Abstract
Phthalic acid ester (PAE) contamination in popular drink bubble tea has been hardly studied in the world. In this work, a liquid–liquid extraction following solid phase extraction (LLE-SPE)-UPLC-MS/MS method was first established for trace determination of ten PAEs in bubble tea. The developed method was validated with respect to linearity (R2 > 0.992), low limit of detections (LODs, 0.49–3.16 µg/L), and satisfactory recoveries (61.8–127.6%) with a low relative standard derivations (RSDs, 1.1–16.4%), which was also validated for commercial milk. Six out of ten PAEs, i.e., diethylhexyl phthalate (DEHP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), diethyl phthalate (DEP), dihexyl phthalate (DHP), and diphenyl phthalate (DPP) were detected in Chinese bubble tea with concentrations ranging from not detection (ND) to 53.43 µg/L, while DEHP, DBP, DIBP, DEP, and dimethyl phthalate (DMP) were detected in commercial milk with concentrations ranging from ND to 110.58 µg/L. The respective average concentrations of DEHP in Chinese bubble tea and commercial milk were 19.40 and 23.46 µg/L, which were over two times that in drinking water quality standards of several countries including Israel, Korea, Oman, and Singapore (i.e., 8 µg/L). Calculated with human estimated daily intake (EDI), the average EDIs of five out of seven PAEs in bubble tea were higher than those in commercial milk. For example, the calculated EDI of DIBP in bubble tea was 5 times that in commercial milk, while their respective corresponding EDIs of DBP and DEHP were over 2.4 and 1.6 times. Based on estrogen equivalence (EEQ) with the unit of ng E2/L, the average EEQs of the ten PAEs in Chinese bubble tea and commercial milk were 14.26 and 17.06 ng E2/L, which were 52.8 and 62.3 times the observed effect concentration that could cause egg mortality of zebrafish. It is evident that the potential estrogenic effect of PAEs in bubble tea and commercial milk cannot be negligible. Given the fact that PAE contamination in bubble tea has been hardly investigated, such study is urgently to be performed in a global view.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalic acid esters (PAEs) as industrial additives have been increasingly employed (Lee et al. 2019). The world annual usage of PAEs has been supposed to increase by 1.3% annually from 2017 to 2022 (Luo et al. 2018), while the total global production of phthalates in 2018 was reported to be approximately 5.5 million tons (Sahoo and Kumar 2023; Zhang et al. 2022). As not chemically bonded to polymeric materials, PAEs can be migrated from the surface of different industrial products during production, usage, and discharge, posing potential risk to human health (Arfaeinia et al. 2019; De-la-Torre et al. 2022; Sungur et al. 2014). Extensive usage and leachable nature contributed to its ubiquity in air and dust (Ma et al. 2014; Yang et al. 2020), aqueous (Hajiouni et al. 2022), soil and sediment (Arfaeinia et al. 2019; Takdastan et al. 2021), biota (He et al. 2020), and food and drink (Kargarghomsheh et al. 2023; Mehraie et al. 2022; Li et al. 2022; Rezaei et al. 2021).
PAEs were widely determined in human serum and adipose tissue, while mono-PAEs were also widely determined in human urine, which illustrated that the vast majority of people around the world are being exposed to PAEs (Liou et al. 2014). Many adverse effects on humans due to exposure of PAEs have been found including endocrine disruption, damage to reproductive, cardiovascular, developmental and immune systems, testicular toxicity, and carcinogenicity (Caldwell 2012; Martino-Andrade and Chahoud 2010; Wang et al. 2014). Due to their potential toxicity, the United States Environmental Protection Agency (US EPA) and the European Union (EU) had listed dibutyl phthalate (DBP), di-(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), dimethyl phthalate (DMP), di-n-octyl phthalate (DOP), butyl benzyl phthalate (BBP) as priority pollutants. To protect human health, China, the USA, and EU have restricted the use of some PAEs in food packaging material including DEHP, DMP, DEP, DBP, and DOP (Huang and Wang 2016; Jeddi et al. 2015; Wei et al. 2022). Meanwhile, DEHP, a widely used PAE, has been listed in the drinking-water quality standard of several countries including China, Canada, Australia, Israel, Japan, the USA, while DBP and DEP have also been included in the Chinese drinking-water quality standard (Liu et al. 2021).
Food safety and quality have received increasing attention in recent years among Chinese population due to the booming economic growth. Bubble tea generally refers to a beverage containing a tea base, milk, and chewy tapioca starch balls, which has been a popular drinking for young Chinese population. The Chinese bubble tea market had increased at an average rate of over 20% in 2014–2018, with an annual sale of 50 billion of Chinese yuan, i.e., proximately 7 billion USD (Prospective Industry Research Institute 2022). There have been many studies in contaminations of PAEs in bottled water and milk (Dobaradaran et al. 2020; Herrero et al. 2021; Korkmaz and Kuplulu 2019; Liu et al. 2016; Selvaraj et al. 2016; Tang et al. 2019; Zelenkin et al. 2018). However, as a popular drink, the contamination situation of PAEs in bubble tea has not been studied yet, which could be an important exposure source to human. Therefore, the main objectives of this work were (1) to develop a sensitive analytical method for trace determination of PAEs in bubble tea, (2) to investigate contamination situation of PAEs in Chinese commercial bubble tea in which a comparison with milk was performed, and (3) to investigate human daily intake based on both concentration and potential estrogenic effects.
