Abstract
Recently, polycyclic aromatic hydrocarbons (PAHs) were found to be linked to various diseases. The current study’s objective was to explore whether or not there was a relation between PAH exposure and poor sleep pattern. We evaluated nine urine PAH metabolites as exposures in our cross-sectional research based on the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2010. Logistic regression, restricted cubic spline regression (RCS) model, weighted quantile sum (WQS) regression, subgroup analysis, and mediation analysis were used to assess the associations between PAH metabolism and poor sleep pattern risk. After controlling for all confounding variables, several primary PAH metabolites, namely 1-hydroxynapthalene (1-NAP, OR 1.32, 95% CI 1.04–1.68), 2-hydroxyfluorene (2-FLU, OR 1.34, 95% CI 1.05–1.71), 1-hydroxyphenanthrene (1-PHE, OR 1.30, 95% CI 1.03–1.64), 9-hydroxyfluorene (9-FLU, OR 1.38, 95% CI 1.09–1.74), and ∑PAHs (OR 1.33, 95% CI 1.05–1.69), compared to the bottom tertile, were associated with increased risk of poor sleep pattern. The WQS regression analysis showed that 9-FLU and 1-NAP comprised the two most important factors related to poor sleep pattern. Mediation analysis revealed that inflammation acted as a mediator between PAHs and the prevalence of poor sleep pattern. In conclusion, exposure to PAHs may be associated with poor sleep pattern. Inflammation is a mediator of the effects of PAH exposure on poor sleep pattern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of sleep to human health is becoming more widely acknowledged. According to a World Health Organization assessment, almost one-third of the world’s population suffers from sleep disorders (Lancet 2022). It is reported that insomnia constitutes a common health concern, with an estimated incidence ranging from 5 to 50% (Morin and Jarrin 2022), which is associated with a number of negative consequences such as hypertension (Jarrin et al. 2018), cardiac events (Zhong et al. 2023), mood/anxiety disorders (Palagini et al. 2022), and cancer (Blask 2009). In addition, our bodies require appropriate sleep duration to perform regular physiological, cognitive and psychological processes, and excessive or insufficient sleep length can be harmful to our health (Diekelmann and Born 2010). Previous research has indicated that insufficient sleep duration is linked to an elevated risk of obesity (Itani et al. 2017), type 2 diabetes (Lee et al. 2017), coronary heart disease (Lao et al. 2018), and hypertension (Guo et al. 2013). In the meantime, studies of epidemiology have revealed that sleeping for lengthy periods of time is closely connected with higher risk of cardiovascular disease (Krittanawong et al. 2019), obesity (Liu et al. 2019), diabetes (Shan et al. 2015), and stroke (He et al. 2017). Sleep quality is affected by many characteristics, conditions, and stimuli such as age, sex, physical exercise, mental or physical health, and the environment (Billings et al. 2020). It has been shown that air pollution disrupts sleep and can cause a variety of issues. According to several studies, longer sleep duration is connected with worsening air pollution (An et al. 2019; An and Yu 2018). Xu et al. (2021a) found that excessive exposure to higher concentrations of PM2.5 or O3 increased the incidence of trouble sleeping. In brief, exposure to air pollutants is a significant environmental risk factor that should be given enough attention due to its potential impact on sleep.
Polycyclic aromatic hydrocarbons (PAHs) comprise a type of air pollution that exerts a negative impact on human health (Marzooghi and Toro 2017). They are principally produced by the incomplete combustion of organic substances like coal, oil, natural gas, wood, waste, and tobacco—as well as by cooking, smoking, and grilling foods (Bostrom et al. 2002; Hoseini et al. 2018; Wang et al. 2016a, b). PAHs can enter the body via the mouth, nose, and epidermis, among other entry points (McClean et al. 2004; Veyrand et al. 2013; Zhang et al. 2014). After a few hours in the body, PAHs are broken down into different products that are primarily excreted in the urine, and the urinary monohydroxy-PAHs such as naphthalene, fluorene, phenanthrene, and pyrene are thought to be able to assess the body’s level of PAH exposure (Dalton et al. 2002). Assessing 1-hydroxypyrene (1-PYR) is the most prevalent means used to assess PAH exposure, so it is often used to analyze how much PAH a person is exposed to (Shi et al. 2021). PAH pollution has been studied widely in recent years and has been linked to diseases such as chronic obstructive pulmonary disease (COPD) (Peng et al. 2023), hypertension (Wang et al. 2022a, b), diabetes (Stallings-Smith et al. 2018), rheumatoid arthritis (Sun et al. 2020), metabolic syndrome (Yang et al. 2022), and cancer (Rahman et al. 2022). Current research demonstrates that PAH is associated with trouble sleeping (Chen et al. 2023) as well as short sleep duration (Han and Wang 2023). However, their studies are limited to a single dimension of sleep, which does not provide a comprehensive assessment of a person’s sleep. At the same time, earlier research has highlighted the importance of including a variety of sleep components in analyses (Hestetun et al. 2018).
In addition, considering that there are many opportunities for people to be exposed to PAHs and that there are fewer studies on the relationship between PAHs and sleep, further research on the relation between the two is imperative. As a result, the aim of this study was to inspect the exposure burden of PAHs and evaluate their effects on poor sleep pattern (a combined sleep behaviors) using the 2005–2010 National Health and Nutrition Examination Survey (NHANES) data. This study provides epidemiologic evidence of the effects of PAHs on public health, and we call for the development of prevention and control policies to reduce the effects of environmental pollutants on the sleep health of the public.
Materials and methods
Study population
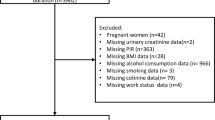
In this study, we utilized data from four NHANES cycles (2005–2006, 2007–2008, and 2009–2010) that involved a total of 31,034 participants. We excluded individuals younger than 20 years or elder than 80 years (n = 15,160) and those with missing co-variables or outcome-variable data (n = 12,234), leaving 3640 adults (Fig. 1). There were 595 participants identified with poor sleep pattern and 3045 non-poor sleep pattern participants. The NHANES is a cross-sectional study of the US’s population that provides a wealth of information to support the formulation of nutrition and health policies. Since the NHANES publishes open-access data, an institutional review board analysis was not necessary for our study.
