuals from each locality
When data from the livers of turtles were compared, higher copper averages were found in specimens belonging to the northern hemisphere (67.45 µg g−1) (SE = 45.15, range 1.3–173 µg g−1), when compared to specimens from the southern hemisphere (16.79 µg/g) (SE = 6.09, range 11.31–28.33 µg g−1), with a significant difference between the two groups (p < 0.05). Graphics were compiled for the liver with the dispersion points (in Fig. 5A), and the medians between the two hemispheres (Fig. 5B).
A Scatter chart with curvilinear carapace length (cm) and copper concentration (µg g−1 wet weight) in liver of green turtles (Chelonia mydas) in the northern hemisphere (USA—Texas, Japan, and Hawaii) (n = 21) and south hemisphere (Brazil) (n = 7). B Median concentrations (µg g−1 wet weight) of copper recorded in liver of juvenile green turtles in the northern and south hemispheres. Boxplot: central line is median, upper lines of the boxes represent the 75th quartile, lower lines of the boxes represent the 25th quartile, end of whiskers are minimum and maximum. Dots represent the individuals from each locality
When analyzing the mean contents found in the kidney in the direct analyses, it was observed that the means of all locations were statistically equal (p > 0.05), United States (4.83 µg g−1) (SE = 4.41, range 0.86–13.26 µg g−1), and Japan (2.47 µg g−1) (SE = 0.62, range 1.60–3.27 µg g−1), and even specimens from southern Brazil (1.89 µg g−1) (SE = 0.94, range 0.20–3.27 µg g−1) (p > 0.05). In the additional analyses for the kidneys when the mean of Hawaii (3.71 µg g−1) was added (SE = 2.95, range 1.5–10.5 µg g−1), this pattern remained of equal means across all locations (p > 0.05). Direct and additional analyses of the kidney with median and scatter points are represented in Fig. 6.
Direct and additional analyses: median concentrations (µg g−1 wet weight) of copper recorded in the kidney of juvenile green turtles of Texas (USA) (n = 7), Japan (n = 7), Hawaii (USA) (n = 7), and Brazil (n = 7). Boxplot: central line is median, upper lines of the boxes represent the 75th quartile, lower lines of the boxes represent the 25th quartile, end of whiskers are minimum and maximum. Dots represent the individuals from each locality
In muscle, statistically equal means (p > 0.05) were found between the USA (0.75 µg g−1) (SE = 0.32, range 0.36–1.25 µg g−1) and Japan (0.75 µg g−1) (SE = 0.90, range 0.11–2.64 µg g−1), while the average for Brazil (0.13 µg g−1) (SE = 0.03, range 0.09–0.19 µg g−1) was the lowest with difference significant compared to the Texas and Japan. No further analysis was performed on muscle due to the lack of muscle data from the other two locations. Muscle analyses with median and scatter points are represented in Fig. 7.
Direct: median concentrations (µg g−1 wet weight) of copper recorded in the muscle of juvenile green turtles of USA (Texas) (n = 7), Japan (n = 7), and Brazil (n = 7). Boxplot: central line is median, upper lines of the boxes represent the 75th quartile, lower lines of the boxes represent the 25th quartile, end of whiskers are minimum and maximum. Dots represent the individuals from each locality
When comparing the copper contents between organs of specimens from Texas, Japan, and Brazil (since there were no data on Cu in muscle in the specimens from Hawaii), it was possible to perceive the highest Cu contents found in the liver (28.33 µg g−1) (SE = 30, range 11.31–103.09 µg g−1), followed by the kidney (2.25 µg g−1) (SE = 2.82 range 0.20–13.26 µg g−1), and having the pectoral muscle (0.33 µg g−1) (SE = 0.60, range 0.09–2.64 µg g−1), the lowest site of Cu bioaccumulation, with significant differences between organs (p < 0.05). The analyses between liver, kidney, and muscle with median and scatter points were represented (Fig. 8).