Materials and methods
Standards and reagents
Ten PAEs were selected as the targeted compounds, which included DEHP, DBP, DEP, DMP, BBP, DOP, diisobutyl phthalate (DIBP), dihexyl phthalate (DHP), diphenyl phthalate (DPP), and dibenzyl phthalate (DBzP). Their basic physiochemical properties as well as estrogenic potencies are shown in Table 1. DEHP, DBP, DEP, DMP, BBP, and DOP were purchased from Dr. Ehrenstorfer Gmbh (Augsburg, Germany). DIBP, DHP, DPP, and DBzP were bought from Sigma-Aldrich (USA). Deuterated diethyl hexyl phthalate (DEHP-D4) was used as the internal standard (IS), which was purchased from ANPEL Laboratory Technologies (Shanghai, China) Inc. The purity of each PAE was over 97%. Acetonitrile (ACN), n-hexane, acetic acid, acetone, and methanol (MeOH) were obtained from Fisher Scientific (USA). All solvents were HPLC grade. Sodium chloride was obtained from Sinopharm Chemical Reagent (Shanghai, China), which was purified in a muffle furnace at 450 °C for 6 h. Stock standard solutions of all eleven standards were prepared with MEOH at a concentration of 20 mg/L and stored in a refrigerator at − 20 °C. The mixed working standard solution (ten PAEs) or the IS (DEHP-D4) were prepared with ACN at a concentration of 2 mg/L by diluting the stock standard solution. The brown screw-capped glass tubes were used to hold all the stock standard and working standard solutions. The ultrapure water (18.2 MΩ·cm) was used for cleaning and preparing for mobile phase A.
Sample collection and pretreatment
In total, seventeen bubble tea samples belonging to 17 different brands were purchased via a Chinese popular app called Meituan. To give a comparison, twenty-one commercial milk samples were purchased from a campus supermarket and campus milk reservation service provider in South China University of Technology, Guangzhou, China, which belonged to 14 national famous brands. The sample information of bubble teas and commercial milks were listed in Table 2. The gathered commercial milks were all pasteurized milk whose shelf lives were normally within 1 month under the storing temperature recommended by their manufacturers. Once the bubble tea and commercial milk samples were delivered to the lab, they were immediately extracted with liquid–liquid extraction and then solid phase extraction (LLE-SPE).
The extraction method was based on Bai et al. (2014) that used for extraction of PAEs in milk with some modifications. In brief, 1-mL bubble tea or commercial milk was added to one 5-mL centrifuge glass tube and 100 ng IS was added to the tube. After gentle mixing with hand for three times, 0.1-g sodium chloride and 3-mL acetonitrile with 5% acetic acid solution were subsequently added to the tube, which was vortexed for 1 min, following with ultra-sonication for 20 min. The tube was centrifuged for 3 min at a speed of 4000 r/min, and the upper layer was transferred into another glass tube. The remainder was re-extracted with the same extraction steps as mentioned above. The two upper layers were combined together for further cleanup with SPE using a Si/PSA GLASS Cartridge (500 mg/500 mg, 6 mL), which was purchased from ANPEL Laboratory Technologies (Shanghai) Inc. In the SPE extraction, the aqueous sample was loaded into the cartridge under vacuum, which was preconditioned with 5-mL dichloromethane, 5-mL acetonitrile, and 5-mL ultrapure water. After the loading, acetonitrile (5 mL) and acetone (1 mL) were used as the elution solvents to elute the ten target compounds from the cartridge. The eluate was evaporated to dryness under a gentle stream of high purity nitrogen (> 99.99%) at 35 °C. Finally, the eluent was re-dissolved with 1-mL acetonitrile. The sample was added to one brown glass vial for UPLC-MS/MS analysis. Each sample was performed in triplicate.
