Abstract
Purpose
Much farmland soil suffers severe cadmium (Cd) pollution. Many studies indicate that amended in-situ passivation economically, practically, and effectively repairs Cd-contaminated soil. However, few have researched the effect of a serpentine and lime combination on soil properties and the transformation of Cd formations.
Materials and methods
We conducted a laboratory incubation experiment. First, the soil samples were mixed with a 10 mg/kg level of Cd and maintained at stable equilibrium for 1 week. Second, the soil was amended in different ways and cultivated for 60 days. Ten treatments were applied: different doses of serpentine (S1, S2, S3), lime (L1, L2, L3), serpentine combined with lime (L2S1, L2S2, L2S3), and control treatment (CK). Finally, the soil was sampled at various culture periods for test analysis.
Results and discussion
The content of available Cd was significantly and negatively correlated with pH (r = −0.751**). The available Cd in the soils subject to S1–S3, L1–L3, and L2S1–L2S3 treatments were 13.85–25.84 %, 23.41–33.07 %, and 27.48–34.57 % lower, respectively, than those under the control treatment when cultured for 60 days. The content of exchangeable Cd generally decreased in all treatments. Opposite trends were observed in relation to Fe–Mn oxide, organic matter, and residual Cd amounts. The L2S3 treatment was optimal, with the highest decreasing amplitude (29.13 %) of exchangeable Cd content.
Conclusions
Applying serpentine and lime can reduce available Cd and convert bioavailable Cd into the potential biological available state and biological non-availability state Cd. On the whole, the combination of serpentine and lime achieves the best effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Much farmland soil suffers severe pollution by heavy metals, particularly cadmium (Cd) pollution. This pollution originates from sewage irrigation, the abuse of pesticides and fertilizers, and the accumulation of mining and industrial waste (Garau et al. 2007; Liu et al. 2009). According to a national contaminated soil survey communique released by Ministry of Environmental Protection of the People’s Republic of China and Ministry of Land and Resources of the People’s Republic of China in 2014 revealed that, among all arable land in China, 19.4 % soil survey points were above the national standards, and Cd was one of the most prevalent pollutants. Cd is generally considered to be a type of heavy metal element that is characterized by strong biological toxicity and mobility. Cd pollution is immutable, nondegradable, and persistent. Thus, it may seriously threaten terrestrial ecosystems and human health (Shi et al. 2009). In natural conditions, the biological availability and mobility of heavy metals are reduced through the mechanisms of precipitation, adsorption, and redox reactions. However, heavy metals are activated; such metals then severely pollute farmland or groundwater when their concentration is higher than the self-purification capacity of the soil (Brown et al. 2004). Therefore, heavy metal pollution is a global problem. Much research has been conducted on the remediation of heavy metal-contaminated soil to reduce the bioavailability of Cd and ensure the quality and safety of agricultural products. The remediation technologies of soil contaminated by heavy metals basically have three kinds, including physical remediation, chemical remediation, and biological remediation (Yao et al. 2012). Amended in situ passivation(Chemical fixation) as a kind of chemical remediation is considered to be an economical, practical, and effective method by which to rehabilitate heavy metal-contaminated soil (Álvarez-Ayuso and Garíia-Sánchez 2003; Brown et al. 2005; Mahabadi et al. 2007; Xu et al. 2010). Selecting valid and affordable amendments becomes the numerous scholars’ research direction. Many inorganic amendments, such as lime, sepiolite, zeolite, etc., have been proved to be very effective in the remediation of soil heavy metal (Li et al. 2009; Sun et al. 2013).
Lime is a common and effective modifier of heavy metal contaminated-soil. It can improve the properties of acidic soil and effectively reduce the concentration of the available Cd in soil (Wharfe 2004). Serpentine has a chemical formula of Mg6[Si4O10](OH)8 and is a hydrous magnesium silicate mineral with a large specific surface area, regular pore structure, and strong surface activity (Arijit et al. 2010; Li et al. 2003). It can increase the pH of the soil (Zhang et al. 2013) and improve the adsorption of heavy metals in the soil. The majority of previous studies have focused on individual applications of lime and serpentine to repair heavy metal-contaminated soil; however, few have examined the combined effect of these two inorganic modifiers on soil properties. This effect forms the transformation rule of heavy metals. The current work evaluates the efficiency of lime and serpentine with respect to immobilizing the Cd presence in contaminated acidic soil. To this end, we assess the following through an incubation experiment: (1) the individual effects of lime and serpentine and their combined influence on soil pH (potential of hydrogen) and available Cd contents; and (2) the influence of different treatments on the transformation and distribution of cadmium formations in soil.