Direct analyses: median concentrations (µg g−1 wet weight) of copper recorded in organs (liver, kidney, and muscle) of juvenile green turtles of USA (Texas) (n = 7), Japan (n = 7), and Brazil (n = 7). Boxplot: central line is median, upper lines of the boxes represent the 75th quartile, lower lines of the boxes represent the 25th quartile, end of whiskers are minimum and maximum. Dots represent the individuals from each locality
Discussion
The present work made possible, in a pioneering way, the uniform comparison of Cu contents when using marine vertebrates of great longevity, of the same species, size class and that inhabit coastal regions of similar characteristics in different parts of the world, which resulted in the possibility of assessment of global Cu distribution through concentrations in animal tissues.
The global distribution of Cu in the oceans differs in patterns in coastal and open ocean regions, where there is a pattern of higher concentration on the coastal surfaces of the Atlantic and Pacific Oceans (Roschan and Wu 2015), mainly due to sources such as river input and deposition of aerosols (Richon and Tagliabue 2019a, b).
The global pattern of trace elements for green turtle juveniles found in the present work was also found for Cd by Fraga et al. (2018), with a pattern of higher levels being found in the Pacific compared to the Atlantic. This pattern is also found for Cu, but for deep oceanic waters (Millero 2006a, b). However, the Cu contents found in the present study were higher than those of Cd found by Fraga et al. (2018), which can be explained by the relevance of the element in metabolic activities and because it is an essential micronutrient (Bremner 1998), which does not occur with Cd, which is an element with toxic properties.
In addition to the global pattern identified in the organisms of the present study, turtles from the northern hemisphere had higher levels of Cu than turtles from the southern hemisphere, with a significant difference (p < 0.05) in the liver and kidney. This difference may be reflecting the global pattern of concentrations of this element in the two hemispheres identified by Richon and Tagliabue (2019a, b), who associated this pattern with the large anthropogenic and natural input in the northern hemisphere through fluvial input and aerosols in relation to the southern hemisphere. However, as we have only one locality representing the southern hemisphere, other local factors may be more relevant, and it is not possible to confirm this difference between the two hemispheres by the turtles in the present study, but it is a plausible hypothesis to be tested in future studies.
When comparing the regions, higher levels of Cu were found in specimens from the Hawaiian Islands and Okinawa in Japan, which despite having lower anthropic activities compared to the great continents, the animals from these places had higher levels of Cu in their organs, when compared to other regions. This fact may be due to the previously mentioned atmospheric and fluvial input, but with great influence from the volcanic processes present in both islands and that contribute to the deposition of Cu in the oceans of these regions. In addition, the presence of large extensions of polymetallic nodules containing Cu in high concentrations south of the Hawaiian Islands (Martins et al. 2006), and hydrothermal activities such as the Okinawa trough in Japan that are rich in Cu (Mello and Quental 2000), may be contributing to the loads of this element in the turtles in the present study.
The lowest levels found in the liver of turtles in Rio Grande do Sul (RS), Brazil, in relation to other locations follow an apparent pattern of low levels of copper in the local biota, with records of invertebrates with lower levels when compared to other locations around the globe within of the same species, such as populations of Perna perna (Baraj et al. 2003) and Amphibalanus improvisus (Garcia 2011), also mentioned by Fraga et al. (2023). In vertebrates, although there are no global comparative studies published, Baraj et al. (2009) found low levels of Cu in liver (7.48 µg g−1 wet wt.) and in kidney (4.55 µg g−1 wet wt.) of the southern fur seal (Arctocephalus australis) in the same region, which is a carnivore with a higher trophic level than green turtles with a diet based on fish and cephalopods (Gerpe et al. 1990; Naya et al. 2002). In addition, this same pattern of low levels of metals including Cu for animals in Rio Grande do Sul compared to other parts of the globe was also found for Zn in animals from that locality, in a study carried out by Fraga et al. (2023). The great variability found between lower copper levels in Brazil in relation to the liver of Hawaiian turtles, kidneys of Texas turtles, and muscles of Japanese turtles may also be reflecting this pattern of lower copper contents found in the biota of Rio Grande do Sul, Brazil.