Sample analysis by UPLC-MS/MS
The ten PAEs were analyzed with a Shimadzu LC-20AD XR series (Kyoto, Japan) coupled to an AB Sciex API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA, USA), which was equipped with an ESI ionization source. The chromatographic separations were achieved with an UHPLC BEH C18 column (2.1 × 100 mm, 1.7 µm). Mobile phase (A) was ultrapure water with 0.1% formic acid and 5 mM ammonium acetate. Mobile phase (B) was acetonitrile. The mobile phase flow rate was set to 0.4 mL/min. The gradient elution program was started at 55% B and continued for 1 min, which then linearly rose to 85% B within 1.5 min. Subsequently, it linearly increased to 97% B within 3 min, which was kept for 2 min. Finally, it linearly returned to 55% B within 0.1 min and maintained for 2.4 min. The entire separation time was 10 min for each injection. The column temperature was maintained at 40 °C, and the sample injection volume was 10 µL. The tandem mass spectrometer (MS/MS) was performed in positive ESI mode with the following operational parameters: MS ion source temperature, 600 °C; ion spray voltage, − 4500 V; curtain gas pressure, 40 psi; collision gas (N2) pressure, 9 psi; gas 1, 85 psi; gas 2, 80 psi. Multiple reaction monitoring (MRM) mode was adopted for quantitative analysis. The optimal MRM parameters for all chemicals are presented in Table 3.
Risk assessment
Daily intake-associated risk assessment
Human daily intake of phthalates via the ingestion of bubble tea or milk can be estimated based on the method of Luo et al. (2018) as shown in Eq. (1):
where \({EDI}_{i}\) is the estimated human daily intake (EDI) for an individual PAE (ng/kg-bw/day); \({C}_{i}\) is measured concentration of the corresponding PAE in bubble tea or commercial milk (µg/L); \(V\) is the human daily ingestion volume (L/day/person) of bubble tea or milk; and bw (kg) was the reference body weight for an adult. The latest dietary guidelines for Chinese residents recommend a daily intake of 300–500 g of milk or dairy products (Chinese Dietary Guidelines 2022). The size of bubble tea cup is generally between 350 and 1000 mL (Henan Shuangjiang Paper Plastic Packaging Co. 2022). According to the European Food Safety Agency, micro-pollutants in food should be overestimated to ensure consumer health (Luo et al. 2020), thus, 1000 and 500 mL were used as the \(V\) values for bubble tea and milk. Seventy kilogram was used as the reference \(bw\) value (Huang et al. 2017).
Estrogenic activity-associated risk assessment
Potential estrogenic effects of PAEs in bubble tea or milk were evaluated based on chemically calculated estrogen equivalence (EEQ) (Liu et al. 2009; 2010), i.e.,
where \({EP}_{i}\) and \({C}_{i}\) mean the estrogenic potency of an individual PAE and its corresponding average concentration in bubble tea or commercial milk (µg/L), respectively. 17β-estradiol (E2), the strongest natural estrogenic compound, is often selected as the standard estrogenic compound, for which its EP is arbitrarily set to 1. Hence, the unit of EEQ is defined as ng E2/L. As can be seen in Table 1, some PAEs showed different EP values from different studies. To avoid possible underestimation, the maximum reported EP of each PAE was selected for the EEQ calculation.
Quality control and quality assurance
To avoid possible contamination during sample preparation and analysis, a stringent cleaning procedure was performed as follows: all glassware used in the experiment were carefully cleaned with detergent and thoroughly rinsed with ultrapure water except for the 2-mL brown glass sample vial, and they were immersed in acetone and sonicated for 30 min. Finally, they were thoroughly rinsed with ultrapure water, dried in oven at 100 °C for at least 3 h before use. All solvents employed were checked by UPLC-MS/MS before use to ensure that only those solvents with the least trace residues of PAEs were used. Three blank controls with ultrapure water were performed with the same pretreatment procedure for bubble tea or milk sample as described above. Injections of blank control samples were performed for every 12 real samples, and the average concentrations of blank control samples were obtained for background subtraction. Each sample was performed in triplicate.
Data analysis
The concentrations marked as not detected (ND) were set to zero for statistical analysis. Nonparametric statistical tests were applied to assess the statistical significance because the data were not normally distributed (Shapiro-Wilk test; p < 0.05). The concentration levels of PAEs in commercial milk and bubble tea were compared using the Mann − Whitney test. Statistical analyses and all figures were created with Origin 2018. The statistical significance was set at p < 0.05.
