Abstract
Coal mining has produced a large amount of coal gangue. It makes the soil around the mining area seriously polluted by heavy metals, affects the growth of crops, and endangers human health. Therefore, there is an urgent need to develop new materials for remediation of Cd in soil. In this study, mercaptosilane-modified sepiolite (Q-Sep) was used as a basic passivator, and it was pretreated with acid (H-Q-Sep) and high temperature (R-Q-Sep) respectively. By analyzing the forms of Cd and pH values in soil after adding modified sepiolite, we compared the remediation effects of two modified methods on Cd in soil. The enrichment of spinach (Spinacia oleracea L) to Cd and changes in physiological and biochemical indexes of spinach were determined, and the effect of modified sepiolite on the growth of spinach was judged. The experimental results showed that the addition of modified sepiolite could significantly increase the soil pH values (p < 0.05); the content of exchangeable Cd in soil decreased by 60.4%; and the maximum increase of residual state was 32.9%. The absorption of Cd in soil by spinach decreased, and root length, plant height, and biomass of spinach all increased. It was proved that the addition of modified sepiolite can improve the productivity of soil, reduce toxicity of heavy metals in soil, and promote growth of plants. As a result, the addition of H-Q-Sep and R-Q-Sep can effectively repair Cd in gangue filled soil, which provides a certain theoretical basis for the passivation remediation of Cd in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is the largest producer and consumer of coal in the world; a large amount of solid waste including coal gangue will be produced in the process of coal mining; heavy metal is one of the typical pollutants of coal gangue (Gao et al. 2021; Sun et al. 2020). The long-term accumulation of coal gangue and the filling of coal mining subsidence areas with coal gangue will lead to the surrounding soil to be polluted by heavy metals in coal gangue, including a variety of heavy metal elements, such as Cd, Hg, Mn, Pb, and Zn (Sun et al. 2021; Zhang et al. 2019). Heavy metal pollution of cultivated land in China is relatively serious, among which Cd pollution is the most serious, with the national point exceeding the standard rate of 7%(Lu et al. 2019; Zhang et al. 2018). Cd is a heavy metal with silver luster, which has strong biological toxicity and mobility, and is very harmful to plants, animals, and microorganisms in soil. It is considered by most scholars an element with serious toxic effects. Cd pollution is nondegradable and persistent. On the one hand, it can poison plant tissues; on the other hand, after being absorbed by plant roots, it will be directly transported to the aboveground parts and in grains, vegetables, stems, leaves, and fruits of food crops, which will reduce yields of crop and endanger human health through the food chain (Shi et al. 2009; Wang et al. 2022).
Because of the seriousness of Cd pollution in soil and the urgency to solve it, it has been widely studied. Soil remediation refers to use methods of transfer, absorption, degradation, and transformation to reduce the concentration of pollutants to an acceptable level of the soil or to convert toxic and harmful pollutants into harmless substances (Hossain et al. 2010). The remediation of heavy metal Cd in soil is generally carried out from two aspects: removal of heavy metals by leaching or phytoremediation. Passivation changes the existing forms of heavy metals in soil and reduces mobility and bioavailable toxicity of heavy metals to organisms (Houben et al. 2013). The technology of in situ passivation for heavy metals has the advantages of quick effect, convenient operation, high repair efficiency, and low cost. It can generally meet the application of heavy metal remediation and agricultural production (Xu et al. 2017). Currently, clay minerals (Liang et al. 2013), phosphate compounds, basic compounds (Ma et al. 2012), biochar, etc. are considered as effective passivators. Zhang et al. (2021) showed that the addition of biochar and steel slag could reduce the content of Cd in soil, reduce the absorption of Cd by rice, and increase the yield of rice. Huang et al. (2020) proved that the addition of slaked lime and sepiolite to Cd contaminated soil can reduce the content of exchangeable Cd by 42.66%, and the content of Cd in brown rice decreased by 49.03%.
Sepiolite is a clay mineral with good adsorption properties, and it has the advantages of low cost and large output. However, the adsorption capacity of natural sepiolite is limited by its narrow internal channels and low self-loading capacity (Sun et al. 2016). Therefore, it is necessary to modify sepiolite properly to improve its repair effect on heavy metals. Existing research had shown that thiol groups can be loaded on the surface of organic and inorganic materials through hydrolytic condensation reactions (Gan et al. 2016). By covalent and electrostatic binding, sulfur-containing groups can form stable complexes with heavy metals to achieve the purpose of adsorption (Yusuke et al. 2009). Karlsson et al. (2007) found that Cd in soil mainly forms complexes with active groups such as sulfhydryl and carboxyl groups on the surface of soil organic matter, to reduce the mobility and bioavailability of Cd. When sepiolite is roasted at high temperature, its crystal water will be taken away, thereby enlarging the internal pores of the sepiolite and enhancing the adsorption performance of sepiolite (Valentin et al. 2007; Kuang et al. 2003). Activation with acid can reduce content of iron and aluminum in clay minerals and increase their specific surface area and porosity, thus enhancing their adsorption capacity (Rusmin et al. 2016). At present, the adsorption properties of sepiolite can be optimized by using acid, heat, and thiol-modified sepiolite, which can enhance the ability of sepiolite to adsorb heavy metals. However, the results of combining acid modification and thermal modification with sulfhydryl modification are unknown.
The study showed that the average concentration of Cd in soil around the Xinzhuangzi mine exceeded the soil background value in Huainan city and it was 2.9 times larger than the soil background value of Huainan city (You et al. 2016). Therefore, this study selected the cultivated soil near the Xinzhuangzi coal mining subsidence area in Huainan as the research samples. Spinach is a leafy vegetable with strong Cd enrichment ability. At present, spinach has been selected as the research object for the experiment (Bakhshayesh et al. 2014). In this study, the combination of acid treatment, heat treatment, and mercaptosilane was used to modify sepiolite; the existing forms of Cd in soil and the physiological indexes of spinach after the addition of two modified sepiolite were analyzed and compared to verify their passivation effect on Cd in soil and their effect on the growth of spinach. The purpose of this study is to clarify the effect of two modified sepiolite on soil environment, compare the passivation effects of two modified sepiolite on Cd in soil, and analyze their effect on the growth of spinach.