Assessment of PAH metabolites
The researchers collected the urine samples from the subjects, refrigerated them, and then sent them to the testing center for pretreatment before analyzing the PAH metabolite concentrations in urine. A total of nine PAH metabolites were analyzed: 1-hydroxynapthalene (1-NAP), 2-hydroxynapthalene (2-NAP), 3-hydroxyfluorene (3-FLU), 2-hydroxyfluorene (2-FLU), 3-hydroxyphenanthrene (3-PHE), 1-hydroxyphenanthrene (1-PHE), 2-hydroxyphenanthrene (2-PHE), 1-hydroxypyrene (1-PYR), and 9-hydroxyfluorene (9-FLU). The levels of urinary PAH metabolites were adjusted in all analyses for the concentration of creatinine (ng/g creatinine). The adjusted PAH levels were equal to the PAH concentration multiplied by 100 and divided by the concentration of creatinine in the urine (Middleton et al. 2019). Given that the values of PAH metabolites in urine were skewed, a logarithmic change was performed before statistical analysis to ensure a normal distribution.
Assessment of poor sleep pattern
The inquiry “How much sleep do you usually get at night on weekdays or workdays?” was used to collect sleep duration data. The responses to the questions “Have you been asked by a doctor or other health professional if you have trouble sleeping?” and “Have you been asked by a doctor or other health professional if you have sleep disorder?” were used to determine whether someone had trouble sleeping or sleep disorder. A poor sleep pattern was defined as having at least two of the following sleep issues: (1) abnormal sleep duration, which was defined as < 7 h or > 9 h per night, (2) trouble sleeping, and (3) self-reported sleep disorder.
Covariates
Covariates included age, sex, race, educational level, marital status, poverty-to-income ratio (PIR), body mass index (BMI), smoking status, and alcohol intake. The categories were as follows: age (20–39, 40–59, 60–79), sex (male, female), race (Mexican American, non-Hispanic White, non-Hispanic Black, others), educational level (less than 9th grade, 9–11th grade, high school graduate or equivalent, some college or AA degree, college graduate or above), marital status (married/living, windowed/divorced/separated, never married), PIR (0–4.99, ≥ 5), BMI (< 25.0, 25.0–29.9, ≥ 30.0), smoking status (yes, no), and alcohol intake (< 12 drinks/year, ≥ 12 drinks/year).
Statistical analysis
For categorical data, counts (%) were used to describe the demographic characteristics. We used the chi-squared test to compare categorical variates between individuals with poor sleep pattern and their peers. The coefficients of association among PAH metabolites were calculated using Spearman correlation analysis. The relation between PAH and poor sleep pattern was evaluated employing multivariate logistic regression analysis. Individual PAHs were classified into tertiles, with the bottom tertile serving as the reference. We first explored the relation between each PAH metabolite and poor sleep pattern in model 1, and then adjusted the regression model by adding age, sex, and race (model 2). Finally, the link between PAH metabolites and poor sleep pattern was further explored in model 3 as adjusted for age, gender, race, educational level, marital status, PRI, BMI, smoking status, alcohol intake. In addition, we adopted restricted cubic spline (RCS) analysis to investigate the dose–response connection between PAH metabolites and poor sleep pattern. We employed weighted quantile sum (WQS) regression to elucidate the total effects and relative weights of each PAH metabolites on poor sleep pattern in order to shed further light on the relationship between PAH and sleep. Finally, mediated effect analysis was employed to determine if PAHs influence the occurrence of poor sleep pattern via inflammation. The average causal mediated effect (ACME) and average direct effect (ADE) are the two most important parameters in mediation analysis. We executed the statistical software R Studio to conduct all data analyses and to generate diagrams. In the current investigation, P values < 0.05 were considered statistically significant.
Results
Demographic information of participants, using data from NHANES, 2005–2010
As shown in Table 1, there were 595 (16.3%) subjects who exhibited poor sleep pattern, and these subjects were more likely to be older (30.9% vs. 26.8%, P < 0.001), non-Hispanic White (55.0% vs. 47.6%, P < 0.001), smokers (57.1% vs. 45.8%, P < 0.001), and to have had a greater BMI (49.4% vs. 36.1%, P < 0.001). However, we detected no differences in gender, PIR or alcohol consumption.
Distribution and correlation of PAH metabolites
The distribution with respect to PAH exposure among the study cases, adjusted for urine creatinine, is shown in Table 2. Participants were most exposed to 2-NAP, with geometric mean concentrations of 4155.7 ng/g and median concentrations of 3713.7 ng/g. The least PAH exposure was noticed for 2-PHE of 69.9 ng/g (median = 63.5 ng/g). When we analyzed the correlations among nine PAH metabolites, our results revealed that the Spearman’s correlation coefficients ranged from 0.40 to 0.95, and that 2-FLU and 3-FLU exhibited the strongest association (r = 0.95, P < 0.001; Fig. 2).