Results and discussion
Performance of the developed methods
The developed LLE-SPE-UPLC-MS/MS method was validated with respect to linearity, sensitivity, recovery, and precision. Standard calibration curves were established based on seven gradient standard concentrations ranging from 5 to 500 µg/L. Satisfactory linearity (R2 > 0.99) for each target compound based on the internal standard method was obtained (Table 4). LODs and limit of quantifications (LOQs) were calculated based on three times and ten times of the standard deviation (SD), in which the SD values were obtained from the seven repeated injections with the lowest concentration of 5 ng/mL used for the standard calibration curve in blank bubble tea or milk sample. The two kinds of blank samples were obtained by passing the bubble tea or milk sample to a SPE cartridge, through which the background PAEs could be removed. As shown in Table 4, the LODs and LOQs of all ten target PAEs in bubble tea and milk samples were 0.64–3.71 and 2.13–12.39 µg/L, respectively. Due to the presence of background contamination, DBP and DEHP showed higher LODs and LOQs. Compared to milk sample, the ten target PAEs in bubble tea sample showed relative higher LODs and LOQs, and the difference might derive from more complex matrix in bubble tea. Recovery experiments were carried out by spiking each PAE with three known concentrations of 50, 100, and 200 µg/L into real bubble tea or milk sample. The same real bubble tea or milk sample without spiking was also analyzed, and the analyzed values were subtracted for the recovery calculation. The recoveries of the 10 target compounds ranged from 61.8 to 118.6% with RSDs ranging from 0.6 to 16.4% (Table 5). The developed analytical method for the ten target compounds in the bubble tea or milk sample showed satisfactory recovery and precision, in which recovery efficiency with 50.0–120.0% and RSD below 20.0% was regarded as acceptable (Tang et al. 2020; Yuan et al. 2019; Wan et al. 2022). All analytical parameters in terms of linearity, sensitivity, recovery, and precision suggested that the developed method is reliable and appropriate for trace determination of the ten PAEs in bubble tea or milk sample.
PAEs in bubble tea and commercial milk
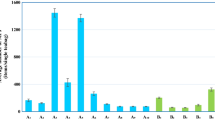
Concentration levels of the ten PAEs in bubble tea and commercial milk samples were shown in Fig. 1, while their original data was provided in Table S1. It could be seen that six out of ten PAEs including DEHP, DIBP, DBP, DEP, DHP, and DPP were detected in the bubble tea samples. Among them, DEHP was the most frequently detected PAE with a detection frequency of 70.6%, followed by DIBP with 47.1%, DBP with 29.4%, DEP with 17.7%, DHP with 11.8%, and with DPP the least with 5.9%. The concentrations of DEHP ranged from 8.64 to 53.43 µg/L in the detected 12 samples with an average concentration of 19.40 µg/L, and the average concentration was over two times that in drinking-water quality standards (i.e., 8 µg/L) in several countries, including Israel, Korea, Oman, Singapore (Liu et al. 2021), which suggested relatively heavy contamination of DEHP in bubble tea. The respective maximum and average concentrations of DBP were 83.6 and 11.6 µg/L, while the respective corresponding concentrations of DIBP were 22.4 and 4.7 µg/L. DEP, DHP, and DPP were only detected in no more than 3 samples and their concentrations ranged from ND-3.76, ND-3.48, to ND-3.31 µg/L, respectively.
For commercial milk samples, five out of ten PAEs including DEHP, DBP, DIBP, DEP, and DMP were detected in commercial milk samples. Similar to bubble tea, DEHP and DBP were the two most frequently detected PAEs, in which their respective concentrations ranged from ND-110.58 to ND-37.19 µg/L with respective average concentrations of 23.46 and 9.5 µg/L. The other three detected PAEs were DIBP, DEP, and DMP, and their respective maximum concentrations were 7.58, 7.13, and 5.14 µg/L. The average concentrations of DEHP, DBP, DIBP, DEP, and DMP in commercial milk samples were 23.46, 9.50, 1.87, 1.49, and 0.89 µg/L, respectively. To give a comparison, related investigations on PAEs in milk samples all over the world in the last decade were summarized. As shown in Table 6, PAEs varied greatly in different milk samples, but DEHP was no doubt the most frequently detected PAE, and on most occasions, it shared the highest maximum concentration.
Differences were seen in bubble tea and commercial milk. For example, DEHP, DBP, DIBP, and DEP were the top four PAEs ranked from high to low based on average concentration in both bubble tea and commercial milk samples. However, the average concentrations of DEHP and DEP in commercial milk samples were higher than those in bubble tea. On the other hand, the opposite results were observed for DBP and DIBP. Nevertheless, no statistical differences were observed for the four PAEs (p > 0.05). Neither were the concentrations of the total ten PAEs (Fig. 2).
The main sources of PAEs in bubble tea or commercial milk include migration from package materials, contamination during production, or product itself. In the commercial milk sample labeled as CM-02, the respective concentrations of DEP, DBP, DEHP, and DBP were 5.86, 6.77, 12.17, and 16.28 µg/L in carton package, while their corresponding concentrations were 3.67, 4.59, 8.89, and 10.54 µg/L in glass package (Table S1). Similar results were observed in milk sample labeled as CM-03 and CM-06. The above results suggested that PAE migration from package material might be one important source, which agreed well with the work of Herrero et al. (2021). However, it is interesting to find that PAEs in bubble tea and milk samples showed excellent linearity (Fig. 3). As all these samples shared different package materials, it suggested that the most important source of PAEs was likely the product itself or contamination during production.