2 Materials and methods
2.1 Test materials
Meadow soil samples were collected from depths of 0–20 cm in the agricultural fields of Xinmin City, Liaoning Province, China. The sampling time was October 2013. The field is an open vegetable plot. The type of tillage was two field vegetable rotations. The crops sequentially cultivated in a two-crops-per-year manner consisted of Chinese cabbage, carrot, lettuce, onion, cucumber, bean, mustard leaf, potato, and pimento. The tillage depth was 20 cm. The farmyard manure used was fowl dung, it was applied at a level of approximately 80 t/hm2 each year. N, P, and K were applied to the crops annually as urea (500 kg/hm2), (NH4)2HPO4 (1200 kg/hm2), and K2SO4 (1000 kg/hm2) fertilizers. The soil properties are shown in Table 1. The tested lime was analytical reagent-grade Ca(OH)2 produced by Sinopharm Group. Its pH, total nitrogen, total phosphorus, total cadmium, and total lead contents were 12.38, 0.02 g/kg, 0.004 g/kg, 0.16 mg/kg, and 0.52 mg/kg, respectively. The serpentine was obtained from Xiuyan County, Anshan City, Liaoning Province, China. A mineral fine grinder was used to grind it, after which it was passed through a 100-mesh sieve prior to use in the test. The pH value of serpentine was 9.55, total cadmium concentration was 0.32 mg/kg, and its CEC was 5.86 cmol/kg. The Brunauer–Emmett–Teller (BET) measurements revealed that serpentine had a surface area of 110.30 m2/g. The rock mineralogy was investigated using X-ray powder diffraction (XRD) (X’Pert Pro). The diagram is shown in Fig. 1. The test sample contains high purity antigorite, and its impurity content is low. Because the antigorite has a relatively stable alternating wavy structure, its X-ray diffraction peaks are sharper and the resolution of reflection line is higher. In XRD spectra, peak a, b, c are three characteristic diffraction peaks of antigorite; there are also two weaker peaks (peak d and e) which are the characteristic diffraction peaks of cordierite; others are some low content of impurities.
2.2 Soil incubation experiment
The soil samples were passed through a 20-mesh sieve after drying naturally. The plant root and stem were first removed and then mixed with 10 mg/kg of Cd (CdCl2·2.5H2O) and maintained at stable equilibrium for 1 week. Finally, soil samples weighing 50 g were placed into each plastic pot. The following treatments were conducted in these pots: control treatment(CK), different dosages of serpentine (S1, S2, and S3 at application rates of 1, 3, and 5 % of soil weight, respectively), lime (L1, L2, and L3 at application rates of 0.1, 0.2, and 0.4 % of soil weight, respectively), and a serpentine-lime combination (L2S1, L2S2, and L2S3 represent treatments containing 0.2 % lime soil and 1, 3, and 5 % serpentine). Each treatment was performed in triplicate, and the soil was thermostat-cultivated for 60 days at 25 ± 2 °C. Lost water was compensated with deionized water (no Cd detected) to reach 70 % of the field-water holding capacity. The humidity was maintained through daily watering throughout the entire cultivation process. Soil samples were obtained at different culture periods (0, 5, 15, 30, and 60 days) and then dried and passed through 20- and 100-mesh sieves prior to analysis.
2.3 Analytical methods
pH level was measured at a soil/water ratio of 1:2.5 using a pH meter (PB-10, Sartorius). Samples of different treatments were collected and evaluated for bioavailability, as well as for the distribution of the various chemical forms of Cd through single and sequential extractions. The available Cd content was extracted with DTPA (0.005 mol/LDTPA, 0.1 mol/LTEA, and 0.01 mol/LCaCl2), and various chemical forms of Cd were determined with the sequential extraction method developed by Tessier et al. (1979). All of the metal concentrations in the soil solutions were determined using an AA800 atomic absorption spectrometer.