Spearman correlation among 9 PAH metabolites. Abbreviation: 1-NAP, 1-hydroxynapthalene; 2-NAP, 2-hydroxynapthalene; 3-FLU, 3-hydroxyfluorene; 2-FLU, 2-hydroxyfluorene; 3-PHE, 3-hydroxyphenanthrene; 1-PHE, 1-hydroxyphenanthrene; 2-PHE, 2-hydroxyphenanthrene; 1-PYR, 1-hydroxypyrene; 9-FLU, 9-hydroxyfluorene. *P < 0.05, **P < 0.01, ***P < 0.001
Associations between PAH metabolites and poor sleep pattern
The tertile analyses for model 1 illustrated that the highest levels of 1-NAP (OR 1.58; 95% CI 1.27–1.96), 2-NAP (OR 1.35; 95% CI 1.09–1.68), 3-FLU (OR 1.26; 95% CI 1.03–1.56), 2-FLU (OR 1.58; 95% CI 1.28–1.96), 3-PHE (OR 1.40; 95% CI 1.14–1.73), 1-PHE (OR 1.55; 95% CI 1.25–1.92), 2-PHE (OR 1.58; 95% CI 1.28–1.95), 9-FLU (OR 1.71; 95% CI 1.38–2.12), and ∑PAHs (OR 1.56; 95% CI 1.26–1.93) were positively correlated with the risk of poor sleep pattern. While in model 3 adjusted for all confounding factors, only highest concentrations of 1-NAP (OR 1.32; 95% CI 1.04–1.68), 2-FLU (OR 1.34; 95% CI 1.05–1.71), 1-PHE (OR 1.30; 95% CI 1.03–1.64), 2-PHE (OR 1.27; 95% CI 1.01–1.60), 9-FLU (OR 1.38; 95% CI 1.09–1.74), and ∑PAHs (OR 1.33; 95% CI 1.05–1.69) still showed considerable favorable associations with poor sleep pattern, compared to the lower concentrations (Table 3).
The dose–response relationships between PAH metabolites and the prevalence of poor sleep pattern
Figure 3 shows a definite connection between PAH metabolites and poor sleep pattern. After log-transformation and adjusting for age, sex, race, educational degree, marital status, PIR, BMI, smoking status, and alcohol consumption, RCS exhibited a linear relationship between 1-NAP, 3-PHE, 1-PHE, and 9-FLU and the risk of poor sleep pattern (P for non-linearity > 0.05). However, 2-NAP, 3-FLU, 2-FLU, 2-PHE, and 1-PYR were nonlinearly associated with poor sleep pattern (P for non-linearity < 0.05).
Association between PAH metabolites and poor sleep pattern evaluated by restricted cubic splines (RCS). Adjusted for age, gender, race, educational level, marital status, PRI, BMI, smoking status, and alcohol intake. 1-NAP, 1-hydroxynapthalene; 2-NAP, 2-hydroxynapthalene; 3-FLU, 3-hydroxyfluorene; 2-FLU, 2-hydroxyfluorene; 3-PHE, 3-hydroxyphenanthrene; 1-PHE, 1-hydroxyphenanthrene; 2-PHE, 2-hydroxyphenanthrene; 1-PYR, 1-hydroxypyrene; 9-FLU, 9-hydroxyfluorene; OR, odds ratio; CI, confidence interval
Associations between combined and individual PAH and poor sleep pattern
We then executed the WQS model to investigate the effects of mixed exposures and the proportional weight of each PAH metabolite with regard to poor sleep pattern (Fig. 4A and B). The WQS results showed that 9-FLU was the most heavily weighted component in both models (weighted 0.443 and 0.441, respectively). In the crude model, 2-PHE and 1-NAP were weighted heavily (weighted 0.187 and 0.168, respectively), while 1-NAP and 2-PHE were weighted heavily in the modified model (weighted 0.180 and 0.109, respectively).
Weights for weighted quantile sum (WQS) regression index for mixed PAH exposure in A crude model; B adjusted for age, gender, race, educational level, marital status, PRI, BMI, smoking status and alcohol intake in NHANES, 2005–2010. Abbreviation: 1-NAP, 1-hydroxynapthalene; 2-NAP, 2-hydroxynapthalene; 3-FLU, 3-hydroxyfluorene; 2-FLU, 2-hydroxyfluorene; 3-PHE, 3-hydroxyphenanthrene; 1-PHE, 1-hydroxyphenanthrene; 2-PHE, 2-hydroxyphenanthrene; 1-PYR, 1-hydroxypyrene; 9-FLU, 9-hydroxyfluorene
Relations between PAH metabolites and poor sleep pattern in different subgroups
We observed heterogeneity in the correlations of specific PAH metabolites with poor sleep pattern across age, gender, and smoking status in subgroup analyses (Table 4). Participants aged 20–39 years showed statistically significant associations of all PAH metabolites except for 1-NAP (OR 1.28; 95% CI 0.94–1.73), with poor sleep pattern. However, there is a borderline link between ∑PAHs (OR 1.50, 95% CI 0.99–2.25) and poor sleep pattern. Among the female participants, we observed a statistically significant association of 1-NAP (OR 1.30; 95% CI 1.08–1.55), 2-NAP (OR 1.50; 95% CI 1.08–2.07), 2-FLU (OR 1.59; 95% CI 1.18–2.14), 3-FLU (OR 1.46; 95% CI 1.12–1.90), 9-FLU (OR 1.47; 95% CI 1.04–2.07), 1-PYR (OR 1.63; 95% CI 1.16–2.30) and ∑PAHs (OR 1.42; 95% CI 1.11–1.82) with poor sleep pattern. In addition, subjects who were smokers manifested a greater association between PAH metabolites and poor sleep pattern, especially for 9-FLU (OR 1.53; 95% CI 1.12–2.10) and ∑PAHs (OR 1.43; 95% CI 1.13–1.82).
Associations between PAH metabolites and poor sleep pattern and its components
Supplementary Table S1 shows the associations between PAH metabolites and poor sleep pattern and its several components in adults. The logistic regression analyses showed significant associations of ∑PAHs with poor sleep pattern (OR 1.29; 95% CI 1.07–1.56), and trouble sleeping (OR 1.33; 95% CI 1.13–1.58) after adjusting for all potential confounders. In addition to being associated with poor sleep pattern, 1-NAP, 2-NAP, 2-FLU, 3-FLU, and 9-FLU had positive correlations with at least one of the components.
Analysis of inflammation as a mediator between PAH exposure and poor sleep pattern
In the present investigation, we conducted a mediation analysis to determine if inflammation acted as a mediator between PAHs and the prevalence of poor sleep pattern. PAHs have been demonstrated to contribute to the development of poor sleep pattern by altering white blood cell (WBC) and neutrophil count (NC) levels (Fig. 5). In detail, WBC-mediated efficacy accounted for 6.67% of the relationship between ∑PAHs and poor sleep pattern prevalence (ACME = 1.17e − 03, 95% CI 8.51e − 05, 0.01; ADE = 1.63e − 02, 95% CI 4.33e − 03, 0.02; Fig. 5A), while NC mediated efficacy accounted for 6.44% (ACME = 1.12e − 03, 95% CI 4.02e − 05, 0.01;ADE = 1.63e − 02, 95% CI 4.54e − 03, 0.02; Fig. 5B).
