Abstract
Evidences on the association between exposure to air pollution and liver enzymes was scarce in low pollution area. We aimed to investigate the association between air pollution and liver enzyme levels and further explore whether alcohol intake influence this association. This cross-sectional study included 425,773 participants aged 37 to 73 years from the UK Biobank. Land Use Regression was applied to assess levels of PM2.5, PM10, NO2, and NOx. Levels of liver enzymes including AST, ALT, GGT, and ALP were determined by enzymatic rate method. Long-term low-level exposure to PM2.5 (per 5-μg/m3 increase) was significantly associated with AST (0.596% increase, 95% CI, 0.414 to 0.778%), ALT (0.311% increase, 0.031 to 0.593%), and GGT (1.552% increase, 1.172 to 1.933%); The results were similar for PM10; NOX and NO2 were only significantly correlated with AST and GGT Significant modification effects by alcohol consumption were found (P-interaction < 0.05). The effects of pollutants on AST, ALT, and GGT levels gradually increased along with the weekly alcohol drinking frequency. In conclusion, long-term low-level air pollutants exposure was associated with elevated liver enzyme levels. And alcohol intake may exacerbate the effect of air pollution on liver enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is a global public health concern, resulting in about 8.9 million deaths each year worldwide (Collaborators 2016). Studies have found air pollution exposure was related to various health outcomes, such as respiratory disease (Gordon et al. 2014), cardiovascular disease (Miller and Newby 2020), and mental disorders (Braithwaite et al. 2019). The mechanisms of health effects induced by air pollution may involve multiple mechanisms, such as oxidative stress and inflammation. Among them, oxidative stress has been found to underlie the pathophysiology of various etiologies of chronic liver disease (Seen 2021).
The liver health, which plays an important role in physical health, can be affected by various factors including age, diet, alcohol consumption, obesity. In current studies, four liver enzymes in the blood including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) are usually considered as indicators of liver injury (Newsome et al. 2018). Recently, experimental researches have observed that exposure to air pollution significantly increases the serum levels in mice, indicating that air pollutants exposure may lead to liver injury (Maglione et al. 2020; Orona et al. 2020). Epidemiological researches have already assessed the relation between air pollution exposure and liver enzyme levels, but the results on the relation turned out to be heterogeneous in comparison with each other (Kim et al. 2019, 2015; Markevych et al. 2013; Pejhan et al. 2019). In addition, these researches on the relationships of air pollution exposure and liver enzymes are mainly conducted in Asia regions with high pollutant concentrations such as South Korea (Kim et al. 2019) and Taiwan (Zhang et al. 2019) (during the study time, the average concentrations of PM2.5 were about 50 μg/m3 and 25 μg/m3, respectively), while there are few relevant studies in areas with low pollutant concentrations. The liver was also found to suffer from greater tissue injury related to air pollution through aggravating liver inflammation after excessive alcohol consumption (Kwon et al. 2014). It suggested that reducing alcohol consumption may be beneficial in reducing the impact of air pollution on liver enzymes.
Therefore, we conducted a large-scale study in Europe with low pollutant concentrations (the average concentration of PM2.5 and PM10 were only 9.98 μg/m3 and 16.22 μg/m3, respectively) using the UK Biobank health datasets. The present study aimed to investigate the associations between long-term low-level ambient air pollutants exposure, including particulate matter with diameters ≤ 2.5 μm (PM2.5) and ≤ 10 μm (PM10), nitrogen oxides (NOx) and nitrogen dioxide (NO2), and levels of AST, ALT, GGT, and ALP, and further explore whether alcohol intake influence this association.
Methods
Study samples
The UK Biobank is a community-based cohort that enrolled about 0.5 million participants at ages 37 to 73 years between 2006 and 2010. Information on demographic, health, and lifestyle of participants was collected by a computer-assisted interview and a touch-screen questionnaire. The protocol of the study and individual tests protocols can be obtained online. The North West Multi-centre Research Ethics Committee provided ethical approval and all written informed consent were already obtained by participants.
