Abstract
This study aimed to examine the associations of pyrethroid exposure with handgrip strength and skeletal muscle mass and potential modification effects in US adults. The data from the National Health and Nutrition Examination Survey was used. Handgrip strength was determined with a handgrip dynamometer, and we quantified muscle mass by using the appendicular skeletal muscle index (ASMI). Urinary 3-Phenoxybenzoic Acid (3-PBA), a validated biomarker for pyrethroid exposure, was used in the primary analysis. After adjusting for other covariates, participants exposed to the highest tertile of 3-PBA exposure had significantly lower handgrip strength (β = –1.88, 95% CI: –3.29, –0.23, P = 0.026) than those exposed to the lowest tertile of 3-PBA. Similarly, the 3-PBA exposure was marginally significantly associated with ASMI (Tertile 3 vs. Tertile 1: β = -0.07, 95% CI: –0.14, –0.01, P = 0.056). Significant interactions were found between 3-PBA and body mass index (BMI) on handgrip strength and ASMI (P interaction < 0.05), which indicated a potential moderation effect of BMI on the associations. In conclusion, pyrethroid exposure was adversely associated with handgrip strength and skeletal muscle mass, especially in overweight and obese populations. Further studies are warranted to confirm our results and to explore the potential mechanisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Handgrip strength and skeletal muscle mass are reliable measurements to evaluate muscle quality and quantity (Roberts et al. 2011). In 2018, the European Working Group of Sarcopenia in Older People 2 (EWGSOP2) recommend to evaluate handgrip strength and appendicular skeletal muscle mass in the diagnosis of sarcopenia (Cruz-Jentoft et al. 2019). Recently, multiple studies have found that weaker handgrip strength is associated with physical health, including cardiovascular disease, type 2 diabetes, respiratory, and cancer outcomes, and mental health (Alfaro-Acha et al. 2006; Celis-Morales et al. 2018; Kim 2019; Fraser et al. 2021). On the other hand, skeletal muscle mass plays an important role in protein metabolism and body glucose metabolism (Wolfe 2006, Merz & Thurmond 2020). It is reported that muscle mass was also inversely related to the risk of cardiovascular disease, metabolic syndrome, diabetes and mortality (Kim et al. 2014, 2015; Burrows et al. 2017, Kelley & Kelley 2017).
As synthetic pesticides extracted from naturally occurring pyrethrins, pyrethroids were widely used to control pests in domestic indoor and agricultural settings (Singh et al. 2022). Nowadays, the use of pyrethroid pesticides has increased dramatically due to the restrictions of acutely toxic insecticides (e.g., organophosphate pesticides) (Williams et al. 2008; Horton et al. 2011). Pyrethroid exposure can be through different ways (e.g., dietary intake and residential application) (Fortes et al. 2013; Lu et al. 2013). Prior studies, including ours, have shown that environmental pyrethroid exposure would increase the risk of cardiovascular disease, diabetes and other health problems (Park et al. 2019; Xue et al. 2021; Zuo et al. 2022). Pyrethroid exposure was known to be related to inflammation and oxidative stress (Mostafalou & Abdollahi 2013, Chrustek et al. 2018). Higher proinflammatory cytokines may result in lower muscle mass and lower handgrip strength (Visser et al. 2002). Similarly, oxidative stress may contribute to fiber atrophy and breakage (McKenzie et al. 2002). However, to our knowledge, the effects of pyrethroid exposure on handgrip strength and skeletal muscle mass are not reported. Additionally, a positive association between endocrine-disrupting chemical mixture (e.g., phthalate metabolites, phenols, parabens, and pyrethroid pesticides metabolite) and dyslipidemia and weight gain have been reported (Gore et al. 2015; Pinos et al. 2021; Kim et al. 2022). This indicated that pyrethroids were more deeply deposited in adipose tissue, persons with overweight and obese might be more susceptible and persistently affected by pyrethroids.
We hypothesized that exposure to pyrethroids is associated with handgrip strength and skeletal muscle mass. Here, using the data from National Health and Nutrition Examination Survey (NHANES), we examined the cross-sectional associations of pyrethroid metabolites [e.g. 3–phenoxybenzoic acid(3-PBA)] with handgrip strength and skeletal muscle mass and explored the potential effect modification.