Materials and methods
Chemicals
All chemicals used in this study were of analytical grade. 3-Mercaptopropyltrimethoxysilane and anhydrous ethanol were purchased from Shanghai Boyle Chemical Co., Ltd. (Shanghai China). Hydrochloric acid (HCl), nitric acid (HNO3), and acetone were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai China). National mineral soil standard material (GSS-3) was supplied by Wuhan Zhong Chang Guo Yan Biao Wu Technology Co., Ltd. (Wuhan, China). Spinach seeds were obtained from Xingyun Vegetable Seed Breeding Center in Qingxian County, Hebei Province. Sepiolite was obtained from Xinlei Mineral Powder Processing Plant, Xingtang County, Shijiazhuang, Hebei Province.
Preparation of modified sepiolite
Sulfhydryl-modified sepiolite
First, 3-mercaptopropyltrimethoxysilane, ethanol, distilled water were mixed in a volume ratio of 1:8:0.5 to obtain a sulfhydryl mixed solution. Then, natural sepiolite was immersed into the sulfhydryl mixed solution at a ratio of 1:1 (g to ml). After shaking for 6 h at a shaker speed of 300 rpm and 25 °C, the mixture was filtered with 0.45-μm filter paper and dried in an oven at 40 °C to obtain sulfhydryl-loaded sepiolite (Q-Sep) (Han et al. 2019).
Acid-sulfhydryl modified sepiolite
The natural sepiolite was mixed with HCl at a ratio of 1:10 (g to ml) (1 g sepiolite:10 ml HCl). After shaking for 8 h at a shaker speed of 300 rpm and 25 °C, the mixture was filtered with 0.45-μm filter paper and then dried in an oven at 40 °C to obtain acidified sepiolite. The concentrations of HCl were 2 M, 3 M, 4 M, and 5 M (Su et al. 2014). Acidified sepiolite and sulfhydryl mixed solution were mixed at a ratio of 1:1 (g to ml). After shaking for 6 h at a shaker speed of 300 rpm and 25 °C, the mixture was filtered with 0.45-μm filter paper and dried in an oven at 40 °C to obtain acidified sulfhydryl-loaded sepiolite (H-Q-Sep).
Thermo-sulfhydryl-modified sepiolite
The natural sepiolite was roasted in a tube furnace (SX-GO7102, Zhonghuan Experimental Electric Furnace, China) at high temperature for 2 h, and the temperature settings were 100 °C, 200 °C, 300 °C, 400 °C, and 500 °C (Biswas et al. 2016). The thermally modified sepiolite was immersed into the sulfhydryl mixed solution at a ratio of 1:1 (g to ml). After shaking for 6 h at a shaker speed of 300 rpm and 25 °C, the mixture was filtered with 0.45-μm filter paper and dried in an oven at 40 °C to obtain sulfhydryl-loaded sepiolite after thermal treatment (R-Q-Sep).
Sample collection and experiment setup
Soil samples were collected from Xinzhuangzi coal gangue filling area, Huainan city, Anhui Province. Soil samples were collected at a depth of 0–20 cm from the soil surface. Soil samples were collected from five different locations within 100 m2 and composited in one sample bag to make representative soil samples. When samples were brought back to the lab, stones and debris in soil were removed. Sep, Q-Sep, H-Q-Sep, and R-Q-Sep at 15 g·kg−1 were mixed with 3-kg soil and placed in flowerpots. After soil in the flowerpots was balanced for 1 week, 30 spinach seeds were sown in each pot. The duration of the whole growth period was 60 days. Three duplicates per processing were set.
Analysis of soil properties
Physiochemical properties and heavy metals in the collected soil and spinach matured (60 days) soil were determined. Soil was air-dried at room temperature (25 °C) and sieved with 2-mm sieve. The soil pH value was measured with a pH meter (MP220, Mettler Toledo, USA) after shaking 5 g of soil and 25 ml of distilled water for 1 h at a shaker speed of 300 rpm and 25 °C. Total nitrogen in soil was determined by the Kjeldahl method (HJ 711-2014), total phosphorus in soil was determined by molybdenum-antimony resistance colorimetry soil (HJ 632-2011), and organic matter (OM) was determined by oxidation potassium dichromate colorimetric method (Nelson et al. 1996). The basic properties of soil are shown in Table 1.
After soil samples were digested with aqua regia (HNO3 to HCl (v to v) =1:3) (Yoon et al. 2019), their content of Cd was measured by an atomic absorption spectrophotometer (Analyst 100, PerkinElmer, USA). The Tessier five-part extraction method (Tessier et al. 1979) divides heavy metals in soil into five forms according to their biological activities, which are exchangeable Cd, carbonate-bound Cd, iron–manganese oxide–bound Cd, organic matter–bound Cd, and residual Cd. Various forms of Cd in soil were extracted and the content of Cd was determined by an atomic absorption spectrophotometer (Analyst100, PerkinElmer, USA). In order to ensure the accuracy of the test process and data, quality control and quality assurance measures were taken. During the test, blank reagents, three samples, and national mineral soil standard material (GSS-3) were included, to verify the accuracy of the digestion and analysis method, and the analysis error was less than 10%.
Plant sample measurement
Measurement of plant height, root length, biomass, and Cd content
After 60 days of culture cycle, three spinach plants of uniform size were selected for each treatment. Plant height and root length were measured after washing with distilled water. Then, the plants were put in an oven at 105 °C for 40 min and dried at 80 °C to constant weight. The dried weights of plants were recorded.
The dried spinach was divided into stems, leaves, and roots; after grinding the 2-mm sieve, they were digested with aqua regia (HNO3 to HCl (v to v) =1:3). After that, the content of Cd in spinach was measured by an atomic absorption spectrophotometer (Analyst 100, PerkinElmer, USA).