Health risk assessment
Human daily intake
To evaluate health risk of PAEs in bubble tea and milk samples, the average and maximum concentrations as shown in Fig. 1 and Table S1 were adopted to calculate human’s EDI and EEQ. As shown in Table 7, the average EDIs of five out of seven PAEs in bubble tea were higher than those in commercial milk. For example, the calculated EDI of DIBP in bubble tea was 5 times that in commercial milk, while their respective corresponding EDIs of DBP and DEHP were over 2.4 and 1.6 times. The calculated EDI of DEP in bubble tea was similar to that in commercial milk. DPP and DHP were detected in bubble tea but not in commercial milk. The reason can be explained by the serious problem of food additives and preservatives, which have been reported to be harmful to human health (Herrero et al. 2021; Wu et al. 2022). DMP was detected in commercial milk sample but not in bubble tea, the reason was unknown. It should be noted that many young adults likely consume two or more cups of bubble tea per day, thus the actual exposure via bubble tea is likely higher than the calculated EDIs (Huang et al. 2022; Wu et al. 2022). The European Food Safety Authority (ESFA) has established tolerable daily intakes (TDIs) for some PAEs, e.g., 50, 500, 10, and 50 µg/kg bw/d for DMP, DEP, DBP, and DEHP, respectively (Dobaradaran et al. 2020). It was evident that the corresponding EDIs of the seven detected PAEs in bubble tea or commercial milk were far below those of their recommended TDIs. However, when compared to bottled water across 20 countries, as summarized in Luo et al. (2018), the respective average EDIs of DEHP in bubble tea and commercial milk were 2.8 and 1.7 times that in bottled water. The above fact suggests that PAEs in bubble tea and commercial milk are two important exposure sources to human. Compared to lots of investigations on PAEs in bottled water worldwide, PAEs in bubble tea has been hardly investigated, which should be paid with more attention.
Potential estrogenic effects of PAEs
Endocrine disrupting compounds (EDCs) can pose adverse effects on fishes and other animals even at environmentally relevant concentrations, which include intersex, infertility, mortality, and disruption to mating behavior (Liu et al. 2017; Tang et al. 2022). To protect human’s health, some EDCs have been listed as the restricted items in the drinking water quality standards of some countries. For example, bisphenol A, DEHP, DEP, and DBP have been listed in the Chinese drinking-water quality standard, while E2 and some other EDCs have been listed in the Japanese drinking-water quality standard (Yuan et al. 2017; 2018). To assess the potential estrogenic effects of PAEs in bubble tea and commercial milk, the average and maximum chemically calculated EEQ levels in bubble tea and milk sample were calculated and summarized in Table 7. The average EEQ levels in bubble tea and commercial milk were 14.26 and 17.06 ng E2/L, while their corresponding respective maximum EEQ levels were 41.52 and 80.15 ng E2/L. Based on the work of Soares et al. (2009), the minimum observed EEQ level of 0.27 ng E2/L could cause the egg mortality in the late gastrulation and/or early organogenesis stage of zebrafish, which suggested that the average EEQ levels in bubble tea and commercial milk were 52.8 and 63.2 times that of the observed effect concentration that could do harm to zebrafish. Moreover, a seven-year long lake experiment illustrated that chronic exposure of fathead minnow to 17α-ethynyl estradiol (EE2) at EEQ level of 7.5–9 ng/L could lead to feminization of male fathead minnow (Kidd et al. 2007; Wang et al. 2020). However, the average chemically calculated EEQ levels in bubble tea and commercial milk were about 2 times that could cause feminization of male fathead minnow. The above facts suggest that the potential estrogenic effects of PAEs in bubble tea and commercial milk should be paid with attention. It should be pointed out that DEHP contributed to over 91% of the total EEQ among the monitored ten PAEs in bubble tea and commercial milk. To decrease the potential estrogenic effect, reduction contamination of DEHP in bubble tea and commercial milk is the most effective way.
Conclusion
This work first established a LLE-SPE-LC–MS/MS method for trace determination of ten PAEs in bubble tea and then investigated their contamination level in seventeen brands of bubble tea in China, along with the contamination situation of the ten PAEs in commercial milk samples. Results of this work suggested that six PAEs including DEP, DPP, DIBP, DBP, DHP, and DEHP were detected in bubble tea, while five PAEs including DEHP, DBP, DIBP, DEP, and DMP were detected in commercial milk. Among them, DEHP was remarkably contaminated in both bubble tea and commercial milk, and its respective average concentrations were over 2.4 and 2.9 times of the regulated concentration in drinking-water quality standard of many countries including Israel, Korea, Oman, and Singapore. The respective average chemically calculated EEQ values of PAEs in bubble tea and commercial milk were 14.26 and 17.06 ng E2/L, which were 52.8 and 62.3 times that could cause egg mortality of zebrafish. The above fact suggests that the potential estrogenic effects of PAEs in bubble tea and commercial milk cannot be ignored. The limitation of this work is that this work only covered 17 bubble tea brands and 14 commercial milk brands, and more brands are necessary to be included so as the overall contamination situations of PAEs in Chinese bubble tea and commercial milk can be more accurately estimated.
Data availability
Original data is provided in supplementary materials.