Material and methods
Study population
Data comes from the NHANES, a cross-sectional study with national representativeness conducted to evaluate the health and nutritional status of the US population. The protocol has been approved by the National Center for Health Statistics Ethics Review Board and all participants provided written informed consent (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Two survey cycles (NHANES2011-2014) were available to examine the association between pyrethroid exposure and handgrip strength. After excluding participants who reported having cancer, and those with missing data on 3–phenoxybenzoic acid (3-PBA), handgrip strength, or on other covariance, 2,317 participants remained (Supplemental Figure 1). Four survey cycles (NHANES1999-2002 and 2011–2014) were available to examine the association between pyrethroid exposure and skeletal muscle mass. Consistently, participants who were ≤ 20 years and had cancer were restricted from our analysis. Those with missing information on skeletal muscle mass, 3-PBA, or missing any information on covariates were further excluded. Finally, a total of 3069 participants remained (Supplemental Figure 2).
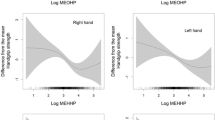
Non-linear relationship of 3-Phenoxybenzoic Acid (3-PBA) with handgrip strength (A) and appendicular skeletal muscle index (ASMI) (B). *Adjusted for age, gender, race/ethnicity, marital status, education, the ratio of family income to poverty, smoking, drinking, urinary creatinine, exercise and total energy intake
The interaction between body mass index (BMI) and 3-Phenoxybenzoic Acid (3-PBA) on handgrip strength (A) and appendicular skeletal muscle index (ASMI) (B). Age, gender, race/ethnicity, marital status, education, the ratio of family income to poverty, smoking, drinking, urinary creatinine, exercise, total energy intake and BMI were adjusted in the model
Pyrethroid exposure assessment
Pyrethroid metabolites were collected from spot urine specimens, stored under –20 ℃ and transported to CDC’s National Center for Environmental Health laboratory for measurement through confirmed methods of high–performance liquid chromatography coupled with electrospray chemical ionization and tandem mass spectrometry (Barr et al. 2010). We used urinary metabolite measurements of 3-PBA, trans-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid (trans-DCCA), cis-3-(2,2-dibromovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid (cis-DCCA), 4-fluoro-3-phenoxybenzoic acid (4-F-3PBA) and cis-3-(2,2-dibromovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid (cis-DBCA). Detailed descriptions of the laboratory procedures were provided elsewhere (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). The metabolite 3-PBA represents exposure to permethrin, cypermethrin, deltamethrin, allethrin, resmethrin, fenvalerate, cyhalothrin, fenpropathrin, and tralomethrin; cis-DCCA and trans-DCCA represent exposure to the cis and trans isomers, respectively, of permethrin, cypermethrin, and cyfluthrin; 4 F-PBA is a specific metabolite of cyfluthrin, and cis-DBCA is a specific metabolite of deltamethrin(Barr et al. 2010). Therefore, we used 3-PBA in the main analysis, as a represent biomarker for a wide range of pyrethroid exposure.
Definition of outcome
Handgrip strength was measured by a dynamometer (Takei Digital Grip Strength Dynamometer, Model T.K.K.5401). The examination excluded participants who were unable to hold the dynamometer with both hands (e.g., missing both arms, both hands, thumbs on both hands, or paralyzed in both hands) and had surgery on either hand or wrist within the last 3 months. After adjusting the grip size properly, the participants were asked to squeeze the dynamometer as hard as they could three times using left and/or right hands, with each interval of 60 s in a standing position unless the participant was physically limited. The combined handgrip strength is in kilograms and was calculated as the total of the maximum readings for each hand.
All body composition measurements were scanned using whole–body dual–energy X–ray absorptiometry (DXA) (Hologic, Inc., Bedford, Massachusetts). Appendicular skeletal mass was calculated based on the sum of the lean soft tissue from the arms and legs. Appendicular skeletal muscle index (ASMI) was used to quantify the muscle mass, which was calculated as ASMI = ASM (kg) / height (m2) (Baumgartner et al. 1998).