Mediation analysis of inflammation indicators A WBC, B NC, C LC, and D CRP on the interaction between PAHs and prevalence of poor sleep pattern. WBC, white blood cell; NC, neutrophil; LC, lymphocyte; CRP, C-reactive protein. The models were adjusted for age, gender, race, educational level, marital status, PRI, BMI, smoking status, and alcohol intake
Discussion
The goal of our study was to examine PAH exposure burden and its link to the risk of poor sleep pattern using the NHANES database, 2005–2010. Our key results were as follows: (1) The geometric mean of urine 2-NAP concentrations was 4155.7 ng/g creatinine, designating it as the most abundant of all the PAH metabolites we evaluated; (2) higher levels of 1-NAP, 2-FLU, 1-PHE, 2-PHE, 9-FLU, and ∑PAHs were positive correlated with poor sleep pattern; (3) the most prevalent PAH metabolites in the WQS model for poor sleep pattern were 9-FLU and 1-NAP; (4) the levels of various PAH metabolites were linked to higher rates of poor sleep pattern in individuals who were women, smoker, and between 20 and 39 years of age; and (4) inflammation is a mediator between PAHs and the incidence of poor sleep pattern.
To our knowledge, this is the first-ever comprehensive study undertaken on the relationship between PAHs and poor sleep pattern. We herein ascertained that there were variations in age, race, smoking status, and BMI between the groups showing poor sleep pattern and those without poor sleep pattern. According to a survey about sleep issues among US adults, non-Hispanic Whites reflected the highest incidence of sleep issues (Wang et al. 2023), which was consistent with our results. Moreover, previous research showed that non-Hispanic Whites were more prone to use sleep medications than non-Hispanic Blacks or Mexican Americans (Ford et al. 2014; Vozoris 2019). Currently, it is believed that differences in healthcare availability, healthcare-seeking behaviors, and cultural norms may partially account for racial disparities in the prevalence of sleep issues (Flack et al. 1995; Gornick et al. 1996; Jean-Louis et al. 2001). We acknowledge that numerous physiological alterations accompany aging (including alterations in sleep and circadian rhythms), elevating the susceptibility of middle-aged and elderly people to sleep disorders (Patel et al. 2018); similarly, our findings revealed that subjects who had trouble sleeping were also elder (≥ 40 years of age). It has been shown that smoking, a prevalent lifestyle risk factor, is associated with poorer sleep quality (Woo et al. 2022), which is consistent with the data in the baseline of this study. A meta-analysis of relations between obesity and insomnia showed a significant weak positive correlation between insomnia and BMI (Chan et al. 2018). In the present study, while we discerned that subjects with poor sleep pattern had a greater BMI (49.4% vs. 36.1%, P < 0.001), a recent study from India illustrated that insomnia symptoms were inversely related to obesity/overweight when compared to older individuals with a normal BMI (Muhammad et al. 2022). This contradicts our findings and might be attributed to the disparity in the study populations. As a result, research is sorely needed in the future to explicate the association between BMI, overweight, or obesity and sleep problems in order to provide a clinical reference.
There is less evidence of a link between air pollution and the likelihood of insomnia or other sleep-related symptoms. According to a Boston longitudinal survey, increased air pollution caused by vehicular traffic was significantly correlated with a diminution in sleep duration (Fang et al. 2015). Xu et al. (2021b) demonstrated that exposure to higher levels of PM2.5 and O3 raised the incidence of insomnia. One cross-sectional study conducted by Wang et al. (2022a, b) on patients with obstructive sleep apnea (OSA) discovered a relationship between sleep-disordered breathing parameters and air pollutants, with PM10 and O3 the most significant effectors. It was previously generally hypothesized that air pollutants impacted the quality of sleep via the central nervous system and/or due to changes in the physiology of the respiratory system (Park et al. 2019). In addition, a UK bank–based study has further confirmed that long-term exposure to air pollution can have an adverse impact on sleep health. (Li et al. 2022). We observed similar results in the current investigation. In a multivariate regression model adjusted for all confounding variates, we found the highest levels of 1-NAP, 2-FLU, 1-PHE, 2-PHE, and 9-FLU still reflected significant positive associations with poor sleep pattern in the general US population, revealing that air-pollutant PAHs may have exerted an adverse effect on sleep. However, additional research is necessitated to further elaborate on the mechanism(s) by which PAH exposure leads to trouble sleeping.
To elucidate the association between PAH and poor sleep pattern, we employed WQS regression to analyze the multiple effects and relative weights of each PAH on poor sleep pattern. The results illustrated that exposure to PAH was associated with an increased prevalence of poor sleep pattern, specifically, 9-FLU and 1-NAP. It is currently believed that 1-NAP and 2-NAP are the two isomers of naphthol, and 1-NAP (as naphthalene’s primary metabolite) is found in cigarette smoke, pesticides, and herbicides containing carbamates, and in the beta-receptor blocker propranolol (Berman et al. 2016; Chen et al. 2020; Zhu et al. 2021). In addition, exposure to high concentrations of PAHs in the diet, cigarette smoke, and in automobile exhaust generate fluorene metabolites such as 2-FLU, 3-FLU, and 9-FLU (Cao et al. 2020; Lotz et al. 2016; Wang et al. 2016a, b). A recent study, based on the NHANES data, examined the relationship between PAHs and COPD, emphasizing the primary sources of each PAH metabolite for the first time (Peng et al. 2023). Therefore, the identification of the primary pollutants and the primary sources of those pollutants can enhance methods for the prevention and management of air pollution and, to a certain extent, provide substantial health benefits for individuals who suffer from sleep issues.