References
Arfaeinia H, Fazlzadeh M, Taghizadeh F, Saeedi R, Spitz J, Dobaradaran S (2019) Phthalate acid esters (PAEs) accumulation in coastal sediments from regions with different land use configuration along the Persian Gulf. Ecotoxicol Environ Saf 169:496–506
Bai S, Wang X, Hu F, Wang T, Cheng P, Zhou Z (2014) Determination of phthalate acid esters in soybean milk using dispersive liquid-liquid microextraction coupled with gas chromatography and mass spectrometric detection. Anal Methods 6:7361–7366
Caldwell JC (2012) DEHP: genotoxicity and potential carcinogenic mechanisms-a review. Mutat Res - Rev Mutat Res 751:82–157
Céspedes R, Petrovic M, Raldúa D et al (2004) Integrated procedure for determination of endocrine-disrupting activity in surface waters and sediments by use of the biological technique recombinant yeast assay and chemical analysis by LC-ESI-MS. Anal Bioanal Chem 378:697–708
Chinese Dietary Guidelines, http://dg.cnsoc.org/article/04/jqRp8FyyRYi2YDh3bSnLKw.html. Access on November 11, 2022
De-la-Torre GE, Dioses-Salinas DC, Dobaradaran S et al (2022) Release of phthalate esters (PAEs) and microplastics (MPs) from face masks and gloves during the COVID-19 pandemic. Environ Res 215
Dobaradaran S, Akhbarizadeh R, Mohammadi MJ, Izadi A, Keshtkar M, Tangestani M et al (2020) Determination of phthalates in bottled milk by a modified nano adsorbent: presence, effects of fat and storage time, and implications for human health. Microchem J 159:105516
Farajzadeh MA, Djozan D, Reza M, Mogaddam A, Norouzi J (2012) Determination of phthalate esters in cow milk samples using dispersive liquid-liquid microextraction coupled with gas chromatography followed by flame ionization and mass spectrometric detection. J Sep Sci 35:742–749
Hajiouni S, Mohammadi A, Ramavandi B, Arfaeinia H, De-la-Torre GE, Tekle-Röttering A et al (2022) Occurrence of microplastics and phthalate esters in urban runoff: a focus on the Persian Gulf coastline. Sci Total Environ 806:150559
He MJ, Lu JF, Wang J, Wei SQ, Hageman KJ (2020) Phthalate esters in biota, air and water in an agricultural area of western China, with emphasis on bioaccumulation and human exposure. Science of the Total Environment 698
Henan Shuangjiang Paper Plastic Packaging Co. http://www.shuangjiangzs.com. Access on November 11, 2022
Herrero L, Quintanilla-López JE, Fernández MA, Gómara B (2021) Plasticisers and preservatives in commercial milk products: a comprehensive study on packages used in the Spanish market. Food Chem 338:128031
Huang ZY, Wang ZW (2016) Determination of six small-molecule compounds in polyethylene terephthalate (PET) used for food packaging by GC-MS. Modern Food Sci Technol 32:297–303
Huang RP, Liu ZH, Yuan SF, Yin H, Dang Z, Wu PX (2017) Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ Pollut 230:143–152
Huang Z, Zhang X, Wang X, Deji Z, Lee HK (2022) Occurrence of perfluoroalkyl and polyfluoroalkyl substances in ice cream, instant noodles, and bubble tea. J Agric Food Chem 70:10836–10846
Jeddi MZ, Rastkari N, Ahmadkhaniha R, Yunesian M (2015) Concentrations of phthalates in bottled water under common storage conditions: do they pose a health risk to children? Food Res Int 69:256–265
Kargarghomsheh P, Naghasha M, Farhadiyan S, Arabameri M, Tooryan F, Shariatifar N (2023) Determination of phthalic acid esters (PAEs) along with probabilistic health risk assessment in fruit juice samples in Tehran. Iran Environ Sci Pollut Res 30:44833–33844
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci 104:8897–8901
Kim YJ, Ryu JC (2006) Evaluation of estrogenic effects of phthalate analogues using in vitro and in vivo screening assays. Mol Cell Toxicol 2:106–113
Korkmaz SD, Kuplulu O (2019) Determination of phthalates in some milk products by liquid chromatography/tandem mass spectrometry. Ank Univ Vet Fak Derg 66:231–236
Lee YM, Lee JE, Choe W, Kim T, Lee JY, Kho Y et al (2019) Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ Int 126:635–643
Li ZY, Liu H, Liu H, Huang W, Chu Y, Huang ZQ et al (2022) Dietary exposure and risk assessment of phthalic acid esters through a total diet study in Shenzhen, South China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 39:1591–1603
Lin J, Chen W, Zhu H, Wang C (2015) Determination of free and total phthalates in commercial whole milk products in different packaging materials by gas chromatography-mass spectrometry. J Dairy Sci 98:8278–8284