Determination of chlorophyll, malondialdehyde content, and antioxidant enzyme activity
The blotted fresh leaves of the plants were used for the estimation of chlorophyll. The leaves were ground into powder with liquid nitrogen in the dark. Absolute ethanol and acetone were mixed with a volume ratio of 1:2 to obtain the extract solution. The extract solution was added to the plant powder, and the mixture was stored in the dark for more than 3 h to extract chlorophyll. Then, the supernatant was measured at 645 nm and 663 nm by an ultraviolet-visible spectrophotometer (UV-5500, Shanghai Metash Instruments, China), and the absorbance value was read for calculation.
Leaf samples (0.2 g) were ground with liquid nitrogen and homogenized with 1.8 ml phosphate buffer. Then, the homogenate was centrifuged at 6000 rpm and 4 °C for 15 min. This supernatant was used to measure the activities of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) and the content of malondialdehyde (MDA) (Huang et al. 2020). These steps were followed by a kit (Nanjing Jiancheng Bioengineering Institute).
Data analysis
There were three duplicates in all processing, and the statistical analysis of experimental data was performed with SPSS (Version 20.0) software. And all values are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze the statistical characteristic differences of sample data, When a significant (p < 0.05) difference was observed between treatments, multiple comparisons were made by the Duncan test. The graphical work was performed with Origin 2022b.
Results
Effects of modified sepiolite on soil pH values
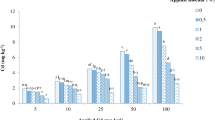
According to Fig. 1, sepiolite modified by HCl and mercaptosilane can increase the pH in soil significantly (p < 0.05) compared with the control group. And with the increase of the concentration of HCl, it showed a trend of first increasing and then decreasing. The highest increase in soil pH (7.65) was observed when the concentration of HCl was 3 M, which was 0.78 higher than the control group. In the group of thermal treatment as pretreatment, the highest increase in soil pH (7.71) was observed when the temperature was 300 °C, which was 0.84 higher than the control group.
Effects of different hydrochloric acid concentration and temperature modified sepiolite on pH values of soil. Letters above the bar diagram refer to the difference at significance level p < 0.05 among different treatments of sepiolite. Note:Sep stands for sepiolite, Q-Sep stands for sulfhydryl modified sepiolite, H-Q-Sep is sulfhydryl modified sepiolite after acidification, and R-Q-Sep is sulfhydryl modified sepiolite after high temperature. In this experiment, the number of samples n= 3, and the level of significance test was p < 0.05
When Q-Sep and Sep were added to soil the pH values of the soil increased by 0.54 and 0.46, respectively. This is because sepiolite contains a large amount of calcium carbonate, which has a certain neutralization effect on the hydrogen ions in the soil (Tang et al. 2016). It can be seen from Fig. 1 that the addition of H-Q-Sep and R-Q-Sep can increase the pH values of soil. However, R-Q-Sep can increase pH values to a greater extent than H-Q-Sep, probably due to the increased concentration of HCl in the H-Q-Sep treatment, which resulted in a similar increase of hydrogen ions in soil, resulting in a slight drop in pH values. Hence, R-Q-Sep increased pH values of soil more significantly.
Wang et al. (2015) added serpentine and lime to contaminated soil, which significantly increased pH values of the soil. The reason was that there existed a large number of internal and external hydroxyl groups in the serpentine structure with strong chemical reactivity. The hydrogen atoms could covalently bond to highly electronegative atoms such as O, F, and N to form hydroxyl groups and then generate an alkaline solution, resulting in an increase of soil pH values (Sharma et al. 2009). In this experiment, sepiolite also contained internal and external hydroxyl groups; the same reaction as serpentine can occur and improve soil pH values. The rise of soil pH values can also convert Cd in soil from exchangeable state to residual state through coordination, precipitation, etc. It is conducive to the substitution of magnesium in the edge structure of sepiolite octahedral sheets, which is beneficial to the adsorption of heavy metals in soil by sepiolite (Biswas et al. 2016). Therefore, increasing soil pH value is also one of the main methods of soil heavy metal remediation.
Effects of modified sepiolite on the occurrence form of Cd in soil
The effects of adding H-Q-Sep and R-Q-Sep to soil with different concentrations of HCl and temperatures on the forms of Cd in soil are shown in Fig. 2. The addition of H-Q-Sep can significantly improve the passivation effect of Cd in soil. In this treatment, the content of exchangeable state Cd was significantly reduced and the content of residual state was increased compared with the control group.
Effects of different hydrochloric acid concentration and temperature modified sepiolite on the speciation of cadmium in soil. Note:Sep stands for sepiolite, Q-Sep stands for sulfhydryl modified sepiolite, H-Q-Sep is sulfhydryl modified sepiolite after acidification, and R-Q-Sep is sulfhydryl modified sepiolite after high temperature. In this experiment, the number of samples n= 3
Among them, the most significant effect was observed when the concentration of HCl was 3 M. Compared with the control group, the content of exchangeable Cd decreased by 60.4% and the residual content increased by 32.9%. The content of exchangeable Cd in soil decreased by 21.9% and 34.2%, when Sep and Q-Sep were added. The content of residual state increased by 4.69% and 16.4%, respectively. On the one hand, this is due to the increase of soil pH values, which converts Cd in soil from the more active exchangeable state to the residual state through coordination, precipitation, etc. (Liang et al. 2014). On the other hand, there are certain pores in sepiolite, which can convert exchangeable and carbonate-bound Cd into residual state by adsorption (Lu et al. 2018). In addition to combining with hydroxyl groups on the surface of sepiolite, active Cd can also complex with sulfhydryl groups, thereby changing its morphology (Liang et al. 2011).
It can be seen from Fig. 2 that the sepiolite modified by high temperature and mercaptosilane also has a passivation effect on Cd in soil, the content of exchangeable Cd decreased, and the content of residual state was also significantly increased. Among them, the best passivation effect was produced when the R-Q-Sep temperature was 300 °C. Compared to the control group with the treatment of additional Sep and Q-Sep, its content of exchangeable state decreased by 58.9%, 47.1%, and 37.5%, respectively. The increase of residual state was 31.2%, 28.0%, and 20.0%, respectively. After high-temperature roasting and HCl activation, the excess water in the sepiolite was lost, the purity was improved, its internal pores were enlarged, and the specific surface area became larger (Liang et al. 2014). The sulfhydryl group was more easily attached to the surface of sepiolite, and the adsorption effect was better. However, when the temperature was too high, a large amount of crystal water in sepiolite may come out, which would lead to the destruction of the structure of sepiolite and the collapse of its internal pores, making its specific surface area smaller. Therefore, too high temperature will lead to a decrease of passivation effect of R-Q-Sep on soil. The results showed that the addition of H-Q-Sep and R-Q-Sep can reduce the content of exchangeable Cd in soil and reduce toxicity of Cd to the environment and organisms.