Other studies already carried out around the world with C. mydas demonstrate that different average levels are found in the liver of these specimens, such as from Japan by Anan et al. (2002) (49.9 µg g−1 wet wt.), Gardner et al. (2006) (60.04 µg g−1 wet wt.) in Mexico, (32.8 µg g−1 wet wt.) by Storelli et al. (2008) in Europe, and in Hawaii (87.6 µg g−1 wet wt.) by Aguirre et al. (1994). However, as these specimens from these studies did not follow a pattern of age, sex, size, among other requirements for constructing the average content, such values can only be used for a more generalized view of copper levels in these animals across the globe, and not a bioindicator standard as in the present study. But they still showed higher copper values in specimens from the northern hemisphere than those found by da Silva et al. (2014) in the southern hemisphere.
The individuals in the present study presented Cu in all analyzed tissues, with the liver being the organ with the highest levels, followed by the kidney and pectoral muscle, with a significant difference between the organs (p < 0.05); this pattern is also common in birds and marine mammals (Caurant et al. 1999). The liver with the highest levels corroborates the study by Tam et al. (2010) with cell lines in C. mydas, where copper is predominantly found in liver cells when compared to other tissues, demonstrating that this organ is relevant in the bioindication of copper. In addition, the liver plays an important role in the metabolism of metals, mainly with its relationship with metallothionein, a protein that aims to detoxify metals by binding to metals, preventing harmful effects on organisms, that is, limiting the toxicity of metals (Jakimska et al. 2011) and targeting homeostasis (Rosa et al. 2008).
For sea turtles, there are no reference values for determining the amount of copper content that brings toxicity and damage to the metabolism of these animals, but studies carried out by Tan et al. (2010) with cell lines demonstrate that the cytotoxic sensitivity in turtles is lower than that of fish, indicating that there is a level of tolerance to some metals. Therefore, in the present study, it is not possible to determine whether the concentrations found harm the marine biota, especially the species C. mydas.
It has already been demonstrated that the presence of toxic trace elements added to other comorbidities such as the presence of herpes virus, low immunity, and other factors can contribute to the emergence and development of fibropapillomatosis in C. mydas that inhabit coastal areas, mainly supported by the idea that contamination by metals such as Cu, Fe, and Pb may be related to the etiology of fibropapillomatosis through the generation of oxidative stress (da Silva et al. 2016). Therefore, works like this one that quantify the presence of these metals are relevant for conservation, because according to Limpus and Miller (1990) this disease leads to the appearance of neoplastic tumors causing weakness and that can lead to the death of these animals. And more than that, important toxicity studies are carried out to determine levels that harm the health of these animals.
Therefore, the present study represents an important step towards the conservation of sea turtles, through the uniform comparison of levels in a global range, by serving for future comparative studies related to the average levels found in this work. In addition, the present study using C. mydas as a bioindicator provided a new view of the distribution of bioavailable copper in the oceans, demonstrating the relevance of anthropogenic and natural factors in copper supply.
Data availability
Data supporting the findings of this study are not openly available as they are data provided by the co-authors and will be available by the corresponding author upon submission request.