Liou S-H, Yang GCC, Wang C-L, Chiu Y-H (2014) Monitoring of PAEMs and beta-agonists in urine for a small group of experimental subjects and PAEs and beta-agonists in drinking water consumed by the same subjects. J Hazard Mater 277:169–179
Liu ZH, Ito M, Kanjo Y, Yamamoto A (2009) Profile and removal of endocrine disrupting chemicals by using an ER/AR competitive ligand binding assay and chemical analyses. J Environ Sci 21:900–906
Liu ZH, Kanjo Y, Mizutani S (2010) A review of phytoestrogens: their occurrence and fate in the environment. Water Res 44:567–577
Liu D, Min S, Ping H, Song X (2016) The application of directly suspended droplet microextraction for the evaluation of phthalic acid esters in cow’s milk by gas chromatography mass spectrometry. J Chromatogr A 1443:66–74
Liu ZH, Dang Z, Yin H (2017) Do estrogenic compounds in drinking water migrating from plastic pipe distribution system pose adverse effects to human? An analysis of scientific literature. Environ Sci Pollut Res 24:2126–2134
Liu ZH, Dang Z, Liu Y (2021) Legislation against endocrine-disrupting compounds in drinking water: essential but not enough to ensure water safety. Environ Sci Pollut Res 28:19505–19515
Luo Q, Liu Z-h, Yin H, Dang Z, Wu P-X, Zhu N-W et al (2018) Migration and potential risk of trace phthalates in bottled water: a global situation. Water Res 147:362–372
Luo Q, Liu Z-h, Yin H, Dang Z, Wu P-x, Zhu N-w et al (2020) Global review of phthalates in edible oil: an emerging and nonnegligible exposure source to human. Sci Total Environ 704:135369
Ma J, Chen LL, Guo Y, Wu Q, Yang M, Wu MH et al (2014) Phthalate diesters in airborne PM2.5 and PM10 in a suburban area of Shanghai: seasonal distribution and risk assessment. Sci Total Environ 497–498: 467–474
Martino-Andrade AJ, Chahoud I (2010) Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54:148–157
Mehraie A, Shariatifar N, Arabameri M, Moazzen M, Mortazavian AM, Sheikh F et al (2022) Determination of phthalate acid esters (PAEs) in bottled water distributed in tehran: a health risk assessment study. Int J Environ Anal Chem
Prospective Industry Research Institute. Research report of Chinese new style tea drink market from 2019–2024. https://bg.qianzhan.com/. Accessed on 10, November, 2022
Rezaei H, Moazzen M, Shariatifar N, Khaniki GJ, Dehghani MH, Arabameri M, Alikord M (2021) Measurement of phthalate acid esters in non-alcoholic malt beverages by MSPE-GC/MS method in Tehran city: chemometrics. Environ Sci Pollut Res 28:51897–51907
Sahoo TP, Kumar MA (2023) Remediation of phthalate acid esters from contaminated environment—insights on the bioremedial approaches and future perspectives. Heliyon 9:e14945
Sajid M, Basheer C, Alsharaa A, Narasimhan K, Buhmeida A, Al Qahtani M et al (2016) Development of natural sorbent based micro-solid-phase extraction for determination of phthalate esters in milk samples. Anal Chim Acta 924:35–44
Sakhi AK, Lillegaard ITL, Voorspoels S, Carlsen MH, Loken EB, Brantsaeter AL et al (2014) Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 73:259–269
Selvaraj KK, Mubarakali H, Rathinam M et al (2016) Cumulative exposure and dietary risk assessment of phthalates in bottled water and bovine milk samples: a preliminary case study in Tamil Nadu, India. Hum Ecol Risk Assess Int J 22:1166–1182
Soares J, Coimbra AM, Reis-Henriques MA, Monteiro NM, Vieira MN, Oliveira JMA et al (2009) Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinylestradiol. Aquat Toxicol 95:330–338
Sungur S, Koroǧlu M, Özkan A (2014) Determination of bisphenol a migrating from canned food and beverages in markets. Food Chem 142:87–91
Takdastan A, Niari MH, Babaei A, Dobaradaran S, Jorfi S, Ahmadi M (2021) Occurrence and distribution of microplastic particles and the concentration of Di 2-ethyl hexyl phthalate (DEHP) in microplastics and wastewater in the wastewater treatment plant. J Environ Manage 280:111851
Tang Z, Han Q, Xie L, Chu L, Wang Y, Sun Y et al (2019) Simultaneous determination of five phthalate esters and bisphenol A in milk by packed-nanofiber solid-phase extraction coupled with gas chromatography and mass spectrometry. J Sep Sci 42:851–861
Tang Z, Liu Z-H, Wang H, Dang Z, Yin H, Zhou Y, Liu Y (2020) Trace determination of eleven natural estrogens and insights from their occurrence in a municipal wastewater treatment plant and river water. Water Res 182:115976
Tang Z, Wan Y-P, Liu Z-H, Wang H, Dang Z, Liu Y (2022) Twelve natural estrogens in urines of swine and cattle: concentration profiles and importance of eight less-studied. Sci Total Environ 803:150042