2.4 Statistical analyses
All treatments were replicated three times in the experiments. The test data were analyzed and graphed using Microsoft Office Excel 2003. A one-way ANOVA was conducted for statistical analysis with SPSS19.0. When a significant (P < 0.05 or P < 0.01) difference was observed between treatments, multiple comparisons were made by the Duncan test.
3 Results and discussion
3.1 Effect of amendments on soil pH
The dynamic changes in soil pH level in each period are listed in Table 2. These levels were significantly higher with different amendments than in the CK treatment. In the same training period, the pH levels of the treatments varied and the soil pH value increased with the increase in the dosage of amendments. The application of serpentine lowered soil pH throughout the entire training process, but the pH level rebounded slightly was observed when the samples were cultured for 15 days. Soil pH level of S1–S3 treatments rised 0.15 to 0.77 units when cultured for 60 days, compared with CK treatment. The individual application of lime can increase soil pH level significantly in the initial stage. These levels then decrease considerably. Finally, they tend to stabilize. Soil pH level of L1–L3 treatments rised 1.01 to 1.83 units when cultured for 60 days, compared with CK treatment. The changing trend of the pH level of the combined serpentine and lime soil was consistent with that of the individual application of the lime treatment. Soil pH level of L2S1–L2S3 treatments rised 1.57 to 1.65 units when cultured for 60 days, compared with CK treatment, but its range of enhancement was insignificant compared with that of the L2 treatment.
The application of amendments increased soil pH level somewhat because of the low pH of the control sample. This result is consistent with that obtained in the research conducted by Cotter-Howells and Caporn (1996). The main reasons as to why serpentine can significantly enhance soil pH are as follows: much internal and external hydroxyl exists in the brucite octahedral layer of serpentine with strong chemical reactivity (Sharma et al. 2009). Hydrogen atoms can combine with a highly electronegative atom (e.g., O, F, and N) through a covalent bond to form hydroxyl, which in turn generates an alkaline solution. The combination of serpentine and lime altered pH significantly; this result was affected by both serpentine and lime. However, lime maybe made a greater contribution to enhancing the soil pH, because the pH level of lime was significantly higher than the serpentine, and with the increase of serpentine dosage in the combined treatment, the difference between the soil pH level was small. Therefore, in the case of the combination of serpentine and lime, the addition of serpentine had little effect on soil pH level.
3.2 Change in available Cd
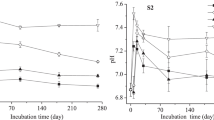
Figure 2 depicts the dynamic changes in DTPA-extracted available Cd content during different culture periods. The available Cd content in the CK treatment soil was constantly high (7.61–8.04 mg/kg), and remained in steady state throughout the entire incubation period. With the increase in the added amounts of different treatments and in incubation times, the available Cd content decreased significantly. The reduction rates of available Cd content increased from 11.71–19.92 %, 18.90–25.61 %, and 21.81–27.79 % (control group) to 13.85–25.84 %, 23.41–33.07 %, and 27.48–34.57 % according to the different dosages of serpentine, lime, and the combination, respectively. In general, the different amendments influence the available Cd content in soil as follows: serpentine + lime > lime > serpentine > CK.