We excluded participants with liver diseases at baseline (n = 2957), and participants with missing data on air pollution (n = 41,011) and the four liver enzymes (n = 32,739). Finally, 425,773 participants were included. The study population flow-chart is described in Fig. 1.
Assessment of air pollution
Air pollution values were estimated by the Small Area Health Statistics Unit. The land use regression model is used for centralized calculation of PM10, PM2.5, NO2, and NOx annual concentrations. The annual spatial variations of the air pollutants were calculated by the LUR model and linked to geocoded residential addresses of UK Biobank participants. More detailed descriptions of the development of these land-use regression models are available elsewhere (Beelen et al. 2013; Eeftens et al. 2012).
Measurement of liver enzymes
The circulating concentrations of GGT, ALP, ALT, and GGT were measured by enzyme rate method, more detailed descriptions of the assay performance can be obtained elsewhere (UK-Biobank 2019). The intra-laboratory mean CV of quality-control specimens ranged from 1.3–2.1% of AST, 1.2–2.9% of ALT, 1.4–2.8% of GGT, and 2.8–3.1% of ALP, showing that these data are qualified and available.
Assessment of covariates
In line with previous scientific literature, the following variables were included as covariates: age (continuous); gender (female or male); ethnicity (white or other); education level (college or university degree; A/AS levels or equivalent; O level/GCSE or CSE equivalent; NVQ or HND or HNC or other professional qualification; or None); employment status; household income (less than £18,000, £18,000 to £52,000, or £52,000 and above); body mass index (BMI) was measured as weight (kg) divided by height squared (m2); smoking status (never; previous; current); the amount of alcohol drinking was assessed according to the frequency of drinking; triglycerides (continuous); Total Metabolic Equivalent Task (MET) minutes per week was used to assess physical activity, containing walking, moderate and vigorous activity (MET-min/week) according to International Physical Activity Questionnaire (IPAQ) guidelines; vascular problems were evaluated on the basis of hypertension, heart attack, angina, and stroke; diabetes (none or yes).
Statistical analysis
The characteristics of participants were recorded in Table 1. Since the distribution of the four liver enzymes was right biased, we performed natural logarithmic transformation before analysis. To explore the association between air pollutants and liver enzymes, the study used the multiple linear regression analyses. We fitted two statistical models: crude Model (unadjusted for covariates); adjusted Model (adjusted for age, gender, employment status, ethnicity, education level, BMI, smoke status, alcohol drinking, physical activity, household income, triglycerides, vascular problems, diabetes). In addition, we performed a stratified analysis by alcohol consumption, gender, and age. The interactions between subgroups were tested by the Wald test. The outcomes were presented as percent changes of liver enzymes with per 5-µg/m3 for PM2.5, per 10-µg/m3 for PM10 and NOx, and per-20 µg/m3 for NO2. All statistical analyses were performed in the R Statistical Software, version 4.0.2. A two-sided P less than 0.05 was considered significant.
Results
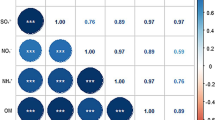
Distribution of air pollutants and characteristics of the study participants were showed in Table 1. The final sample comprised of UK Biobank 425,773 participants. The estimate mean (SD) of PM10, PM2.5, NOx, and NO2 were 16.22 (1.90), 9.98 (1.06), 43.90 (15.61), and 26.58 (7.62) μg/m3, respectively. The Pearson correlation coefficients among the four air pollutants are shown in Supplemental Fig. 1.