Covariates
NHANES collected information on age, sex, race/ethnicity, education, poverty income ratio (PIR), smoking status, alcohol intake, physical activity and body mass index (BMI) by using standardized questionnaires. Age and BMI were treated as continuous variables. Ethnic groups contained non–Hispanic white, non–Hispanic black, Mexican American and others. Education was categorized as less than 9th grade, 9–11th grade and high school education or higher. Marital status was classified as married/cohabiting, widowed/divorced/separated, and never married. PIR was classified as < 1.3, 1.3–3.5 and > 3.5 (Bao et al. 2020). Participants were grouped as never smokers (smoked less than 100 cigarettes in their lifetime), current smokers (had smoked more than 100 cigarettes and smoked during the research) and former smokers (had smoked more than 100 cigarettes and quitted smoking during the research) (Xue et al. 2021). Drinkers were defined as consuming ≥ 12 alcoholic drinks in a lifetime. The exercise was considered as participants who have at least once a month ≥ 10 min of moderate or severe activities (Hong et al. 2021). Continuous simulation of total energy intake based on data collected from 24–hour dietary recall interviews. Hypertension was defined as diastolic blood pressure ≥ 90 mmHg and (or) systolic blood pressure ≥ 140 mmHg, reporting the use of anti-diabetic medication (Kim et al. 2021). The participants whose answer of “Has a doctor or other health professional ever told you that you had congestive heart failure/coronary heart disease/angina pectoris/stroke?” was “yes” were defined as cardiovascular disease (Liao et al. 2020). Diabetes was defined as self–reported use of hypoglycemic medicines, the level of fasting glucose ≥ 126 mg/dL or HbA1c ≥ 6.5% (Zuo et al. 2022). Participants who answered “yes” to the question “Have you ever been told by a doctor or health professional that you have arthritis” was defined as having arthritis (Brooks et al. 2018).
Statistical analysis
The geometric mean and its 95% confidence interval (CI) were reported for urinary 3-PBA concentrations and creatinine-corrected 3-PBA. The normal distribution of urinary 3-PBA was obtained by log transformation. Multivariable linear regression was used to estimate the associations of 3-PBA exposure (categorized by tertiles) with handgrip strength and ASMI. Model 1 was adjusted for age, sex and urinary creatinine, model 2 was further adjusted for race/ethnicity, education, poverty income ratio, marital status, smoking, drinking, BMI, exercise and total energy intake, model 3 was additionally adjusted for hypertension, diabetes, cardiovascular disease and arthritis. Meanwhile, linear regression based on three-knots restricted cubic splines was performed to examine the nonlinear regression. Further, we conducted subgroup analyses by age (≤ 44, 45–59, ≥ 60 years), sex, and BMI (< 25 and ≥ 25 kg/m2), and additionally detected the potential effect modifications. All statistical analyses were conducted with STATA, version 17.1 using the survey command. P < 0.05 was considered to be statistically significant.
Results
Characteristics of participants
Among 2317 eligible participants included in the analyses of the association between 3-PBA and handgrip strength, 1148 (49.55%) were females, the mean age was 46.57 years, and the mean handgrip strength was 72.62 kg. There were statistical differences in smoking, drinking, marital status, cardiovascular disease, total energy intake and handgrip strength between males and females. In the analysis of the relationship between 3-PBA and skeletal muscle mass, 3069 participants were included, 1478 (48.16%) of them were female, the average age was 41.47 years, and the mean ASMI was 7.89 kg/m2. There were similar trends for the differences between males and females (Table 1).
Association of pyrethroid metabolites with handgrip strength
After adjusting for age, sex and urine creatinine, participants exposed to the highest tertile of 3-PBA exposure had significantly lower handgrip strength (β = –2.11, 95% CI: –3.88, –0.34, P = 0.021) than those exposed to the lowest tertile of 3-PBA. Further adjusting for race/ethnicity, education, poverty income ratio, marital status, smoking, drinking, BMI, exercise and total energy intake, the negative association was observed (β = –1.73, 95% CI: –3.34, –0.13, P = 0.035). When further adjusting for hypertension, diabetes, cardiovascular disease and arthritis, the association remained unchanged (β = –1.88, 95% CI: –3.29, –0.23, P = 0.026) (Table 2). Consistently, we did not observe a nonlinear association between 3-PBA and handgrip strength (Fig. 1A).