Tuncel SG, Senlik D (2016) Determination of phthalates in milk by ultrasound-assisted dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry. Anal Lett 49:1334–1343
Wan YP, Chai BW, Wei Q, Hayat W, Dang Z, Liu ZH (2022) 17α-ethynylestradiol and its two main conjugates in seven municipal wastewater treatment plants: analytical method, their occurrence, removal and risk evaluation. Sci Total Environ 812:152489
Wang IJ, Lin CC, Lin YJ, Hsieh WS, Chen PC (2014) Early life phthalate exposure and atopic disorders in children: a prospective birth cohort study. Environ Int 62:48–54
Wang H, Liu ZH, Tang Z, Zhang J, Yin H, Dang Z, Wu PX, Liu Y (2020) Bisphenol analogues in Chinese bottled water: quantification and potential risk analysis. Sci Total Environ 713:136583
Wei D, Zhang C, Pan A, Guo M, Lou C, Zhang J et al (2022) Facile synthesis and evaluation of three magnetic 1,3,5-triformylphloroglucinol based covalent organic polymers as adsorbents for high efficient extraction of phthalate esters from plastic packaged foods. Food Chemistry: X 14:100346
Wu Y, Lu Y, Xie G (2022) Bubble tea consumption and its association with mental health symptoms: An observational cross-sectional study on Chinese young adults. J Affect Disord 299:620–627
Yan H, Cheng X, Liu B (2011) Simultaneous determination of six phthalate esters in bottled milks using ultrasound-assisted dispersive liquid-liquid microextraction coupled with gas chromatography. J Chromatogr, B: Anal Technol Biomed Life Sci 879:2507–2512
Yang C, Harris SA, Jantunen LM, Kvasnicka J, Nguyen LV, Diamond ML (2020) Phthalates: relationships between air, dust, electronic devices, and hands with implications for exposure. Environ Sci Technol 54:8186–8197
Yuan SF, Liu ZH, Lian HX, Yang CT, Lin Q, Yin H, Dang Z (2017) Simultaneous determination of eleven estrogenic and odorous chloro- and bromo-phenolic compounds in surface water through an automated online headspace SPME followed by on-fiber derivatization coupled with GC-MS. Anal Methods 9:4819–4827
Yuan SF, Liu Z-H, Lian H-X, Yang C-T, Lin Q, Yin H, Lin Z, Dang Z (2018) Fast trace determination of nine odorant and estrogenic chloro- and bromo-phenolic compounds in real water samples through automated solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. Environ Sci Pollut Res 25:3813–3822
Yuan SF, Liu Z-H, Yin H, Dang Z, Wu P-X, Zhu N-W, Lin Z (2019) Trace determination of sulfonamide antibiotics and their acetylated metabolites via SPE-LC-MS/MS in wastewater and insights from their occurrence in a municipal wastewater treatment plant. Sci Total Environ 653:815–821
Zelenkin SE, Shur TZ, Ulanova TS, Karnazhitskaya TD, Khoroshavin VA, Ukhabov VM (2018) Assessment of health risk caused by phthalates penetrating a body with milk in polymer and polymer-containing package. Health Risk Anal 1:32–38
Zhang Z, Hu Y, Zhao L, Li J, Bai H, Zhu D et al (2011) Estrogen agonist/antagonist properties of dibenzyl phthalate (DBzP) based on in vitro and in vivo assays. Toxicol Lett 207:7–11
Zhang Y, Lyu L, Tao Y, Ju H, Chen J (2022) Health risks of phthalates: a review of immunotoxicity. Environ Pollut 313:120173
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21577040; no. 21107025); Science and Technology Program of Guangzhou, China (no. 201904010100; no. 201510010162); Special Funds for Public Welfare Research and Capacity Building in Guangdong Province (no. 2015A020215003), Zhongshan Public Water Co. LTD for water micropollutant project (no. ZPW-2020-A-010), and the Guangdong Science and Technology Program (no. 2020B121201003).
Author information
Authors and Affiliations
Contributions
De-kang Huang: data analysis and original draft preparation. Ze-hua Liu: supervisor, funding support, and writing review. Yi-ping Wan: sample preparation. Zhi Dang: writing review.
Corresponding author
Ethics declarations
Ethical approval
Not available.
Consent to participate
All authors have given consent to their contribution.
Consent for publication
All authors have agreed with the content and all have given explicit consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D.k., Liu, Zh., Wan, Yp. et al. Analysis and contamination levels of ten phthalic acid esters (PAEs) in Chinese commercial bubble tea: a comparison with commercial milk. Environ Sci Pollut Res 30, 103153–103163 (2023). https://doi.org/10.1007/s11356-023-29728-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29728-7