Table 2 shows the relationships of air pollutants with liver enzymes. In the crude Model, exposure to air pollution was significantly related to increased liver enzyme levels, except for the association of ALT with NO2 (P = 0.816). After adjusting for potential confounders, per 5-μg/m3 increases of PM2.5 were correlated with elevated AST (0.596% increase; 95% confidence interval (CI), 0.414 to 0.778%), ALT (0.311% increase; 95% CI, 0.031 to 0.593%), and GGT (1.552% increase; 1.172 to 1.933%) levels. PM10 (per 10-μg/m3) were correlated with AST (0.451% increase; 0.252 to 0.650%), ALT (0.520% increase; 0.212 to 0.828%), and GGT (0.692% increase; 0.280 to 1.106%) levels. NOx (per 10-μg/m3) were correlated with AST (0.174% increase; 0.125 to 0.223%) and GGT (0.369% increase; 0.267 to 0.472%) levels. NO2 (per 10-μg/m3) were correlated with AST (0.222% increase; 0.172 to 0.273%) and GGT (0.301% increase; 0.196 to 0.406%) levels.
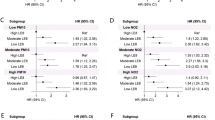
Figure 2 presents the association between pollutants with liver enzymes stratified by alcohol drinking frequency. With the increase of drinking frequency, the influence of pollutants on AST, ALT, and GGT levels gradually increased (P-interaction < 0.05) while substantial changes of ALP were not observed. In addition, the results of stratified analyses by age and gender are shown in Supplemental Table 1 and Supplemental Table 2, respectively. There were interactions of the four air pollutants with gender on all liver enzymes levels (all P-interaction < 0.05) while the only significant interactions of NOx, PM2.5, and NO2 with age on ALP levels.
Associations between air pollution and liver enzymes in subgroups stratified by alcohol drinking. a Represents non-drinker, b represents alcohol drinking less than once a week, c represents alcohol drinking at least once a week. P-interaction was evaluated for the product term between air pollutants and alcohol consumption. Model were adjusted for age, gender, ethnicity, education level, employment status, household income, BMI, smoke status, alcohol drinking, physical activity, triglycerides, vascular problems, diabetes. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; and GGT, gamma-glutamyl transferase. PM2.5, fine particulate matter with diameter ≤ 2.5 μm; PM10, particulate matter with diameter ≤ 10 μm; NO2, nitrogen dioxide; NOx, nitrogen oxides
Discussion
In this large population-based longitudinal study in the UK, we found a positive association between air pollutants and liver enzyme (AST, ALT, and GGT) levels. We also evaluated the stratified effects of alcohol drinking frequency on these associations, and found that with the frequency of alcohol drinking, the air pollutants effects on AST, ALT, and GGT levels increased.
To date, research on the role of air pollution exposure in the serum liver enzyme levels is limited and inconsistent. Several studies have concluded that exposure to environmental pollution was linked with elevated liver enzyme levels, which are in agreement with our findings (Kim et al. 2019, 2015; Li et al. 2022; Pejhan et al. 2019; Zhang et al. 2019). A population-based longitudinal study found that long-term exposure to ambient air pollution including PM2.5, PM10, NO2, CO, and O3 was significantly associated with increased serum liver enzyme levels in older adults (Li et al. 2022). Another study in Korea also found a positive significant association of PM10, NO2, and CO with levels of ALT and AST (Kim et al. 2019). And a Taiwanese study in adults reported significant effect of PM2.5 on elevated GGT, ALT, and AST levels (Zhang et al. 2019) However, a study conducted in Germany reported completely different results that there was no any significant association of PM2.5, PM10, or NO2 with ALT or AST (Markevych et al. 2013), and a panel study of elderly Koreans found no significant association of O3 with AST or ALT. The inconsistency among these researches was probably owing to discrepancies in the research population, research site, exposure levels, and/or exposure assessment strategies.