References
Aguirre AA, Balazs GH, Zimmerman B, Galey FD (1994) Organic contaminants and trace metals in the tissues of green turtles (Chelonia mydas) afflicted with fibropapillomas in the Hawaiian Islands. Mar Pollut Bull 28(2):109–114
Anan Y, Kunito T, Sakai H, Tanabe S (2002) Subcellular distribution of trace elements in the liver of sea turtles. Mar Pollut Bull 45(1–12):224–229
ATSDR (Agency for Toxic Substances and Disease Registry) (2022) Atlanta toxicological profile for copper; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA
Baraj B, Niencheski LF, Corradi C (2003) Trace metal content trend of mussel Perna perna (Linnaeus, 1758) from the Atlantic coast of southern Brazil. Water Air Soil Pollut 145:205–214
Baraj B, Niencheski LF, Windom H, Hermanns L (2009) Concentração de metais traço no fígado, rim e coração de focas sul-americanas (Arctocephalus australis) do sul do Brasil. Boletim De Poluição Marinha 58:1922–1952
Barbieri E (2009) Concentration of heavy metals in tissues of green turtles (Chelonia mydas) sampled in the Cananeia estuary, Brazil. Braz J Oceanogr 57:243–248. http://www.scielo.br/pdf/bjoce/v57n3/v57n3a07.pdf
Bremner I (1998) Manifestations of copper excess. Am J Clin Nutr 67(Suppl):1069–73S
Caurant F, Bustamante P, Bordes M, Miramand P (1999) Bioaccumulation of cadmium, copper and zinc in some tissues of three species of marine turtles stranded along the French Atlantic coasts. Mar Pollut Bull 38(12):1085–1091
da Silva CC, Varela Jr AS, Barcarolli IF, Bianchini A (2014) Concentrations and distributions of metals in tissues of stranded green sea turtles (Chelonia mydas) from the southern Atlantic coast of Brazil. Sci Total Environ 466:109–118
Embassy of Japan (2012) Japan regions. https://www.br.emb-japan.go.jp/itprtop_pt/index.Html. Accessed 15 Apr 2020
Espejo W, Celis JE, González-Acuña D, Banegas A, Barra R, Chiang G (2017) A global overview of exposure levels and biological effects of trace elements in penguins. Rev Environ Contam Toxicol 245:1–64
Faust DR, Hooper MJ, Cobb GP, Barnes M, Ertolacci DSS, Smith PN (2014) Inorganic elements in green sea turtles (Chelonia mydas): relationships among external and internal tissues. Environ Toxicol Chem 33:2020–2027. https://doi.org/10.1002/etc.2650
Fraga NS, Martins AS, Faust DR, Sakai H, Bianchini A, da Silva CC, Aguirre AA (2018) Cadmium in tissues of green turtles (Chelonia mydas): a global perspective for marine biota. Sci Total Environ 637:389–397
Fraga NS, Martins AS, Bianchini A, Faust DR, Sakai H, Da Silva CC, Aguirre AA (2023) Pantropical distribution of zinc in green turtles (Chelonia mydas): marine vertebrates as sentiel species. Environ Sci Pollut Res 30(17):50509–50519
Garcia JG (2011) Acumulação de elementos traços em organismos no estuário da Lagoa dos Patos. Universidade Federal do Rio Grande, Rio Grande, RS, Dissertação de Mestrado, p 105
Gardner SC, Fitzgerald SL, Vargas BA, Rodríguez LM (2006) Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. Biometals 19:91–99
Gerpe MS, de Leon AP, Bastida R, Moreno VJ, Rodriguez DH (2009) Sharp accumulation of heavy metals after weaning in the South American fur seal Arctocephalus australis. Mar Ecol-Prog Ser 375:239
Gerpe M, Moreno J, Prrez A, Bastida R, Rodriguez D, Marcovecchio J (1990) Trace metals in the southamerican fur seal, Arctocephalus australis (Zimmermann, 1783). In Proceedings of the 4th International Conference on Environmental Contamination, Barcelona, pp 591–593
Godley BJ, Thompson DR, Furness RW (1999) Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull 38:497–502
IUCN (2023) The IUCN red list of threatened species. Version 2022–2. https://www.iucnredlist.org. Accessed 2 Jan 2023.