Dynamic changes in the DTPA-extracted available Cd content. S1, S2, and S3 represent application rates of 1, 3, and 5 % soil weight of serpentine, respectively. L1, L2, and L3 denote the application rates of 0.1, 0.2, and 0.4 % soil weight of lime, respectively. L2S1, L2S2, and L2S3 correspond to the treatments containing 0.2 % lime soil and 1, 3, and 5 % of serpentine, respectively
Various treatments reduce the available Cd content in soil through different mechanisms. The structural unit layer of serpentine is composed of a silicon-oxygen tetrahedron hexagonal network layer (T layer) and an octahedral brucite layer (O layer) at a ratio of 1:1. These layers form a variable charge surface because of the magnesium alcohol (Mg-OH) hydroxyl in its mineral structure. Cd ions can be enhanced in the mineral surface on the basis of the complex hydroxyl reaction. They can also be adsorbed in the unit layer (Sharma et al. 2009). Thus, the available Cd content in soil decreases significantly. Lime can effectively reduce available Cd content because of different reasons. The negative charge on the surface of soil clay minerals and organic matter increased with the increase in pH level after lime application under alkaline conditions, along with surface adsorption point. Generating the hydroxy state of Cd [Cd (OH)+] is simple. This state has a stronger affinity with soil adsorption point than the free state of metal ions does (Naidu et al. 1994). As a result, available Cd content is reduced. Similar results were reported by Paola et al. (2005), who noted that adding lime to the heavy metal-contaminated soil can increase soil pH level. Moreover, the particle radius of Ca2+ is close to that of Cd2+, which can affect the chemical behavior of Cd2+ in soil considerably. This phenomenon ultimately promotes the available cadmium content. The combination of serpentine and lime can make two different mechanisms complement each other, resulting in positive interaction, thus achieving a better efficiency of Cd immobilization than single application, the actual experimental data also proved its good effects.
Soil pH has a critical effect on available Cd content. Studies have inferred that the immobilization of most heavy metals through surface complexation can be promoted when soil pH increases (Querol et al. 2006). Each unit of increase in pH also reduced heavy metal (e.g., Cd, Zn, and Ni) concentrations in the soil solution by approximately twofold (Madejón et al. 2006). pH level was positively correlated (r = −0.751**) with Cd DTPA concentration (P < 0.05). This finding is consistent with that of the research conducted by A. R. Jafarnejadi et al. (2013).
3.3 Distribution and transformation between different forms of Cd
Various forms of heavy metals in the soil change with the shift in soil environmental factors. This modification is typically in a state of dynamic balance (Tessier et al. 1979). Figure 3 shows the changing trend of various forms of soil Cd after applying the amendments. The concentration of the exchangeable Cd initially decreased differentially. The trend then stabilized. The concentration of carbonate-bound Cd increased early in the culture period (the Cd increase in the three dosages of the combination treatments was particularly significant) and then generally decreased in the middle of the culture period (15–60 days). The contents of the Fe-Mn oxide-bound, the organic matter-bound, and the residual Cd increased throughout the entire training process, but the increase in the organic matter-bound Cd was indistinct because its content accounted for only approximately 1.07 % of total Cd levels. Correlation analysis between soil exchangeable Cd and other forms of Cd were made; relevant analysis results showed the relationships were significant negative correlation (p < 0.01) between exchangeable Cd and carbonate-bound, Fe-Mn oxide-bound, organic matter-bound, residual Cd (r = −0.828**, −0.935**, −0.831**, −0.694**). From these results, we can conclude that the bioavailable Cd (exchangeable Cd) can be transformed into the potential biological available state (carbonate-bound, Fe-Mn oxide-bound, organic matter-bound Cd) and biological non-availability state Cd (residual Cd). This finding is consistent with that of the research conducted by Chen et al. (2000). Nonetheless, researchers reported that the application of amendments can increase the exchangeable Cd content and decrease residual Cd content (Liu et al. 2013). These results contradicted those obtained in the current research, and this discrepancy may be attributed to the different pollution levels of exogenous Cd (5 mg/kg) that promoted the microbial activity in the soil. Therefore, the type and dosage of amendments should be fully considered in future studies. Moreover, research should be conducted on the different contamination levels of soil to provide a solid foundation for the remediation of heavy metal-contaminated soil.