Association of pyrethroid metabolites with ASMI
After adjusting for age, sex and urine creatinine, race/ethnicity, education, poverty income ratio, marital status, smoking, drinking, BMI, hypertension, diabetes, cardiovascular disease and arthritis, the 3-PBA exposure was marginally significantly associated with ASMI (Tertile(T)3 vs. T1: β = -0.07, 95% CI: –0.14, –0.01, P = 0.056) (Table 2). Further, we found a non-linear association between 3-PBA and ASMI, and ASMI started to decrease when the 3-PBA level reached 0.146 μg/L (P for non-linearity = 0.041) (Fig. 1B).
Subgroup analysis
Significant interactions were found between 3-PBA and BMI on handgrip strength and ASMI (P interaction < 0.001), which indicated a potential moderation effect of BMI on the associations (Fig. 2). When stratified by BMI, the result showed significant differences between 3-PBA and handgrip strength in overweight and obese participants (T2, β = –0.93, 95% CI: –3.19, 1.35, P = 0.415; T3, β = –3.29, 95% CI: –5.27, –1.30, P = 0.002), but not observed among lean ones (T2, β = 1.27, 95% CI: –0.99, 3.53, P = 0.262; T3, β = 2.44, 95% CI: –0.72, 5.60, P = 0.126). Consistently, there was a significant association between 3-PBA and ASMI among the population with overweight and obesity (T2, β = –0.06, 95% CI: –0.13, 0.02, P = 0.143; T3, β = –0.12, 95% CI: –0.21, –0.04, P = 0.005), but not in lean ones (Table 3). We did not find significant interactions for age and sex (Supplemental Tables 1 and 2).
Sensitivity analysis
We found a significantly negative association between urinary trans-DCCA and ASMI but did not find any significant associations for cis-DBCA, cis-DCCA or 4-F-3PBA (Table 4). The associations of trans-DCCA and 4-F-3PBA with handgrip strength also did not reach significance. Associations of urinary cis-DBCA with handgrip strength were not examined because urinary cis-DBCA and cis-DCCA were not measured in NHANES 2011–2014. We also performed sensitivity analyses for the association of 3-PBA with handgrip strength and skeletal muscle mass by excluding participants with diabetes, cardiovascular disease or arthritis. All results remained similar trend of 3-PBA with handgrip strength and skeletal muscle mass (Supplemental Table 3). In addition, we excluded smokers and drinkers (523 participants for handgrip strength and 551 participants for ASMI were available) from the population and also found negative associations of 3-PBA with handgrip strength (T2, β = 0.31, 95% CI: –3.39, 4.01, P = 0.865; T3, β = –1.05, 95% CI: –5.24, 3.13, P = 0.612) and ASMI (T2, β = –0.26, 95% CI: –3.84, 3.33, P = 0.885; T3, β = –0.91, 95% CI: –5.07, 3.25, P = 0.658), although did not reach a statistical significance.
Discussion
In this cross-sectional study among a representative population of adults from NHANES, we demonstrated that higher concentrations of urinary 3-PBA were associated with weaker handgrip strength and lower ASMI. Meanwhile, interactions between BMI and 3-PBA exposure on handgrip strength and ASMI were identified. In the subgroup analysis, we found a significant association between urinary pyrethroid exposure and handgrip strength and ASMI in overweight and obese populations, but not in lean ones.
To the best of our knowledge, this was the first study to explore the associations of pyrethroid exposure with handgrip strength and skeletal muscle mass. Prior studies found a significant association between 3-PBA exposure and type 2 diabetes (Park et al. 2019; Liang et al. 2022). Another study among the US adult population from NHANES showed that exposure to 3-PBA was longitudinally associated with an increased risk of all-cause and cardiovascular disease mortality (Bao et al. 2020). In addition, the associations between 3-PBA and BMI have been reported in the adult population (Yoshinaga et al. 2014). Our findings suggested that skeletal muscle may play a role in the associations between pyrethroid exposure and cardiometabolic diseases.
In this study, significant differences between higher concentrations of 3-PBA with weaker handgrip strength and ASMI were found especially in people with overweight and obesity. Similarly, Xue Q et al. found the relationships of 3-PBA with coronary heart disease and diabetes were more significant among individuals with higher BMI, (Xue et al. 2021). In a cohort study, the results indicated that the risk of all-cause mortality was stronger among participants exposed to pyrethroids who were obese (Bao et al. 2020).