The chemical forms of heavy metals in soil vary significantly in terms of their effectiveness and harmfulness to organisms (Kim et al. 2003). Among them, the higher the exchangeable content is, the stronger the toxic effect on organisms. The mutual transformation of different forms of heavy metal Cd in soil is the main process of passivation remediation. The addition of H-Q-Sep and R-Q-Sep can transform Cd from a more active form to a less active form or a form that is difficult for biology to use, thus achieving the purpose of heavy metal remediation.
Effects of modified sepiolite on physiology and biochemistry of spinach
Effects of modified sepiolite on Cd absorption of spinach
The effect of different modified sepiolite on the absorption of Cd by spinach is shown by Fig. 3. The addition of passivating agents can reduce the absorption of Cd by spinach, and there were significant differences (p < 0.05) compared with the control group. The addition of Q-Sep can reduce the content of Cd in the aboveground and underground parts of spinach by 35.83% and 47.76%, respectively. In the treatment of H-Q-Sep, when the concentration of HCl was 3 M, spinach had the lowest concentration of Cd. The content of Cd in aboveground and underground parts of spinach decreased by 65.0% and 59.2%, respectively. The content of Cd in the aboveground part decreased from 0.24 mg·kg−1 in the control group to 0.08 mg·kg−1; the content of Cd in the underground part decreased from 0.30 mg·kg−1 to 0.12 mg·kg−1.
Effects of different hydrochloric acid concentration and temperature modified sepiolite on cadmium accumulation in spinach. Letters above the bar diagram refer to the difference at significance level p < 0.05 among different treatments of sepiolite. Note:Sep stands for sepiolite, Q-Sep stands for sulfhydryl modified sepiolite, H-Q-Sep is sulfhydryl modified sepiolite after acidification, and R-Q-Sep is sulfhydryl modified sepiolite after high temperature. In this experiment, the number of samples n= 3, and the level of significance test was p < 0.05
In the group with R-Q-Sep addition, spinach had the lowest concentration of Cd when the modified temperature was 300 °C, in which the content of Cd in aboveground part decreased to 0.07 mg·kg-1, and the content of Cd in the underground part was reduced to 0.11 mg·kg-1, with the declines of 69.2% and 60.7% respectively. The transfer coefficient of spinach was lower than other groups and the content of Cd in leaves was lower when the modified temperature of sepiolite was 400 °C, which might be due to the randomness of sampling. The location of spinach young leaves was determined by sampling; when the modified temperature was 500 °C, the available content of Cd in soil was greater, and plants enriched more Cd, so the content of aboveground part was lower than at 500 °C (Shamshad et al. 2018).
The absorption of heavy metals by plants mainly occurs when heavy metals in the form of ions are adsorbed by the roots of plants and transferred to other parts such as stems, leaves, and fruits. During the process of enrichment, the uptake of heavy metals by roots depends on soil properties, such as soil pH values, soil content of organic matter, and soil nutrients (Zulfiqar et al. 2019). In general, the increased soil pH value promotes the formation of heavy metal carbonate and hydroxide precipitation, thereby reducing the utilization of heavy metals by organisms (Qin et al. 2020). Bashir et al. (2018) added straw biochar to soil, which significantly increased pH value and reduced availability of Cd in soil and absorption of Cd by water spinach. Hong et al. (2020) showed that the concentration of Cd in lettuce was negatively correlated with soil pH, and an increase of soil pH could promote the immobilization of Cd in soil.
Effects of modified sepiolite on plant height, root length, and biomass of spinach
The effects of adding modified sepiolite to soil under different conditions on the root length, plant height, and biomass of spinach are shown in Table. 2. The root length, plant height, and biomass of spinach in each group had different degrees of improvement. In the group pretreated with HCl, when the concentration of HCl was 3 M, the root length, plant height, and biomass increased by 43.8%, 63.3%, and 88.2%, respectively. In the group pretreated at high temperature, when the temperature was 300 °C, the growth index of spinach increased most. And compared to H-Q-Sep and Q-Sep, the addition of R-Q-Sep can reduce toxicity of Cd and increase pH values of soil to a greater extent; it also increases productivity of soil and growth of spinach. HCl can improve the purity of sepiolite and high-temperature roasting can remove the excess crystal water in sepiolite (Rusmin et al. 2016). It can increase the specific surface area of sepiolite and expand its porosity. Therefore, it is more convenient for sulfhydryl to load and more conducive to the adsorption of heavy metals in soil by sepiolite, thus reducing toxicity of Cd to organisms in soil. Anjum et al. (2011) found that the dried weight of mung bean seedlings was reduced by 59.8% in soil containing 100 mg·kg−1 Cd. Gu et al. (2020) confirmed that adding passivating agents can increase the nutrients in soil, reduce toxicity of Cd to plant roots, and increase the biomass of beet roots by 267%.
Effects of modified sepiolite on chlorophyll content of spinach
Photosynthesis is the basis of plant growth. When heavy metal Cd contaminates plants, it will affect photosynthetic pigments of plants and thus affect the photosynthesis of plants (Qin et al. 2018). As shown in Fig. 4, the addition of H-Q-Sep and R-Q-Sep increased the total amount of chlorophyll in spinach leaves. The highest points were at a concentration of HCl which was 3 M and modified temperature which was 300 °C, and there was a significant difference between them and the control group (p < 0.05). This is due to the excessive stress of Cd in the control group, and heavy metal ions were absorbed by plants and accumulated in plants continuously, resulting in destruction of chloroplast structure. The addition of modified sepiolite reduced toxicity of Cd in soil to plants and increased the content of chlorophyll in plants. It can weaken the effect of Cd on plant photosynthesis and improve the ability of plant photosynthesis and promote growth of plant (Lu et al. 2018). Singh et al. (2006) found that heavy metals can replace magnesium ions in chlorophyll, decrease the activity of chlorophyll synthase, and cause chlorophyll synthesis to be blocked. At the same time, the activity of chlorophyll decomposing enzymes is increased and the decomposition of chlorophyll is accelerated. The decrease of chlorophyll content had an adverse effect on growth of plants, which was consistent with the previously recorded indicators of plant growth.