Contents of Cd fractions during the soil incubation period (n = 3). EXE-Exchangeable Cd, CAB-Carbonate Cd, OX-Fe–Mn oxide Cd, OM-Organic matter Cd, and RES-Residual Cd. S1, S2, and S3 represent the application rates of 1, 3, and 5 % soil weights of serpentine, respectively. L1, L2, and L3 denote the application rates of 0.1, 0.2, and 0.4 % soil weight of lime, respectively. L2S1, L2S2, and L2S3 correspond to the treatments containing 0.2 % lime soil and 1, 3, and 5 % of serpentine, respectively
Following 60 days of cultivation, the major chemical speciation of Cd in the control-tested soil was presented in an exchangeable-bound form with a proportion of 73.15 %. The Fe-Mn oxide- and carbonate-bound forms accounted for 11.31 and 8.37 %, respectively, whereas the sum of the organic matter- and residual-bound forms constituted only 7.17 % (Fig. 4). The results suggest that other treatments can reduce the proportion of exchangeable Cd more than the CK treatment can and that they do so in varying degrees. Specifically, the individual applications of serpentine and lime lowered this proportion by 12.74–20.72 % and 18.39–26.78 %, respectively, whereas the combination of serpentine and lime reduced the proportion by 22.57–29.13 %. Furthermore, the influence of different amendments on various Cd forms varied. The individual application of serpentine affected residual Cd considerably and increased its proportion by 11.79–14.44 %. The separate application of lime treatments significantly increased the proportions of carbonate- (5.09–9.35 %) and Fe-Mn oxide-bound (4.62–7.13 %) forms. The combination of serpentine and lime considered the effects of both types of treatments. Specifically, the proportions of three Cd forms described above increased by 6.88–8.51 %, 5.52–8.73 %, and 9.63–11.27 %, respectively. However, the Cd content in organic matter accounted for a small proportion of total Cd; thus, its effect on the distribution of various Cd forms was negligible.
Distribution of various Cd forms (60 days). EXE exchangeable Cd, CAB carbonate Cd, OX Fe–Mn oxide Cd, OM organic matter and Cd, RES residual Cd. S1, S2, and S3 represent the application rates of 1, 3, and 5 % soil weight of serpentine, respectively. L1, L2, and L3 denote the application rates of 0.1, 0.2, and 0.4 % soil weights of lime. L2S1, L2S2, and L2S3 correspond to the treatments containing 0.2 % lime soil and 1, 3, and 5 % of serpentine, respectively
A certain amount of octahedral vacancies is generated between two opposite brucite octahedral layers in the mineral structure unit layer of serpentine. Cd2+ can enter these lattice defects and form a co-ordination octahedral, thereby stabilizing the structure so that the content of residual Cd increases. The addition of lime increased soil pH significantly and promoted precipitation reactions by using the Cd2+ and CO3 2− in the soil. At the same time, coprecipitation can be generated through a Ca2+ (derived from lime hydrolysis) reaction with Cd2+, this process is also helpful to converting the exchangeable Cd to carbonate-bound Cd (Prasad 1995). When the soil pH level increases to more than the zero point charge of the colloidal Fe-Mn oxide, the adsorption capacity of heavy metal ions increases significantly, thus reducing the risk of heavy metal contamination. The combination of serpentine and lime can make the above-described various reactions proceed simultaneously, thus reducing the bioavailable Cd to the maximum extent.
4 Conclusions
The addition of serpentine, lime, and serpentine + lime reduces the available Cd in exogenously contaminated meadow soil by different degrees. The content of available Cd is significantly and negatively correlated with soil pH. The effect of remediation was strengthened with the increase in the amount of amendment added. In particular, high doses of the combination treatment displayed the best result.
The application of amendments can convert bioavailable Cd into the potential biological available state and biological non-availability state Cd. Individual applications of lime can significantly increase the content of carbonate-bound and Fe-Mn oxide-bound Cd, whereas individual applications of serpentine increase residual Cd content considerably. The combination treatment can combine the advantages of both individual treatments and improve the conversion and distribution of the various Cd forms. Therefore, the combined application of serpentine and lime can be considered in in situ restorations of Cd-contaminated soil.