The mechanisms underlying the deleterious association of air pollution with the liver remain unknown. The oxidative stress response is one of the most well-known mechanisms; it has been shown to be a reliable mechanism by which air pollution contributes to cardiovascular disease (Brook et al. 2010), respiratory disease (Barreiro et al. 2005), diabetes (20), and mental illness (Buoli et al. 2018, 2017). Studies have also found exposure to air PM can increase generation of reactive oxygen species (ROS) (Cichoz-Lach and Michalak 2014, Giacco and Brownlee 2010), disrupt the hepatic redox steady state, and enhance oxidative stress (Yang et al. 2020), which could promote hepatic inflammatory infiltration and hepatocellular injury, and further induce chronic organic damage (Danielsen et al. 2010; Xu et al. 2019). Another potential mechanism is that air pollutants may promote liver injury etiopathogenesis through direct and indirect inflammatory responses (Chan et al. 2020; Robinson et al. 2016). Evidence showed that inhaled PM can translocate in the lungs to activate immune cells, so as to stimulate the secretion of inflammatory cytokines (Kawaratani et al. 2013). Kim et al. had proposed that PM may be translocated into the extra-pulmonary circulation and organ including the liver, directly stimulating the immune response in the liver and causing damage (Kim et al. 2014). In addition, PM2.5 has been reported to increase lipid peroxidation to affect liver health (Bourdon et al. 2012).
Studies also explored the impact of more alcohol consumption on the effects of air pollution on the liver and the results showed that more alcohol consumption can exacerbate the effect of air pollution on the liver, leading to abnormal liver enzyme levels (Kim et al. 2019, 2015; Li et al. 2022). Similar results were also found in the stratified analysis of our study. There was a greater relationship between air pollution and liver enzymes among participants who drank more than once a week. Excessive alcohol consumption can enhance oxidative stress by increasing the generation of ROS (Kessova et al. 2003; Sid et al. 2013), inflammatory response and lipid peroxidation, leading to the amplification of the adverse effect of air pollution on liver enzymes. Thus, both air pollution exposure and alcohol consumption are correlated with elevated liver enzyme levels, and there may exist a synergistic impact on liver enzymes. These finding suggested that in order to prevent the increase of liver enzymes caused by air pollution, intervention for alcohol abstinence is necessary. Our researches findings also observed some evidence on the effect modification by gender. The effects of air pollution on liver enzyme levels were stronger in men than in women. It is known that significant differences exist in the lifestyles between men and women. Men were found to smoke more, to consume more alcohol than women, and are more likely to be dangerous drinkers (Erol and Karpyak 2015). Moreover, the liver has been regarded as a sexually organ, which can express sex hormone receptors, and women have higher levels of estrogen than men, which can reduce liver injury (Toyoda et al. 2011).
There are several strengths in our study. Based on a good quality control cohort, our study has strengths including exceptionally large sample sizes in the UK biobank, the well-validated air pollution metrics and various available and acceptable liver enzymes data (Huang et al. 2021; Liu et al. 2022; Strak et al. 2021). Furthermore, our analysis was conducted on the whole population rather than the specific population. Thus, our findings have good statistical power and generalizability. There are also some limitations. First, owing to the cross-sectional design, this study cannot affirm the causal relationship between air pollution and alcohol consumption on liver enzymes. Second, common to most previous ambient air pollution studies, air pollution exposure was estimated based on a single place of residence, which cannot rule out the potential exposure misclassification caused by outside activities. Lastly, despite adjusting some potential confounding variables for the analysis, residual confounding from other unmeasured or unavailable factors cannot be excluded.
Conclusions
To the best of our knowledge, this is the first large-scale research to explore the effect of air pollution on liver enzyme levels in the UK. In summary, the current study found that low air pollution exposures, even within the regulatory limits, were associated with increased levels of liver enzymes including AST, ALT, and GGT, suggesting that exposure to ambient air pollution contributes to hepatocellular injury in adults. Furthermore, the association was stronger among those who drink alcohol more. Notably, our study included nearly 0.5 million individuals and used air pollution data based on the UK Biobank, which has been consistently low for many years. All of these is completely different from previous literatures. The results obtained in this way have added to the currently new evidence-base.