Effects of different concentrations of hydrochloric acid and temperature modified sepiolite on chlorophyll content of spinach. Letters above the bar diagram refer to the difference at significance level p < 0.05 among different treatments of sepiolite. Note:Sep stands for sepiolite, Q-Sep stands for sulfhydryl modified sepiolite, H-Q-Sep is sulfhydryl modified sepiolite after acidification, and R-Q-Sep is sulfhydryl modified sepiolite after high temperature. In this experiment, the number of samples n= 3, and the level of significance test was p < 0.05
Effects of modified sepiolite on antioxidant enzyme activity and malondialdehyde content of spinach
When plants are subjected to external stress, they produce oxidative stress and a large amount of reactive oxygen species (Soares et al. 2019). Reactive oxygen species can interact with proteins, lipids, and nucleic acids in cells to change their structure and function and eventually lead to membrane lipid peroxidation. The content of MDA is an important index that reflects the strength of membrane lipid peroxidation (Zhang et al. 2007). Studies have shown that when plants are under stress, the activities of antioxidant enzymes in plants will increase rapidly to maintain their vitality under stress (Yadhu et al. 2017). According to Fig. 5, the activities of SOD, POD, CAT, and the content of MDA in the control group were highest, while those in other treatments decreased to varying degrees and there were significant differences from the control group (p < 0.05). The most significant effect was observed when the concentration of HCl was 3 M and the modified temperature was 300 °C, because the stress of Cd on spinach was the lowest at this time. The addition of modified sepiolite reduced the stress of Cd to spinach to different degrees. Therefore, the activity of antioxidant enzymes and the content of MDA also decreased to varying degrees.
Effects of different hydrochloric acid concentration and temperature modified sepiolite on antioxidant enzyme activity and malondialdehyde content of spinach. Letters above the bar diagram refer to the difference at significance level p < 0.05 among different treatments of sepiolite. Note:Sep stands for sepiolite, Q-Sep stands for sulfhydryl modified sepiolite, H-Q-Sep is sulfhydryl modified sepiolite after acidification, and R-Q-Sep is sulfhydryl modified sepiolite after high temperature. In this experiment, the number of samples n= 3, and the level of significance test was p < 0.05
Discussion
Spinach is a common and nutritious leafy vegetable (Zubair et al. 2019). Cd pollution in agricultural land poses a threat to growth of crop and human health. Cd in farmland is easily absorbed by plants and transferred to edible parts of plants. The concentration of Cd in spinach in the control group exceeded the Chinese national standard GB 2762-2012 (the highest level of pollutants in food) by 0.2 mg·kg−1 (Fig. 3). Hence, two different modified sepiolite were added to contaminated soil, and the chemical forms of Cd in the treated soil and the growth of spinach were analyzed and compared by pot experiment. The results showed that the addition of modified sepiolite could reduce toxicity of Cd in soil, inhibit the stress of Cd on spinach, and promote the growth of spinach.
Soil pH value can affect the absorption of Cd by plant roots (Huang et al. 2020). Sepiolite is an alkaline substance that can be hydrolyzed to release hydroxide ions to raise soil pH value and thus fix Cd in the soil (Inkham et al. 2019). We used sulfhydryl to modify sepiolite and loaded the sulfhydryl functional group on the surface of sepiolite to form a stable complex, thus enhancing the adsorption performance of sepiolite. Sepiolite pretreatment work (acidification or high temperature) was performed to improve its purity. Our results showed that the addition of passivating agents can reduce the content of exchangeable Cd in soil and reduce the toxicity of Cd (Fig. 2). By using modified sepiolite as a passivating agent, pH values in soil can be significantly increased, thus reducing the content of Cd in spinach roots and leaves (Fig. 3) (Shangguan et al. 2019; Sanderson et al. 2015). Many studies have shown that the addition of passivating agents can reduce the enrichment of heavy metals by plants in heavy metal polluted soil. For example, the addition of hydrated lime and sepiolite significantly reduced the content of Cd in rice grains (Huang et al. 2020). The addition of passivating agents increased the soil pH values (Fig. 1) and reduced the content of exchangeable Cd in the soil. The results showed that a higher soil pH value would make the Cd in the soil fixed, thus reducing the absorption of Cd by plants in soil.
The most common plant response to stress is growth slowdown. Root is the first part of plants to contact pollutants in the soil environment, which can most obviously reflect the absorption of heavy metals in plants. Pollutants also have a relatively obvious inhibitory effect on roots, so the root length can better reflect the toxic effect of Cd on spinach (Xu et al. 2019). After the addition of modified sepiolite, the plant height, root length, and biomass of spinach in each treatment were significantly higher than those in the control group (Table 2) because the stress of Cd on spinach was relieved to some extent, which made the growth of spinach tend to be normal. We analyzed on the correlation between plant height and root length, and there was a certain correlation between them, but the correlation was not significant. We analyzed the main reason why the correlation between them was not significant, mainly because the root length of spinach was shorter when the concentration of modified HCl was 2 M and the modified temperature was 100 °C. In the measurement, we found that the fibrous roots of the two groups of spinach were relatively exuberant and therefore, the straight roots were shorter (Liu et al. 2006). After the two groups of data had been removed, we conducted correlation analysis on the plant height and root length of spinach again and found a significant correlation between them (p < 0.05). We analyzed the correlation between plant height and biomass of spinach and found a significant correlation, which was related to the variety of spinach. The variety of spinach planted in this study was large-leaf spinach, so for biomass, the aboveground part accounted for the dominant biomass, and there was a significant correlation between plant height and biomass (p < 0.05).