In subgroup analysis, participants aged 20 to 39 years were found to be more likely to suffer from poor sleep pattern due to PAH exposure. This might be because such people have a greater likelihood to be exposed to PAH due to factors such as work requirements, food habits, and smoking status. The sex-specific subgroup analysis revealed statistically significant associations between PAH metabolites and poor sleep pattern in the female population. According to an earlier study, this may have been attributable to the women’s dietary habits or their different rates of PAH metabolism (Jain 2015). In addition, women are more vulnerable to chromosomal damage and oxidative stress due to PAH exposure than men (Guo et al. 2014). In addition, tobacco smoke is one of the major indoor sources of PAHs (Kim et al. 2013), and studies have shown that smokers have significantly higher concentrations of PAH metabolites in their urine than non-smokers (Cao et al. 2020). Exposure to tobacco leads to inflammation and oxidative stress in the body (Kuang et al. 2013; Lietz et al. 2013), which may be a possible pathway for the development of poor sleep pattern. The subgroup analysis results indicated that poor sleep pattern caused by PAH exposure was more prevalent in female, 20–39 years old, and smokers, providing a foundation for initiatives targeting reductions in air pollution.
Despite the fact that the mechanisms underlying the association between PAH exposure and poor sleep pattern have not been completely elucidated, there are a few lines of evidence to support the association. First, it was shown that exposure to PAHs is a factor in the onset of oxidative stress and systematic inflammation (Alshaarawy et al. 2013; Andersen et al. 2018; Bortey-Sam et al. 2017; Liu et al. 2018) that can affect the sleep–wake cycle (Pan et al. 2013). Second, numerous studies have shown that PAHs can regulate blood lipids and cause atherosclerosis (Holme et al. 2019; Ranjbar et al. 2015; Xu et al. 2021a, b, c; Yang et al. 2019). In a prior investigation, insomnia was linked to increased vascular stiffness and carotid atherosclerosis, and as the length of sleeplessness increased, the link became more significant (Pan et al. 2022). Third, Xing et al. applied data from NHANES 2011–2012 and found that individuals who were exposed to PAH had higher levels of thyroid hormones and thyroid autoantibodies (Xing et al. 2023), and Sridhar et al. (Sridhar et al. 2011) found an association between high levels of thyroid hormone and numerous aspects of sleep disorders such as increased sleep latency and trouble maintaining sleep. Furthermore, thyroid-stimulating hormone (TSH) levels, as well as T3 and T4, were found to be related to the severity of insomnia in a study by Xia et al. (Xia et al. 2013). To verify whether inflammation mediated the relationship between PAH and sleep patterns, we performed mediation analyses. The results revealed that WBC and NC partly mediated the association between PAH exposure and poor sleep pattern. The results show that PAH exposure is associated with the development of systemic inflammation, which in turn disrupts the normal sleep cycle and increases the incidence of sleep problems. However, other possible mechanisms could not be further investigated due to incomplete information in the NHANES database, and additional clinical studies and animal experiments are needed in the future to demonstrate the precise mechanism by which PAH exposure causes sleep issues.
The strengths of the study were as follows: First, this study entailed a large sample size from the NHANES database, controls for numerous confounding variables to verify the validity of the findings, and offers new proof that exposure to PAH is a risk factor for poor sleep pattern. Second, to determine the contribution of each pollutant in the mixture and to identify special groups, WQS regression and subgroup analysis were utilized. Third, mediation analysis was employed in this study to elucidate inflammation acted as a mediator between PAHs and the prevalence of poor sleep pattern.
There were also some limitations to our study. First, because this was a cross-sectional study, no causal relationship could be drawn. However, with this study, we only explored the link between PAH exposure and poor sleep pattern in order to offer suggestions for future research. Second, although we controlled for several confounding factors, there are numerous factors that influence sleep, and therefore, additional research is needed in the future to confirm the link between PAH and sleep. Third, concentrations of PAH metabolites in urine were used to represent PAH exposure in humans in this study. Nevertheless, each individual’s renal metabolism of PAHs may influence the assessment of PAH exposure in humans. It is thus imperative to develop novel techniques and methods for the detection of blood PAH markers. Lastly, this study’s participants were Americans with distinctive cultural and behavioral norms. Therefore, care must be taken when extrapolating this study’s findings to other nations.
Conclusion
According to the results of this investigation, 2-NAP was the predominant among the PAH metabolites in urine. Several PAH metabolites were found to reflect positive correlations with the risk of poor sleep pattern in US adults, with 9-FLU and 1-NAP achieving the greatest weight. Finally, we found that inflammation acted as a mediator between PAHs and the prevalence of poor sleep pattern. More research, including longitudinal studies, is required to confirm the precise relationship between PAH and sleep and to explore the potential mechanisms underlying this relationship. Our findings suggest that efforts should be undertaken to reduce PAH emissions in order to diminish the risk of poor sleep pattern due to environmental pollution.
Data availability
The corresponding author will provide the data used and analyzed during the present investigation upon reasonable request.