These findings indicated that reducing personal ambient air pollution exposure may help prevent hepatocellular injuries and liver diseases, which can help clinical practitioners and public health policy makers develop targeted measures accordingly. More epidemiology researches should be conducted to validate this finding. Meanwhile, toxicological experiments are needed to elucidate potential biological mechanisms.
Data availability
The data and materials will be sent based on request.
References
Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, Hussain SN, Gea J (2005) Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171:1116–1124
Beelen R et al (2013) Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe – the ESCAPE project. Atmos Environ 72:10–23
Bourdon JA, Saber AT, Jacobsen NR, Jensen KA, Madsen AM, Lamson JS, Wallin H, Moller P, Loft S, Yauk CL, Vogel UB (2012) Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part Fibre Toxicol 9:5
Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF (2019) Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ Health Perspect 127:126002
Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD, American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition PA, Metabolism (2010) Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121:2331-78
Buoli M, Serati M, Caldiroli A, Cremaschi L, Altamura AC (2017) Neurodevelopmental versus neurodegenerative model of schizophrenia and bipolar disorder: comparison with physiological brain development and aging. Psychiatr Danub 29:24–27
Buoli M, Grassi S, Caldiroli A, Carnevali GS, Mucci F, Iodice S, Cantone L, Pergoli L, Bollati V (2018) Is there a link between air pollution and mental disorders? Environ Int 118:154–168
Chan SL, Wong LL, Chan KA, Chow C, Tong JH, Yip TC, Wong GL, Chong CC, Liu PH, Chu CM, Wong VW, To KF, Reeves HL, Chan AW (2020) Development of a novel inflammation-based index for hepatocellular carcinoma. Liver Cancer 9:167–181
Cichoz-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20:8082–8091
Collaborators GBDRF (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1659–1724
Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, Wallin H, Møller P (2010) Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol Sci: Off J Soc Toxicol 118:574–585
Eeftens M et al (2012) Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46:11195–11205
Erol A, Karpyak VM (2015) Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Gordon SB et al (2014) Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2:823–860
Huang Y, Zhu M, Ji M, Fan J, Xie J, Wei X, Jiang X, Xu J, Chen L, Yin R, Wang Y, Dai J, Jin G, Xu L, Hu Z, Ma H, Shen H (2021) Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK Biobank. Am J Respir Crit Care Med 204:817–825
Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M, Fukui H (2013) The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm 2013:495156
Kessova IG, Ho YS, Thung S, Cederbaum AI (2003) Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology 38:1136–1145
Kim JW, Park S, Lim CW, Lee K, Kim B (2014) The role of air pollutants in initiating liver disease. Toxicol Res 30:65–70
Kim KN, Lee H, Kim JH, Jung K, Lim YH, Hong YC (2015) Physical activity- and alcohol-dependent association between air pollution exposure and elevated liver enzyme levels: an elderly panel study. J Prev Med Public Health 48:151–169
Kim HJ, Min JY, Seo YS, Min KB (2019): Association of ambient air pollution with increased liver enzymes in Korean adults. Int J Environ Res Public Health 16:1213
Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, Matsumoto A, Singh S, Abdelmegeed MA, Song BJ, Kawamoto T, Vasiliou V, Thiele GM, Gao B (2014) Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology 60:146–157
Li Y, Yuan X, Wei J, Sun Y, Ni W, Zhang H, Zhang Y, Wang R, Xu R, Liu T, Yang C, Chen G, Xu J, Liu Y (2022) Long-term exposure to ambient air pollution and serum liver enzymes in older adults: a population-based longitudinal study. Ann Epidemiol 74:1–7
Liu S et al (2022) Long-term air pollution exposure and pneumonia-related mortality in a large pooled European cohort. Am J Respir Crit Care Med 205:1429–1439