Chlorophyll is essential for plants to capture light energy and plays a central role in photosynthesis. Its content can directly affect the photosynthesis of plants and therefore affect the accumulation of net photosynthetic energy of plants, thus affecting the yield and biomass of plants (Golan et al. 2015). The stress of Cd is not conducive to the synthesis of photosynthetic pigments, resulting in the decrease of the photosynthetic rate of plants. Therefore, the synthesis of chlorophyll under the stress of Cd has a significant inhibitory effect (Zhang et al. 2021). In this experiment, the addition of modified sepiolite to the soil can improve the pH values and reduce the toxicity of Cd in soil, thus promoting the synthesis of chlorophyll in spinach (Fig. 4). The results showed that when two kinds of modified sepiolite were added, the content of chlorophyll in spinach was the highest when the concentration of HCl and the temperature were 3 M and 300 °C, respectively. This is because at this time, the stress of Cd in soil to plants was the lowest, and the accumulation of heavy metals in spinach body was the lowest. Therefore, the addition of modified sepiolite is beneficial to improve plant photosynthesis and promote the accumulation of carbohydrates in plants, which is necessary to reduce the stress of Cd on plants.
Malondialdehyde is the product of peroxidation of plant membrane lipids. Studies have shown that content of MDA in plants can be significantly increased under Cd stress (Zhang et al. 2021). In our study, the addition of modified sepiolite significantly reduced the content of MDA in spinach (Fig. 5a). Antioxidant enzymes (SOD, POD, CAT) are substances produced by plants to resist various external stresses. They protect plants from oxidative stress by removing reactive oxygen species (Geslin et al. 2001). Under heavy metal stress, plants produce excessive reactive oxygen species, which stimulates the production of antioxidant enzymes. Our results showed that the activities of SOD, POD, and CAT in spinach decreased after passivating agent was added (Fig. 5b–d), indicating that the addition of modified sepiolite increased soil pH value and inhibited the absorption of Cd by spinach roots, and plant roots did not need to produce more antioxidant enzymes to resist the stress of Cd (Huang et al. 2020).
Conclusion
Sepiolite is a good material for passivation and remediation of heavy metals in soil. The sulfhydryl group can be loaded on the surface of sepiolite modified by mercaptosilane to enhance its adsorption capacity. The purity of sepiolite can be increased by HCl and high-temperature pretreatment, the internal pores become larger, and the specific surface area increases, which is more conducive to the loading of sulfhydryl groups. The modified sepiolite has stronger adsorption and passivation ability for heavy metals. The following conclusions are drawn from the experimental results: (1) the addition of modified sepiolite can change the soil environment and increase the pH values of soil; (2) the addition of modified sepiolite can transform Cd in soil from exchangeable state to residual state, thus reducing the toxicity of Cd in soil; and (3) The addition of modified sepiolite can reduce the absorption of Cd in the soil by spinach, promote the growth of spinach, increase the content of chlorophyll in spinach, and reduce the activity of antioxidant enzyme and the content of MDA in spinach. Therefore, the addition of sepiolite modified by acid, heat, and sulfhydryl is an effective method to remediate Cd in soil and can promote the growth of spinach.
Data availability
The data collected are property of our research center but will be made available by the corresponding author when requested.
References
Bakhshayesh BE, Delkash M, Scholz M (2014) Response of vegetables to cadmium-enriched soil. Water 6(5):1246–1256. https://doi.org/10.3390/w6051246
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 58(1):92–99. https://doi.org/10.1134/S1021443710061019
Bashir S, Zhu J, Fu QL, Hu HQ (2018) Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–587. https://doi.org/10.1016/j.chemosphere.2017.11.162
Biswas B, Sarkar B, Naidu R (2016) Influence of thermally modified palygorskite on the viability of polycyclic aromatic hydrocarbon-degrading bacteria. Appl Clay Sci 134:153–160. https://doi.org/10.1016/j.clay.2016.07.003
Gan WT, Gao LK, Zhan XX, Li J (2016) Preparation of thiol-functionalized magnetic sawdust composites as an adsorbent to remove heavy metal ions. RSC Adv 6(44):37600–37609. https://doi.org/10.1039/c6ra02285e
Gao HG, Huang YL, Li W, Li JM, Ouyang SY, Song TQ, Lv FY, Zhai W, Ma K (2021) Explanation of heavy metal pollution in coal mines of China from the perspective of coal gangue geochemical characteristics. Environ Sci Pollut Res 28(46):65363–65373. https://doi.org/10.1007/s11356-021-14766-w
Geslin C, Llanos J, Prieur D, Jeanthon C (2001) The manganese and iron super-oxide dismutases protect Escherichia coli from heavy metal toxicity. Res Microbiol 152(10):901–905. https://doi.org/10.1016/S0923-2508(01)01273-6
Golan K, Rubinowska K, Kmie´c K, Kot I, Górska-Drabik E, Lagowska B, Michałek W (2015) Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Interact 9(1):55–65. https://doi.org/10.1007/s11829-014-9339-7
Gu PX, Zhang YM, Xie HH, Wei J, Zhang XY, Huang X, Wang JY, Luo XY (2020) Effect of cornstalk biochar on phytoremediation of Cd-contaminated soil by Beta vulgaris var. cicla L. Ecotoxicol Environ Saf 205:111144. https://doi.org/10.1016/j.ecoenv.2020.111144