References
Álvarez-Ayuso E, Garíia-Sánchez A (2003) Sepiolite as a feasible soil additive for the immobilization of cadmium and zinc. Sci Total Environ 305:1–12
Arijit S, Kadam RM, Rajeswari B et al (2010) Characterization of Indian serpentine by X-ray diffraction, photoacoustic spectroscopy and electron paramagnetic resonance spectroscopy. Appl Clay Sci 50:305–310
Brown S, Chaney R, Hallfrisch J, Ryan JA, Berti WR (2004) In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc and cadmium. J Environ Qual 33:522–531
Brown S, Christensen B, Lombi E, McLaughlin M, McGrath S, Colpaert J, Vangronsveld J (2005) An inter-laboratory study to test the ability of amendments to reduce the availability of Cd, Pb, and Zn in situ. Environ Pollut 138:34–45
Chen ZS, Lee GJ, Liu JC (2000) The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soil. Chemosphere 41:235–242
Cotter-Howells J, Caporn S (1996) Remediation of contaminated land by formation of heavy metal phosphates. Appl Geochem 11:335–342
Garau G, Castaldi P, Santona L, Deiana P, Melis P (2007) Influence of redmud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 142:47–57
Jafarnejadi AR, Sayyad G, Homaee M, Davamei AH (2013) Spatial variability of soil total and DTPA-extractable cadmium caused by long-term application of phosphate fertilizers, crop rotation, and soil characteristics. Environ Monit Assess 185:4087–4096
Li XJ, Wang LJ, Lu AH (2003) A discussion on activation mechanism of atom groups in serpentine. ACTA Petrol Minerol 22:386–390
Li H, Shi WY, Shao HB, Shao MA (2009) The remediation of the lead-polluted garden soil by natural zeolite. J Hazard Mater 169:1106–1111
Liu W, Yang YS, Li PJ et al (2009) Risk assessment of cadmium-contaminated soil on plant DNA damage using RAPD and physiological indices. J Hazard Mater 161:878–883
Liu LJ, Dong YH, Liu Y, Ge Y (2013) Effects of various amendments on the fractions of cadmium in a polluted soil. J Agro Environ Sci 32:1778–1785
Madejón E, de Mora AP, Felipe E, Burgos P, Cabrera F (2006) Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environ Pollut 139:40–52
Mahabadi AA, Hajabbasi MA, Khademi H, Kazemian H (2007) Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 137:388–393
Naidu R, Bolan NS, Kookana RS et al (1994) Ionic-strength and pH effects on the adsorption of cadmium and lead on the surface charge of soils. Eur J Soil Sci 45:419–429
Paola C, Laura S, Pietro M (2005) Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60:365–371
Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35:525–545
Querol X, Alastury A, Moreno N, Alvarez-ayuso E, Garía-Sánchez A, Cama J, Ayora C, Simón M (2006) Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 62:171–180
Sharma PK, Basu H, Kalkar VM, Avhad DK, Bassan MKT, Singhal RK (2009) Chemical and thermal characterization of serpentine group mineral. Symposium on Indian Analytical Science Congress, pp 97–98
Shi WY, Shao HB, Li H et al (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170:1–6
Sun YB, Sun GH, Xu YM, Wang L, Liang XF, Lin DS (2013) Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 193–194:149–155
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Wharfe J (2004) Hazardous chemicals in complex mixtures-a role for direct toxicity assessment. Ecotoxicology 13:81–88
Xu YM, Liang XF, Sun GH, Sun Y, Qin X, Wang L, Dai XH (2010) Effects of acid and heating treatments on the structure of sepiolite and its adsorption of lead and cadmium. Environ Sci 31:1560–1567
Yao ZT, Li JH, Xi HH, Yu CH (2012) Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci 16:722–729
Zhang ZM, Huang ZB, Shan RJ (2013) Adsorption and desorption of environmental materials on Pb, Cd and As in contaminated soil. Environ Eng 31:122–126
Acknowledgments
The research was supported by the National Natural Science Foundation of China (31171997), the National Science & Technology Pillar Program of China (2015BAD05B03), the Fifth Session of Geping Green Action—123 Project of Liaoning Environmental Research and Education (No. CEPF2012-123-1-4), and the Innovative Graduate Training Program of Shenyang Agricultural University of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ravi Naidu
Rights and permissions
About this article
Cite this article
Wang, X., Liang, CH. & Yin, Y. Distribution and transformation of cadmium formations amended with serpentine and lime in contaminated meadow soil. J Soils Sediments 15, 1531–1537 (2015). https://doi.org/10.1007/s11368-015-1105-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1105-7