Abbreviations
- ACME:
-
Average causal mediated effect
- ADE:
-
Average direct effect
- 1-NAP:
-
1-Hydroxynapthalene
- 2-NAP:
-
2-Hydroxynapthalene
- 3-FLU:
-
3-Hydroxyfluorene
- 2-FLU:
-
2-Hydroxyfluorene
- 3-PHE:
-
3-Hydroxyphenanthrene
- 1-PHE:
-
1-Hydroxyphenanthrene
- 2-PHE:
-
2-Hydroxyphenanthrene
- 1-PYR:
-
1-Hydroxypyrene
- 9-FLU:
-
9-Hydroxyfluorene
- PAH:
-
Polycyclic aromatic hydrocarbon
- NHANES:
-
National Health and Nutrition Examination Survey
- COPD:
-
Chronic obstructive pulmonary disease
- PIR:
-
Poverty-to-income ratio
- BMI:
-
Body mass index
- RCS:
-
Restricted cubic spline
- WQS:
-
Weighted quantile sum
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- WBC:
-
White blood cell
- NC:
-
Neutrophil
- LC:
-
Lymphocyte
- CRP:
-
C-reactive protein
- TSH:
-
Thyroid-stimulating hormone
References
Alshaarawy O, Zhu M, Ducatman A, Conway B, Andrew ME (2013) Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ Res 126:98–104. https://doi.org/10.1016/j.envres.2013.07.006
An R, Yu H (2018) Impact of ambient fine particulate matter air pollution on health behaviors: a longitudinal study of university students in Beijing, China. Public Health 159:107–115. https://doi.org/10.1016/j.puhe.2018.02.007
An R, Shen J, Ying B, Tainio M, Andersen ZJ, de Nazelle A (2019) Impact of ambient air pollution on physical activity and sedentary behavior in China: a systematic review. Environ Res 176:108545. https://doi.org/10.1016/j.envres.2019.108545
Andersen M, Saber AT, Pedersen JE et al (2018) Assessment of polycyclic aromatic hydrocarbon exposure, lung function, systemic inflammation, and genotoxicity in peripheral blood mononuclear cells from firefighters before and after a work shift. Environ Mol Mutagen 59:539–548. https://doi.org/10.1002/em.22193
Berman T, Goen T, Novack L et al (2016) Urinary concentrations of organophosphate and carbamate pesticides in residents of a vegetarian community. Environ Int 96:34–40. https://doi.org/10.1016/j.envint.2016.08.027
Billings ME, Hale L, Johnson DA (2020) Physical and social environment relationship with sleep health and disorders. Chest 157:1304–1312. https://doi.org/10.1016/j.chest.2019.12.002
Blask DE (2009) Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 13:257–264. https://doi.org/10.1016/j.smrv.2008.07.007
Bortey-Sam N, Ikenaka Y, Akoto O et al (2017) Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (pahs) in kumasi, ghana. Environ Pollut 228:311–320. https://doi.org/10.1016/j.envpol.2017.05.036
Bostrom CE, Gerde P, Hanberg A et al (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110(Suppl 3):451–488. https://doi.org/10.1289/ehp.110-1241197
Cao L, Wang D, Wen Y et al (2020) Effects of environmental and lifestyle exposures on urinary levels of polycyclic aromatic hydrocarbon metabolites: a cross-sectional study of urban adults in China. Chemosphere 240:124898. https://doi.org/10.1016/j.chemosphere.2019.124898
Chan WS, Levsen MP, Mccrae CS (2018) A meta-analysis of associations between obesity and insomnia diagnosis and symptoms. Sleep Med Rev 40:170–182. https://doi.org/10.1016/j.smrv.2017.12.004
Chen ZJ, Liu XX, Xiao ZL et al (2020) Production of a specific monoclonal antibody for 1-naphthol based on novel hapten strategy and development of an easy-to-use elisa in urine samples. Ecotoxicol Environ Saf 196:110533. https://doi.org/10.1016/j.ecoenv.2020.110533
Chen J, Li Y, Yin X et al (2023) Dose-response association of polycyclic aromatic hydrocarbon with self-reported trouble sleeping in adults: evidence from the national health and nutrition examination survey. Environ Sci Pollut Res Int 30:82613–82624. https://doi.org/10.1007/s11356-023-28218-0
Dalton TP, Puga A, Shertzer HG (2002) Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem Biol Interact 141:77–95. https://doi.org/10.1016/s0009-2797(02)00067-4
Diekelmann S, Born J (2010) The memory function of sleep. Nat Rev Neurosci 11:114–126. https://doi.org/10.1038/nrn2762
Fang SC, Schwartz J, Yang M, Yaggi HK, Bliwise DL, Araujo AB (2015) Traffic-related air pollution and sleep in the boston area community health survey. J Expo Sci Environ Epidemiol 25:451–456. https://doi.org/10.1038/jes.2014.47
Flack JM, Amaro H, Jenkins W et al (1995) Epidemiology of minority health. Health Psychol 14:592–600. https://doi.org/10.1037/0278-6133.14.7.592
Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB (2014) Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among us adults: findings from the national ambulatory medical care survey 1999–2010. Sleep 37:1283–1293. https://doi.org/10.5665/sleep.3914
Gornick ME, Eggers PW, Reilly TW et al (1996) Effects of race and income on mortality and use of services among medicare beneficiaries. N Engl J Med 335:791–799. https://doi.org/10.1056/NEJM199609123351106
Guo X, Zheng L, Wang J et al (2013) Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med 14:324–332. https://doi.org/10.1016/j.sleep.2012.12.001
Guo H, Huang K, Zhang X et al (2014) Women are more susceptible than men to oxidative stress and chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environ Mol Mutagen 55:472–481. https://doi.org/10.1002/em.21866
Han L, Wang Q (2023) Urinary polycyclic aromatic hydrocarbon metabolites were associated with short sleep duration and self-reported trouble sleeping in us adults: data from nhanes 2005–2016 study population. Front Public Health 11:1190948. https://doi.org/10.3389/fpubh.2023.1190948
He Q, Sun H, Wu X et al (2017) Sleep duration and risk of stroke: a dose-response meta-analysis of prospective cohort studies. Sleep Med 32:66–74. https://doi.org/10.1016/j.sleep.2016.12.012
Hestetun I, Svendsen MV, Oellingrath IM (2018) Sleep problems and mental health among young norwegian adolescents. Nord J Psychiatry 72:578–585. https://doi.org/10.1080/08039488.2018.1499043