Han YX, He TR, Wang ZB (2019) Remediation of methylmercury in paddy soil by modified montmorillonite (In Chinese). Environmental. Science 40(11):5107–5113. https://doi.org/10.13227/j.hjkx.201901124
Hong YK, Kim JW, Lee SP, Yang JE, Kim SC (2020) Heavy metal remediation in soil with chemical amendments and its impact on activity of antioxidant enzymes in lettuce (Lactuca sativa) and soil enzymes. Appl Biol Chem 63(1):1–10. https://doi.org/10.1186/s13765-020-00526-w
Hossain MK, Strezov V, Chan KY, Nelson PF (2010) Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78(9):1167–1171. https://doi.org/10.1016/j.chemosphere.2010.01.009
Houben D, Pircar J, Sonnet P (2013) Heavy metal immobilization by cost-effective amendments in a contaminated soil: effects on metal leaching and phytoavailability. J Geochem Explor 123(12):87–94. https://doi.org/10.1016/j.gexplo.2011.10.004
Huang SH, Rao GS, Ashraf U, He LX, Zhang ZZ, Zhang HL, Mo ZW, Pan SG, Tang XR (2020) Application of inorganic passivators reduced Cd contents in brown rice in oilseed rape-rice rotation under Cd contaminated soil. Chemosphere 259:127404. https://doi.org/10.1016/j.chemosphere.2020.127404
Inkham R, Kijjanapanich V, Huttagosol P, Kijjanapanich P (2019) Low-cost alkaline substances for the chemical stabilization of cadmium-contaminated soils. J Environ Manage 250:109395. https://doi.org/10.1016/j.jenvman.2019.109395
Karlsson T, Elgh-Dalgren K, Bjorn E, Skyllberg U (2007) Complexation of cadmium to sulfur and oxygen functional groups in an organic soil. Geochim Cosmochim Acta 71(3):604–614. https://doi.org/10.1016/j.gca.2006.10.011
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Technol 37(1):189–195. https://doi.org/10.1021/es020799+
Kuang W, Facey GA, Detellier C, Casal B, Serratosa M, Ruiz-Hitzky E (2003) Nanostructured hybrid materials formed by sequestration of pyridine molecules in the tunnels of sepiolite. Chem Mater 15(26):4956–4967. https://doi.org/10.1021/cm034867i
Liang XF, Xu YM, Sun GH, Wang L, Sun YB, Sun Y, Qin X (2011) Preparation and characterization of mercapto functionalized sepiolite and their application for sorption of lead and cadmium. Chem Eng J 174(1):436–444. https://doi.org/10.1016/j.cej.2011.08.060
Liang XF, Xu YM, Wang L, Sun YB, Lin DS, Sun Y, Qin X, Wan Q (2013) Sorption of Pb2+ on mercapto functionalized sepiolite. Chemosphere 90(2):548–555. https://doi.org/10.1016/j.chemosphere.2012.08.027
Liang XF, Han J, Xu YM, Sun YB, Wang L, Tan X (2014) In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 235:9–18. https://doi.org/10.1016/j.geoderma.2014.06.029
Liu P, Qu WZ, Wang JX, Yan XL, Liao H (2006) Phosphorus availability and genesis and development of plant lateral roots (In Chinese). Plant Physiol J 03:395–400 CNKI:SUN:ZWSL.0.2006-03-001
Lu QQ, Zhang TT, Zhang W, Su CL, Yang YR, Hu D, Xu QS (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508. https://doi.org/10.1016/j.ecoenv.2017.09.015
Lu N, Li G, Han JC, Wang HY, Yang W, Sun YY (2019) Investigation of lead and cadmium contamination in mine soil and metal accumulation in selected plants growing in a gold mining area. Appl Ecol Env Res 17(5):10587–10597. https://doi.org/10.15666/aeer/1705-1058710597
Ma XM, Li LP, Zhou JG, Su CY, Wang K, Yuan SB, Zhou JG (2012) Adsorption of heavy metal ions using hierarchical CaCO3-maltose macroporous hybrid materials: adsorption isotherms and kinetic studies. J Hazard Mater 209-210:467–477. https://doi.org/10.1016/j.jhazmat.2012.01.054
Nelson DW, Sommers LE, Sparks DL (1996) Total carbon, organic carbon, and organic matter, methods of soil analysis. Part 3. Chem Methods, pp 961–1010
Qin XM, Nie ZJ, Liu HE, Zhao P, Qin SY, Shi ZW (2018) Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ Exp Bot 150:232–239. https://doi.org/10.1016/j.envexpbot.2018.03.024
Qin SY, Liu HG, Nie ZJ, Rengel Z, Gao W, Li C, Zhao P (2020) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180. https://doi.org/10.1016/S1002-0160(20)60002-9
Rusmin R, Sarkar B, Biswas B, Churchman J, Liu Y, Naidu R (2016) Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Appl Clay Sci 134:95–102. https://doi.org/10.1016/j.clay.2016.07.012
Sanderson P, Naidu R, Bolan N, Lim JE, Ok YS (2015) Chemical stabilisation of lead in shooting range soils with phosphate and magnesium oxide: synchrotroninvestigation. J Hazard Mater 299:395–403. https://doi.org/10.1016/j.jhazmat.2015.06.056
Shamshad S, Shahid M, Rafid M, Khalid S, Dumat C, Sabir M, Murtaza B, Farooq ABU, Shah NS (2018) Effect of organic amendments on cadmium stress to pea: a multivariate comparison of germinating vs young seedlings and younger vs older leaves. Ecotoxicol Environ Saf 151:91–97. https://doi.org/10.1016/j.ecoenv.2018.01.002
Shangguan Y, Qin Y, Yu H, Chen K, Wei Y, Zeng X, Zhou Z, Guo S, He S (2019) Lime application affects soil cadmium availability and microbial community composition in different soils. Clean Soil Air Water 47(6):1800416. https://doi.org/10.1002/clen.201800416
Sharma PK, Basu H, Kalkar VM, Avhad DK, Bassan MKT, Singhal RK (2009) Chemical and thermal characterization of serpentine group mineral. In: Symposium on Indian Analytical Science Congress, pp 97–98
Shi WY, Shao HB, Li H, Shao MA, Du S (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170(1):1–6. https://doi.org/10.1016/j.jhazmat.2009.04.097
Singh S, Eapen S, Dsouza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62(2):233–246. https://doi.org/10.1016/j.chemosphere.2005.05.017
Soares C, Carvalho MEA, Azevedo RA, Fidalgo F (2019) Plants facing oxidative challenges-a little help from the antioxidant networks. Environ Exp Bot 161:4–25. https://doi.org/10.1016/j.envexpbot.2018.12.009