Maglione GA, Kurtz ML, Orona NS, Astort F, Brites F, Morales C, Berra A, Tasat DR (2020) Changes in extrapulmonary organs and serum enzyme biomarkers after chronic exposure to Buenos Aires air pollution. Environ Sci Pollut Res Int 27:14529–14542
Markevych I, Wolf K, Hampel R, Breitner S, Schneider A, von Klot S, Cyrys J, Heinrich J, Döring A, Beelen R, Koenig W, Peters A (2013) Air pollution and liver enzymes. Epidemiology (Cambridge, Mass) 24:934–5
Miller MR, Newby DE (2020) Air pollution and cardiovascular disease: car sick. Cardiovasc Res 116:279–294
Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, Hall R, Harrower U, Hudson M, Langford A, Mackie A, Mitchell-Thain R, Sennett K, Sheron NC, Verne J, Walmsley M, Yeoman A (2018) Guidelines on the management of abnormal liver blood tests. Gut 67:6–19
Orona NS, Astort F, Maglione GA, Ferraro SA, Martin M, Morales C, Mandalunis PM, Brites F, Tasat DR (2020) Hazardous effects of urban air particulate matter acute exposure on lung and extrapulmonary organs in mice. Ecotoxicol Environ Saf 190:110120
Pejhan A, Agah J, Adli A, Mehrabadi S, Raoufinia R, Mokamel A, Abroudi M, Ghalenovi M, Sadeghi Z, Bolghanabadi Z, Bazghandi MS, Hamidnia M, Salimi F, Pajohanfar NS, Dadvand P, Rad A, Miri M (2019) Exposure to air pollution during pregnancy and newborn liver function. Chemosphere 226:447–453
Robinson MW, Harmon C, O’Farrelly C (2016) Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 13:267–276
Seen S (2021) Chronic liver disease and oxidative stress - a narrative review. Expert Rev Gastroenterol Hepatol 15:1021–1035
Sid B, Verrax J, Calderon PB (2013) Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic Res 47:894–904
Strak M et al (2021) Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ (clinical Research Ed) 374:n1904
Toyoda Y, Miyashita T, Endo S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T (2011) Estradiol and progesterone modulate halothane-induced liver injury in mice. Toxicol Lett 204:17–24
UK-Biobank (2019) UK biobank biomarker project-companion document to accompany serum biomarker data. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf. Accessed 3 Nov 2019
Xu MX, Ge CX, Qin YT, Gu TT, Lou DS, Li Q, Hu LF, Feng J, Huang P, Tan J (2019) Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radical Biol Med 130:542–556
Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, Heinrich J (2020) Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res 180:108817
Zhang Z, Guo C, Chang LY, Bo Y, Lin C, Tam T, Hoek G, Wong MC, Chan TC, Lau AK, Lao XQ (2019) Long-term exposure to ambient fine particulate matter and liver enzymes in adults: a cross-sectional study in Taiwan. Occup Environ Med 76:488–494
Acknowledgements
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 69741.
Author information
Authors and Affiliations
Contributions
R-L and Y.H-T contributed to the conception and design of the study. R-L, D.K-L, L.L-W, and Y.H-T advised on all statistical aspects and interpreted the data. R-L and J.Q-X performed the literature search and the analyses. All authors critically reviewed this and previous drafts. All authors approved the final draft for submission, with final responsibility for publication. All authors approved the final version of the manuscript. The corresponding author attests that all the listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethical approval
UK Biobank received ethical approval from the North West Multicenter Research Ethics Committee (REC reference: 16/NW/0274).
Consent to participate
All written informed consent were already obtained by participants, which was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
This work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. Manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Li, D., Xie, J. et al. Air pollution, alcohol consumption, and the risk of elevated liver enzyme levels: a cross-sectional study in the UK Biobank. Environ Sci Pollut Res 30, 87527–87534 (2023). https://doi.org/10.1007/s11356-023-28659-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28659-7