Holme JA, Brinchmann BC, Refsnes M, Lag M, Ovrevik J (2019) Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles. Environ Health 18:74. https://doi.org/10.1186/s12940-019-0514-2
Hoseini M, Nabizadeh R, Delgado-Saborit JM et al (2018) Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a middle eastern area. Environ Pollut 240:781–792. https://doi.org/10.1016/j.envpol.2018.04.077
Itani O, Jike M, Watanabe N, Kaneita Y (2017) Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 32:246–256. https://doi.org/10.1016/j.sleep.2016.08.006
Jain RB (2015) Trends and concentrations of selected polycyclic aromatic hydrocarbons in general us population: data from nhanes 2003–2008. Cogent Environ Sci 1:1031508. https://doi.org/10.1080/23311843.2015.1031508
Jarrin DC, Alvaro PK, Bouchard MA, Jarrin SD, Drake CL, Morin CM (2018) Insomnia and hypertension: a systematic review. Sleep Med Rev 41:3–38. https://doi.org/10.1016/j.smrv.2018.02.003
Jean-Louis G, Magai CM, Cohen CI et al (2001) Ethnic differences in self-reported sleep problems in older adults. Sleep 24:926–933. https://doi.org/10.1093/sleep/24.8.926
Kim KH, Jahan SA, Kabir E, Brown RJ (2013) A review of airborne polycyclic aromatic hydrocarbons (pahs) and their human health effects. Environ Int 60:71–80. https://doi.org/10.1016/j.envint.2013.07.019
Krittanawong C, Tunhasiriwet A, Wang Z et al (2019) Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 8:762–770. https://doi.org/10.1177/2048872617741733
Kuang D, Zhang W, Deng Q et al (2013) Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to dna and lipid in coke oven workers. Environ Sci Technol 47:7446–7456. https://doi.org/10.1021/es401639x
Lancet (2022) Waking up to the importance of sleep. Lancet 400:973. https://doi.org/10.1016/S0140-6736(22)01774-3
Lao XQ, Liu X, Deng HB et al (2018) Sleep quality, sleep duration, and the risk of coronary heart disease: a prospective cohort study with 60,586 adults. J Clin Sleep Med 14:109–117. https://doi.org/10.5664/jcsm.6894
Lee S, Ng KY, Chin WK (2017) The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev 31:91–101. https://doi.org/10.1016/j.smrv.2016.02.001
Li D, Wang L, Yang Y et al (2022) Associations of long-term exposure to ambient air pollution and road traffic noise with sleep health in uk biobank. J Affect Disord 310:1–9. https://doi.org/10.1016/j.jad.2022.04.136
Lietz M, Berges A, Lebrun S et al (2013) Cigarette-smoke-induced atherogenic lipid profiles in plasma and vascular tissue of apolipoprotein e-deficient mice are attenuated by smoking cessation. Atherosclerosis 229:86–93. https://doi.org/10.1016/j.atherosclerosis.2013.03.036
Liu Y, Zhang H, Zhang H et al (2018) Mediation effect of ahr expression between polycyclic aromatic hydrocarbons exposure and oxidative dna damage among chinese occupational workers. Environ Pollut 243:972–977. https://doi.org/10.1016/j.envpol.2018.09.014
Liu W, Zhang R, Tan A et al (2019) Long sleep duration predicts a higher risk of obesity in adults: a meta-analysis of prospective cohort studies. J Public Health (oxf) 41:e158–e168. https://doi.org/10.1093/pubmed/fdy135
Lotz A, Pesch B, Dettbarn G et al (2016) Metabolites of the pah diol epoxide pathway and other urinary biomarkers of phenanthrene and pyrene in workers with and without exposure to bitumen fumes. Int Arch Occup Environ Health 89:1251–1267. https://doi.org/10.1007/s00420-016-1160-4
Marzooghi S, Di Toro DM (2017) A critical review of polycyclic aromatic hydrocarbon phototoxicity models. Environ Toxicol Chem 36:1138–1148. https://doi.org/10.1002/etc.3722
Mcclean MD, Rinehart RD, Ngo L, Eisen EA, Kelsey KT, Herrick RF (2004) Inhalation and dermal exposure among asphalt paving workers. Ann Occup Hyg 48:663–671. https://doi.org/10.1093/annhyg/meh062
Middleton D, Watts MJ, Polya DA (2019) A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a uk population exposed to arsenic in drinking water. Environ Int 130:104721. https://doi.org/10.1016/j.envint.2019.03.069
Morin CM, Jarrin DC (2022) Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin 17:173–191. https://doi.org/10.1016/j.jsmc.2022.03.003
Muhammad T, Gharge S, Meher T (2022) The associations of bmi, chronic conditions and lifestyle factors with insomnia symptoms among older adults in india. PLoS ONE 17:e274684. https://doi.org/10.1371/journal.pone.0274684
Palagini L, Hertenstein E, Riemann D, Nissen C (2022) Sleep, insomnia and mental health. J Sleep Res 31:e13628. https://doi.org/10.1111/jsr.13628
Pan W, Wu X, He Y et al (2013) Brain interleukin-15 in neuroinflammation and behavior. Neurosci Biobehav Rev 37:184–192. https://doi.org/10.1016/j.neubiorev.2012.11.009
Pan XL, Nie L, Zhao SY, Zhang XB, Zhang S, Su ZF (2022) The association between insomnia and atherosclerosis: a brief report. Nat Sci Sleep 14:443–448. https://doi.org/10.2147/NSS.S336318
Park M, Lee JS, Park MK (2019) The effects of air pollutants on the prevalence of common ear, nose, and throat diseases in south korea a national population-based study. Clin Exp Otorhinolaryngol 12:294–300. https://doi.org/10.21053/ceo.2018.00612
Patel D, Steinberg J, Patel P (2018) Insomnia in the elderly: a review. J Clin Sleep Med 14:1017–1024. https://doi.org/10.5664/jcsm.7172
Peng K, Li Z, Gao TR et al (2023) Polycyclic aromatic hydrocarbon exposure burden: individual and mixture analyses of associations with chronic obstructive pulmonary disease risk. Environ Res 222:115334. https://doi.org/10.1016/j.envres.2023.115334
Rahman HH, Niemann D, Munson-Mcgee SH (2022) Association between environmental toxic metals, arsenic and polycyclic aromatic hydrocarbons and chronic obstructive pulmonary disease in the us adult population. Environ Sci Pollut Res Int 29:54507–54517. https://doi.org/10.1007/s11356-022-19695-w