There are several potential pathways for the effect of pyrethroids on handgrip strength and skeletal muscle mass. First, many studies have confirmed the associations between oxidative stress and muscle cells atrophy and necrosis (Powers & Jackson 2008, Musarò et al. 2010; Powers et al. 2011; Murakami et al. 2012), and pyrethroid exposure potentially increase oxidative stress (Chrustek et al. 2018, Ravula & Yenugu 2021). Second, a cohort study among adults and children in the Czech found that pyrethroid metabolites were associated with DNA methylation (DNAm) biomarkers (Janoš et al. 2023), while DNAm age acceleration had a negative relationship with handgrip strength (Peterson et al. 2023). Third, studies have indicated exposure to pyrethroids may cause inflammation (Meng & Yu 2010). Intracellular redox imbalance might active signalling pathways such as NF–κB, which induced the release of proinflammatory factors such as IL–6 and TNF–a, thus leading to the loss of muscle mass and muscle atrophy (Li et al. 1998; Langen et al. 2001; Chung et al. 2009). Consistently, we also found that C-reactive protein (CRP) could explain 5.6%(95%CI: 3.3–16.9%) of the association between 3-PBA and ASMI (data not shown). Last, estrogens and androgens play a role to maintain mass and strength in bones and muscles (Almeida et al. 2017), while the function of hormone receptors might be disrupted by pyrethroid metabolites (Du et al. 2010, Castiello & Freire 2021). Therefore, estrogens and/or androgens receptors might have a mediating influence when skeletal muscle is exposed to pyrethroids.
One of the major strengths was that we for the first time explored the associations of pyrethroid exposure with handgrip strength and skeletal muscle mass. There were serval limitations in our study. First, as a cross-sectional study, the reverse causality between the pyrethroid exposures and handgrip strength was hard to exclude. Second, due to the short half-life of pyrethroid metabolites and the lack of long-term urine pyrethroid measurements, the spot urine samples may not well reflect realistic exposure to pyrethroids. Third, besides the covariates adjusted in our regression analyses model, some potential confounded factors still existed and influenced our result. Finally, the effects of specific types of pyrethroids on cardiometabolic risk should be further studied.
Conclusion
There are negative associations of pyrethroid exposure with handgrip strength and ASMI, particularly in overweight and obese populations. Further studies are required to confirm the results and to examine the underlying mechanisms.
Data Availability
The data are available from NHANES.
References
Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ (2006) Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci 61:859–865. https://doi.org/10.1093/gerona/61.8.859
Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC (2017) Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev 97:135–187. https://doi.org/10.1152/physrev.00033.2015
Bao W, Liu B, Simonsen DW, Lehmler HJ (2020) Association Between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern Med 180:367–374. https://doi.org/10.1001/jamainternmed.2019.6019
Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL (2010) Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ Health Perspect 118:742–748. https://doi.org/10.1289/ehp.0901275
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520
Brooks JM, Titus AJ, Bruce ML, Orzechowski NM, Mackenzie TA, Bartels SJ, Batsis JA (2018) Depression and Handgrip Strength Among U.S. Adults Aged 60 Years and Older from NHANES 2011–2014. J Nutr Health Aging 22:938–943. https://doi.org/10.1007/s12603-018-1041-5
Burrows R, Correa-Burrows P, Reyes M, Blanco E, Albala C, Gahagan S (2017) Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatr Diabetes 18:895–902. https://doi.org/10.1111/pedi.12505
Castiello F, Freire C (2021) Exposure to non-persistent pesticides and puberty timing: a systematic review of the epidemiological evidence. Eur J Endocrinol 184:733–749. https://doi.org/10.1530/EJE-20-1038
Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR (2018) Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361:K1651. https://doi.org/10.1136/bmj.k1651
Chrustek A, Hołyńska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, Olszewska-Słonina D (2018): Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina (Kaunas) 54 https://doi.org/10.3390/medicina54040061
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30. https://doi.org/10.1016/j.arr.2008.07.002