Jakimska A, Konieczka P, Skora K, Namieśnik J (2011) Bioaccumulation of metals in tissues of marine animals, part I: the role and impact of heavy metals on organisms. Pol J Environ Stud 20:1117–1125
Lam JCW, Tanabe S, Chan SKF, Yuen EKW, Lam MHW, Lam PKS (2004) Trace element residues in tissues of green turtles (Chelonia mydas) from South China waters. Mar Pollut Bull 48:164–192
Limpus CJ, Miller JD (1990) The use of measured Scutes of Hawksbill Turtles, Eretmochelys-Imbricata, in the Management of the Tortoiseshell (Bekko) Trade. Wildlife Res 17(6):633–639
Maia CB, Almeida ACM, Moreira FR (2006) Avaliação do teor de chumbo em mexilhões da espécie Perna perna na região metropolitana da cidade do Rio de Janeiro. J Braz Soc Ecotoxicol 1(2):195–198. https://doi.org/10.5132/jbse.2006.02.020
Marcovecchio JE, Moreno VJ, Bastida RO, Gerpe MS, Rodriguez DH (1990) Tissue distribution of heavy metals in small cetaceans from the Southwestern Atlantic Ocean. Mar Pollut Bull 21(6):299–304. https://doi.org/10.1016/0025-326X(90)90595-Y
Marrugo-Negrete J, Pinedo-Hernández J, Marrugo-Madrid S, Navarro-Frómeta E, Díez S (2021) Sea cucumber as bioindicator of trace metal pollution in coastal sediments. Biol Trace Elem Res 199(5):2022–2030. https://doi.org/10.1007/s12011-020-02308-3
Martins LR, Barboza EG, Rosa MLCC (2006) Nódulos Polimetálicos e outros Depósitos de Mar Profundo: o Retorno do Interesse. Centro De Estudos De Geologia e Oceânica Porto Alegre N 4:125–131
Mello SLM, Quental SHAJ (2000) Depósitos de sulfetos metálicos no fundo dos oceanos. Braz J Geophysics 18(3)
Millero FJ (2006a) Minor elements in sea water. Chem Oceanogr 3:99–113
Millero FJ (2006b) Chemical Oceanography. 623 Primary Production in the 624 Oceans. Third ed, CRC Taylor and Frances Group, Boca Raton, FL, pp 496
Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW et al (2013) Processes and patterns of oceanic nutrient limitation. Nat Geosci 6(9):701–710. https://doi.org/10.1038/ngeo1765
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Naya DE, Arim M, Vargas R (2002) Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos. Uruguay Mar Mamm Sci 18:734–745
Niencheski LFH (2015) Propriedades químicas da água de mar.g. In: Castello LC, Krug (eds) Introdução às ciências do mar. Editora Textos, Pelotas, pp 140–171
Okamoto K, Yamamoto Y, Fuwa K (1978) Pepperbush powder, a new standard reference material. Anal Chem 50:1950–1951
Prashanth L, Kattapagari KK, Chitturi RT, Baddam VR, Prasad LK (2015) A review on role of essential trace elements in health and disease. J NTR Univ Health Sci 4:75–85. https://doi.org/10.4103/2277-8632.158577
Richon C, Tagliabue A (2019a) Insights into the major processes driving the global distribution of copper in the ocean from a global model. Glob Biogeochem Cycles 33:1594610. https://doi.org/10.1029/2019GB006280
Richon C, Tagliabue A (2019b) Insights into the major processes driving the global distribution of copper in the ocean from a global model. Glob Biogeochem Cycles 2019(33):1594–1610. https://doi.org/10.1029/2019GB006280
Rivera-Duarte G, Rosen D, Lapota DB, Chadwick L, Kear-Padilla AZ (2005) Copper toxicity to larval stages of three marine invertebrates and copper complexation capacity in San Diego Bay. Calif Environ Sci Technol 39:1542–1546
Rosa C, Blake JE, Bratton GR, Dehn LA, Gray MJ, O’Hara TM (2008) Heavy metal and mineral concentrations and their relationship to histopathological findings in the bowhead whale (Balaena mysticetus). Sci Total Environ 399:165–178
Roshan S, Wu J (2015) The distribution of dissolved copper in the tropical-subtropical north Atlantic across the GEOTRACES GA03 transect. Mar Chem 176:189–198
Roshan S, Devries T, Wu J (2020) Constraining the global ocean Cu cycle with a data-assimilated diagnostic model. Global Biogeochem Cycles 34:e2020GB006741. https://doi.org/10.1029/2020GB006741
Sakai H, Saeki K, Ichihashi H, Suganuma H, Tanabe S, Tatsukawa R (2000) Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar Pollut Bull 40:701–709
SEPLAN (2022) Secretaria do Planejamento, Mobilidade e Desenvolvimento. Atlas socioeconômico do Rio Grande do Sul. http://www.atlassocioeconomico.rs.gov.br/conteudo.asp?cod_menu_filho=818&cod_menu=817&tipo_menu=ECONOMIA&cod_conteudo=1468. Accessed 3 Jan 2022.