Ranjbar M, Rotondi MA, Ardern CI, Kuk JL (2015) Urinary biomarkers of polycyclic aromatic hydrocarbons are associated with cardiometabolic health risk. PLoS ONE 10:e137536. https://doi.org/10.1371/journal.pone.0137536
Shan Z, Ma H, Xie M et al (2015) Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 38:529–537. https://doi.org/10.2337/dc14-2073
Shi J, Zhao Y, Xue L et al (2021) Urinary metabolites of polycyclic aromatic hydrocarbons after short-term fine particulate matter exposure: a randomized crossover trial of air filtration. Environ Pollut 285:117258. https://doi.org/10.1016/j.envpol.2021.117258
Sridhar GR, Putcha V, Lakshmi G (2011) Sleep in thyrotoxicosis. Indian J Endocrinol Metab 15:23–26. https://doi.org/10.4103/2230-8210.77578
Stallings-Smith S, Mease A, Johnson TM, Arikawa AY (2018) Exploring the association between polycyclic aromatic hydrocarbons and diabetes among adults in the united states. Environ Res 166:588–594. https://doi.org/10.1016/j.envres.2018.06.041
Sun L, Ye Z, Ling Y et al (2020) Relationship between polycyclic aromatic hydrocarbons and rheumatoid arthritis in us general population, nhanes 2003–2012. Sci Total Environ 704:135294. https://doi.org/10.1016/j.scitotenv.2019.135294
Veyrand B, Sirot V, Durand S et al (2013) Human dietary exposure to polycyclic aromatic hydrocarbons: results of the second French total diet study. Environ Int 54:11–17. https://doi.org/10.1016/j.envint.2012.12.011
Vozoris NT (2019) Benzodiazepine and opioid co-usage in the us population, 1999–2014: an exploratory analysis. Sleep 42. https://doi.org/10.1093/sleep/zsy264
Wang Q, Liu M, Yu Y, Li Y (2016a) Characterization and source apportionment of pm(2.5)-bound polycyclic aromatic hydrocarbons from Shanghai City. China Environ Pollut 218:118–128. https://doi.org/10.1016/j.envpol.2016.08.037
Wang S, Bai Y, Deng Q et al (2016b) Polycyclic aromatic hydrocarbons exposure and lung function decline among coke-oven workers: a four-year follow-up study. Environ Res 150:14–22. https://doi.org/10.1016/j.envres.2016.05.025
Wang F, Wang Y, Wang Y et al (2022a) Urinary polycyclic aromatic hydrocarbon metabolites were associated with hypertension in us adults: data from nhanes 2009–2016. Environ Sci Pollut Res Int 29:80491–80501. https://doi.org/10.1007/s11356-022-21391-8
Wang J, Wang W, Zhang W et al (2022b) Co-exposure to multiple air pollutants and sleep disordered breathing in patients with or without obstructive sleep apnea: a cross-sectional study. Environ Res 212:113155. https://doi.org/10.1016/j.envres.2022.113155
Wang S, Rossheim ME, Nandy RR (2023) Trends in prevalence of short sleep duration and trouble sleeping among us adults, 2005–2018. Sleep 46. https://doi.org/10.1093/sleep/zsac231
Woo DH, Park M, Jang SY, Park S, Jang SI (2022) Association between smoking status and subjective quality of sleep in the south korean population: a cross-sectional study. Sleep Breath. https://doi.org/10.1007/s11325-022-02726-8
Xia L, Chen GH, Li ZH, Jiang S, Shen J (2013) Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research. PLoS ONE 8:e71065. https://doi.org/10.1371/journal.pone.0071065
Xing W, Gu W, Liang M et al (2023) Sex-specific effect of urinary metabolites of polycyclic aromatic hydrocarbons on thyroid profiles: results from nhanes 2011–2012. Environ Sci Pollut Res Int 30:47168–47181. https://doi.org/10.1007/s11356-023-25693-3
Xu C, Liu Q, Liang J et al (2021a) Urinary biomarkers of polycyclic aromatic hydrocarbons and their associations with liver function in adolescents. Environ Pollut 278:116842. https://doi.org/10.1016/j.envpol.2021.116842
Xu J, Zhou J, Luo P et al (2021b) Associations of long-term exposure to ambient air pollution and physical activity with insomnia in chinese adults. Sci Total Environ 792:148197. https://doi.org/10.1016/j.scitotenv.2021.148197
Yang L, Guo W, Zeng D et al (2019) Heart rate variability mediates the association between polycyclic aromatic hydrocarbons exposure and atherosclerotic cardiovascular disease risk in coke oven workers. Chemosphere 228:166–173. https://doi.org/10.1016/j.chemosphere.2019.04.101
Yang X, Xue Q, Wen Y et al (2022) Environmental polycyclic aromatic hydrocarbon exposure in relation to metabolic syndrome in us adults. Sci Total Environ 840:156673. https://doi.org/10.1016/j.scitotenv.2022.156673
Zhang Y, Ding J, Shen G et al (2014) Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites–a controlled case study in Beijing, China. Environ Pollut 184:515–522. https://doi.org/10.1016/j.envpol.2013.10.005
Zhong Q, Qin Z, Wang X et al (2023) Healthy sleep pattern reduce the risk of cardiovascular disease: a 10-year prospective cohort study. Sleep Med 105:53–60. https://doi.org/10.1016/j.sleep.2023.03.003
Zhu H, Martinez-Moral MP, Kannan K (2021) Variability in urinary biomarkers of human exposure to polycyclic aromatic hydrocarbons and its association with oxidative stress. Environ Int 156:106720. https://doi.org/10.1016/j.envint.2021.106720
Acknowledgements
We appreciate the excellent contributions made by the participants and staff of the National Health and Nutrition Survey. We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and writing: Qian Zhang and Kun Peng; data extraction and statistical analyses: Li-Hong Xin; reviewing and editing: Jie Zhao and Yu-Jie Li. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NHANES publishes open-access data; an institutional review board analysis was not necessary for our study.
Consent for publication
Consent was given by all authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Peng, K., Xin, LH. et al. Exposure to polycyclic aromatic hydrocarbons increases the risk of poor sleep pattern in US adults: results from the NHANES (2005–2010). Environ Sci Pollut Res 30, 114406–114419 (2023). https://doi.org/10.1007/s11356-023-30419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30419-6