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older P, the Extended Group for E (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Du G, Shen O, Sun H, Fei J, Lu C, Song L, Xia Y, Wang S, Wang X (2010) Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol Sci 116:58–66. https://doi.org/10.1093/toxsci/kfq120
Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C (2013) The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem Toxicol 52:91–96. https://doi.org/10.1016/j.fct.2012.10.035
Fraser BJ, Blizzard L, Buscot MJ, Schmidt MD, Dwyer T, Venn AJ, Magnussen CG (2021) The Association Between Grip Strength Measured in Childhood, Young- and Mid-adulthood and Prediabetes or Type 2 Diabetes in Mid-adulthood. Sports Med 51:175–183. https://doi.org/10.1007/s40279-020-01328-2
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT (2015) EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36:E1–E150. https://doi.org/10.1210/er.2015-1010
Hong D, Min JY, Min KB (2021) Association between pyrethroids and prostate endpoints; stratified according to renal function. Environ Int 153:106489. https://doi.org/10.1016/j.envint.2021.106489
Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, Whyatt RM (2011) Characterization of residential pest control products used in inner city communities in New York City. J Expo Sci Environ Epidemiol 21:291–301. https://doi.org/10.1038/jes.2010.18
Janoš T, Ottenbros I, Bláhová L, Šenk P, Šulc L, Pálešová N, Sheardová J, Vlaanderen J, Čupr P (2023) Effects of pesticide exposure on oxidative stress and DNA methylation urinary biomarkers in Czech adults and children from the CELSPAC-SPECIMEn cohort. Environ Res 222:115368. https://doi.org/10.1016/j.envres.2023.115368
Kelley GA, Kelley KS (2017) Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol 96:100–103. https://doi.org/10.1016/j.exger.2017.06.008
Kim JH (2019) Effect of grip strength on mental health. J Affect Disord 245:371–376. https://doi.org/10.1016/j.jad.2018.11.017
Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW (2014) Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int 14(Suppl 1):115–121. https://doi.org/10.1111/ggi.12189
Kim BC, Kim MK, Han K, Lee SY, Lee SH, Ko SH, Kwon HS, Merchant AT, Yim HW, Lee WC, Park YG, Park YM (2015) Low muscle mass is associated with metabolic syndrome only in nonobese young adults: the Korea National Health and Nutrition Examination Survey 2008–2010. Nutr Res 35:1070–1078. https://doi.org/10.1016/j.nutres.2015.09.020
Kim B, Park B, Kim CH, Kim S, Park B (2022) Association between endocrine-disrupting chemical mixture and metabolic indices among children, adolescents, and adults: A population-based study in Korea. Environ Pollut 315:120399. https://doi.org/10.1016/j.envpol.2022.120399
Kim Y, Shin S, Hong N, Rhee Y (2021): Low Serum Vitamin E Level Associated with Low Hand Grip Strength in Community-Dwelling Adults: Korean National Health and Nutrition Examination Survey (KNHANES VII) 2016–2018. Nutrients 13 https://doi.org/10.3390/nu13051598
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2001) Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J 15:1169–1180. https://doi.org/10.1096/fj.00-0463
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12:871–880. https://doi.org/10.1096/fasebj.12.10.971
Liang R, Yu L, Liu W, Dong C, Tan Q, Wang M, Ye Z, Zhang Y, Li M, Wang B, Feng X, Zhou M, Chen W (2022) Associations of bifenthrin exposure with glucose homeostasis and type 2 diabetes mellitus in a general Chinese population: Roles of protein carbonylation. Environ Pollut 315:120352. https://doi.org/10.1016/j.envpol.2022.120352
Liao S, Zhang J, Shi S, Gong D, Lu X, Cheang I, Zhang H, Li X (2020) Association of aldehyde exposure with cardiovascular disease. Ecotoxicol Environ Saf 206:111385. https://doi.org/10.1016/j.ecoenv.2020.111385
Lu C, Adamkiewicz G, Attfield KR, Kapp M, Spengler JD, Tao L, Xie SH (2013) Household pesticide contamination from indoor pest control applications in urban low-income public housing dwellings: a community-based participatory research. Environ Sci Technol 47:2018–2025. https://doi.org/10.1021/es303912n
McKenzie D, Bua E, McKiernan S, Cao Z, Aiken JM, Jonathan W (2002) Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur J Biochem 269:2010–2015. https://doi.org/10.1046/j.1432-1033.2002.02867.x
Meng SJ, Yu LJ (2010) Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11:1509–1526. https://doi.org/10.3390/ijms11041509
Merz KE, Thurmond DC (2020) Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr Physiol 10:785–809. https://doi.org/10.1002/cphy.c190029