Shaw KR, Lynch JM, Balazs GH, Jones TT, Pawloski J, Rice MR et al (2021) Trace element concentrations in blood and scute tissues from wild and captive Hawaiian green sea turtles (Chelonia mydas). Environ Toxicol Chem 40(1):208–18
Silva C, Da C, Klein RD, Barcarolli IF, A. (2016) Bianchini. Metal contamination as a possible etiology of fibropapillomatosis in juvenile female green sea turtles Chelonia mydas from the southern Atlantic Ocean. Aquat Toxicol 170:42–51. https://doi.org/10.1016/j.aquatox.2015.11.007
Storelli MM, Ceci E, Marcotrigiano GO (1998) Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimens beached along the Adriatic Sea (Italy). Bull Environ Contam Toxicol 60:546–552
Storelli MM, Barone G, Storelli A, Marcotrigiano GO (2008) Total and subcellular distribution of trace elements (Cd, Cu and Zn) in the liver and kidney of green turtles (Chelonia mydas) from the Mediterranean Sea. Chemosphere 70(5):908–913
Tan F, Wang M, Wang W, Aguirre AA, Lu Y (2010) Validation of an in vitro cytotoxicity test for four heavy metals using cell lines derived from a green sea turtle (Chelonia mydas). Cell Biol Toxicol 26:255–263
USEPA (1977) United state environmental protection agency. Copper, Office of Research Laboratory and Development U.S. Research Triangle Park, NC, USA
Acknowledgements
We thank A. Gordon for providing primary data.
Funding
Nairana S. Fraga was partly supported by a grant from Coordenação de Aperfeiçoamento de Ensino Superior (CAPES). Agnaldo S. Martins was partly supported by a grant from the Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES) (0280/2019) and from the Brazilian Research Council (CNPq) (308860/2018–7).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. The conceptualization, material preparation, data collection, analysis, preparation of writing the first version of the manuscript, and review were performed by Nairana Santos Fraga. The conceptualization, methodologies, sources of financial support, review of the manuscript, resources, and supervision of the work were carried out by Agnaldo S. Martins. Data provision and manuscript review were performed by A.A. Aguirre, Derek R. Faust, H. Sakai, and Cinthia C. da Silva. The supply of data was also carried out by Adalto Bianchini, and the latter also carried out the validation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The present work did not directly use the animals for the study; only biometric data and concentrations of Cu contents provided by the co-authors were used.
Consent to participate
The present work has the consent for participation of Nairana S. Fraga, Agnaldo S. Martins, Adalto Bianchini, Derek R. Faust, H. Sakai, Cinthia Carneiro da Silva, and A. Alonso Aguirre.
Consent for publication
The present work has the consent for publish of Nairana S. Fraga, Agnaldo S. Martins, Adalto Bianchini, Derek R. Faust, H. Sakai, Cinthia Carneiro da Silva, and A. Alonso Aguirre.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• There is a global distribution of copper in the biota, noted by the green turtle (Chelonia mydas) as a bioindicator.
• The highest levels of copper are found in liver tissue when compared to kidney and muscle, which has the lowest bioaccumulation of copper when compared to liver and kidney.
• The copper levels found in turtles from the northern hemisphere in the present study are higher than those from the southern hemisphere.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fraga, N.S., Martins, A.S., Faust, D.R. et al. Distribution of copper in the Atlantic and Pacific Oceans using green turtles (Chelonia mydas) as a bioindicator. Environ Sci Pollut Res 31, 31967–31977 (2024). https://doi.org/10.1007/s11356-024-33366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33366-y