Su CY, Li WG, Liu XZ, Zhang L (2014) Adsorption property of direct red brown onto acidthermal-modified sepiolite and optimization of adsorption conditions using Box-Behnken response surface methodology. Desalin Water Treat 52(4-6):880–888. https://doi.org/10.1080/19443994.2013.826856
Sun YB, Sun GH, Xu YM, Liu WT, Liang XF, Wang L (2016) Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J Environ Manage 166:204–210. https://doi.org/10.1016/j.jenvman.2015.10.017
Sun Q, Zhang JX, Qi WY, Li M (2020) Backfill mining alternatives and strategies for mitigating shallow coal mining hazards in the western mining area of China. Q J Eng Geol Hydrogeol 53(2):217–226. https://doi.org/10.1144/qjegh2017-026
Sun YQ, Xiao K, Wang XD, Lv ZH (2021) Evaluating the distribution and potential ecological risks of heavy metal in coal gangue. Environ Sci Pollut R 28(15):18604–18615. https://doi.org/10.1007/s11356-020-11055-w
Tang X, Li Q, Wu M, Lin L, Scholz M (2016) Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J Environ Manage 181:646–662. https://doi.org/10.1016/j.jenvman.2016.08.043
Tessier A, Campbell P, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851. https://doi.org/10.1021/ac50043a017
Valentin JL, Lopez-Manchado MA, Rodriguez A, Posadas P, Ibarra L (2007) Novel anhydrous unfolded structure by heating of acid pre-treated sepiolite. Appl Clay Sci 36(4):245–255. https://doi.org/10.1016/j.clay.2006.10.005
Wang X, Liang CH, Yin Y (2015) Distribution and transformation of cadmium formations amended with serpentine and lime in contaminated meadow soil. J Soil Sediment 15(7):1531–1537. https://doi.org/10.1007/s11368-015-1105-7
Wang YQ, Lei Z, Ye RB, Zhou W, Zhou Y, Zou ZK, Li JL, Yi LC, Dai ZY (2022) Effects of cadmium on physiochemistry and bioactive substances of muskmelon (Cucumis melo L.). Molecules 27(9):2913. https://doi.org/10.3390/molecules27092913
Xu Y, Liang XF, Xu YM, Qin X, Huang QQ, Wang L, Sun YB (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27(2):193–204. https://doi.org/10.1016/S1002-0160(17)60310-2
Xu C, Qi J, Yang W, Chen Y, Yang C, He YL, Yang J, Lin AJ (2019) Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci Total Environ 686:476–483. https://doi.org/10.1016/j.scitotenv.2019.05.330
Yadhu S, Anil KG, Navtej SB (2017) Bread wheat progenutors:Aegilops tauschii (DD genome) and Triticum dicoccoides (AABB genome) reveal differential antioxidative response under water stress. Physiol Mol Biol Plants 23(1):99–114. https://doi.org/10.1007/s12298-016-0409-4
Yoon DH, Choi WS, Hong YK, Lee YB, Kim SC (2019) Effect of chemical amendments on reduction of bioavailable heavy metals and ecotoxicity in soil. Appl Biol Chem 62(1):1–7. https://doi.org/10.1186/s13765-019-0460-2
You M, Huang YE, Lu J, Li CP (2016) Fractionation characterizations and environmental implications of heavy metal in soil from coal mine in Huainan, China. Environ Earth Sci 75(1):1–9. https://doi.org/10.1007/s12665-015-4815-7
Yusuke Y, Suzuki N, Sato K, Fukata N, Murakami M, Shimizu T (2009) Active mercury (II) ion removal: stoichiometrically controlled thiol-functionalized mesoporous silica by a mass production spray dry system. Bull Chem Soc Jpn 82(8):1039–1043. https://doi.org/10.1246/bcsj.82.1039
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67(1):44–50. https://doi.org/10.1016/j.chemosphere.2006.10.007
Zhang XY, Zhong TY, Liu L, Ouyang XY (2018) Impact of soil heavy metal pollution on food safety in China. PloS One 10(8):e0135182. https://doi.org/10.1371/journal.Pone.0135182
Zhang JH, Xu YN, Wu YG, Hu SH, Zhang YJ (2019) Dynamic characteristics of heavy metal accumulation in the farmland soil over Xiaoqinling gold-mining region, Shaanxi, China. Environ. Environ Earth Sci 78:1–11. https://doi.org/10.1007/s12665-018-8013-2
Zhang YH, Zeng H, Dong XW, Huang HL, Zheng Q, Dai ZH, Zhang ZW, Li ZY, Feng QM, Xiong SL, Cao MH, Tu SX (2021) In situ cadmium removal from paddy soils by a reusable remediation device and its health risk assessment in rice. Environ Technol Innov 23:101713. https://doi.org/10.1016/j.eti.2021.101713
Zubair M, Khan QU, Mirza N, Sarwar R, Khan AA, Baloch MS, Fahad S, Shah AN (2019) Physiological response of spinach to toxic heavy metal stress. Environ Sci Pollut Res 26(31):31667–31674. https://doi.org/10.1007/s11356-019-06292-7
Zulfiqar U, Farooq M, Hussain S, Maqsood M, Ishfaq M, Ahmad M, Anjum MZ (2019) Lead toxicity in plants: impacts and remediation. J Environ Manage 250:109557. https://doi.org/10.1016/j.jenvman.2019.109557
Acknowledgements
We thank the editors and reviewers for providing suggestions regarding the language and extensive discussion.
Funding
This work was supported by the National Natural Science Foundation of China (No. 42072201) and the University Synergy Innovation Program of Anhui Province (No. GXXT-2021-017).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yuchen Li: methodology, formal analysis, software, formal analysis, writing original draft, and visualization. Xing Chen and Liqun Zhang: data curation, validation, writing, review, and editing. Jie Hu and Chunlu Jiang: writing, review, and editing. Yongchun Chen and Shikai An: supervision. Liugen Zheng: conceptualization, resources, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors equally participate in the study.
Consent for publication
All authors allow the publication of the paper.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zheng, L., Chen, X. et al. Restoration effect of sulfhydryl-modified sepiolite on cadmium in contaminated soil and its effect on the growth of spinach (Spinacia oleracea L). Environ Sci Pollut Res 30, 66598–66609 (2023). https://doi.org/10.1007/s11356-023-27102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27102-1