Mostafalou S, Abdollahi M (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol 268:157–177. https://doi.org/10.1016/j.taap.2013.01.025
Murakami H, Guillet C, Tardif N, Salles J, Migné C, Boirie Y, Walrand S (2012) Cumulative 3-nitrotyrosine in specific muscle proteins is associated with muscle loss during aging. Exp Gerontol 47:129–135. https://doi.org/10.1016/j.exger.2011.11.007
Musarò A, Fulle S, Fanò G (2010) Oxidative stress and muscle homeostasis. Curr Opin Clin Nutr Metab Care 13:236–242. https://doi.org/10.1097/MCO.0b013e3283368188
Park J, Park SK, Choi YH (2019) Environmental pyrethroid exposure and diabetes in U.S. adults. Environ Res 172:399–407. https://doi.org/10.1016/j.envres.2018.12.043
Peterson MD, Collins S, Meier HCS, Brahmsteadt A, Faul JD (2023) Grip strength is inversely associated with DNA methylation age acceleration. J Cachexia Sarcopenia Muscle 14:108–115. https://doi.org/10.1002/jcsm.13110
Pinos H, Carrillo B, Merchán A, Biosca-Brull J, Pérez-Fernández C, Colomina MT, Sánchez-Santed F, Martín-Sánchez F, Collado P, Arias JL, Conejo NM (2021): Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review. Int J Environ Res Public Health 18 https://doi.org/10.3390/ijerph18137170
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276. https://doi.org/10.1152/physrev.00031.2007
Powers SK, Smuder AJ, Criswell DS (2011) Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15:2519–2528. https://doi.org/10.1089/ars.2011.3973
Ravula AR, Yenugu S (2021) Pyrethroid based pesticides - chemical and biological aspects. Crit Rev Toxicol 51:117–140. https://doi.org/10.1080/10408444.2021.1879007
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429. https://doi.org/10.1093/ageing/afr051
Singh S, Mukherjee A, Jaiswal DK, de Araujo Pereira AP, Prasad R, Sharma M, Kuhad RC, Shukla AC, Verma JP (2022) Advances and future prospects of pyrethroids: Toxicity and microbial degradation. Sci Total Environ 829:154561. https://doi.org/10.1016/j.scitotenv.2022.154561
Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB (2002) Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57:M326–M332. https://doi.org/10.1093/gerona/57.5.m326
Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM (2008) Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ Health Perspect 116:1681–1688. https://doi.org/10.1289/ehp.11367
Wolfe RR (2006) The underappreciated role of muscle in health and disease. Am J Clin Nutr 84:475–482. https://doi.org/10.1093/ajcn/84.3.475
Xue Q, Pan A, Wen Y, Huang Y, Chen D, Yang CX, Hy WuJ, Yang J, Pan J, Pan XF (2021) Association between pyrethroid exposure and cardiovascular disease: A national population-based cross-sectional study in the US. Environ Int 153:106545. https://doi.org/10.1016/j.envint.2021.106545
Yoshinaga J, Imai K, Shiraishi H, Nozawa S, Yoshiike M, Mieno MN, Andersson AM, Iwamoto T (2014) Pyrethroid insecticide exposure and reproductive hormone levels in healthy Japanese male subjects. Andrology 2:416–420. https://doi.org/10.1111/j.2047-2927.2014.00202.x
Zuo L, Chen L, Chen X, Liu M, Chen H, Hao G (2022) Pyrethroids exposure induces obesity and cardiometabolic diseases in a sex-different manner. Chemosphere 291:132935. https://doi.org/10.1016/j.chemosphere.2021.132935
Acknowledgements
We thank all the participants and their important contributions to this study.
Formatting of funding sources
None.
Data statement
The data that support the findings of this study are available at https://www.cdc.gov/nchs/nhanes/index.htm.
Author information
Authors and Affiliations
Contributions
GH was responsible for the conceptualization and supervision. The first draft of the manuscript was written by ZF. XC, MLL, LZ, BYZ, GJZ and HYC critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
We affirm that all authors have agreed for submission of the paper to ESPR and are fullyaware of ethical responsibilities.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• We firstly explored the association of pyrethroid exposure with muscle quality.
• Pyrethroid exposure is adversely associated with muscle quality.
• Stronger association of pyrethroid exposure with muscle quality in obese people.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, Z., Chen, X., Liu, M. et al. Associations of pyrethroid exposure with skeletal muscle strength and mass. Environ Sci Pollut Res 30, 89651–89660 (2023). https://doi.org/10.1007/s11356-023-28784-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28784-3