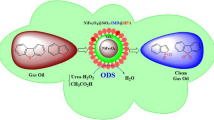
Abstract
Magnetic nanoparticles surrounded with a silica shell are useful materials to immobilize active agents on their surface. Here, a heteropolyacid-functionalized hybrid nanomaterial (NiFe2O4@SiO2-DETA@POM) was prepared and characterized by X-ray powder diffraction patterns (XRD), Fourier-transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA/DTG), vibrating sample magnetometer (VSM), the field emission scanning electron microscopy (FE-SEM), and the electron-dispersive X-ray spectroscopy (EDS). The synthesized hybrid nanostructure was used as a solid nanocatalyst in oxidative desulfurization (ODS) of real fuel and simulated gasoline samples. The ODS process of benzothiophene (BT) and dibenzothiophene (DBT) as model compounds in the presence of NiFe2O4@SiO2-DETA@POM and by using urea-hydrogen peroxide/acetic acid as a safer oxidizing agent was investigated. A good result was obtained by removing 97% of benzothiophene and 98% of dibenzothiophene. Also, 96% of the sulfur compounds were eliminated when the ODS process was tested on a real crude oil sample (600 ppm) under an optimized dosage of nanocatalyst, urea-hydrogen peroxide/acetic acid (0.1 g, 1 g/4 ml) at 50 ºC for 60 min. NiFe2O4@SiO2-DETA@POM could be recycled for five consecutive oxidation runs without significant deterioration in its catalytic activity. The UHP’s safety and efficiency as an oxidant, high removal efficacy, short transformation times, easy workup procedure, catalyst reusability, simple separation of nanocatalyst, green conditions, and environmental compatibility and sustainability. The obtained results prove that NiFe2O4@SiO2-DETA@POM is a suitable and efficient hybrid catalyst for the oxidative desulfurization of simulated and real fuels.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur exists in both organic and inorganic forms in fossil fuels. The harmful SOx gases emitted during fuel combustion led to the pollution of the atmosphere. Due to the high toxicity, corrosive nature, serious environmental problems, and human health risks of sulfur-containing impurities, the main concern in the refinery industry has been the production of fuels with an ultra-low level of sulfur content (Rezvani and Imani 2021). The sulfur content of fuels is removed conventionally by hydrodesulfurization (HDS). Dibenzothiophene (DBT) as a polyaromatic organosulfur compound and similar derivatives indicates lower activity in the hydrodesulfurization process (Rezvani and Fereyduni 2019). As a result, the HDS process operated under elevated pressures and temperatures to attain deep desulfurization, which increases the process cost. Due to the drawbacks of HDS, alternative desulfurization techniques such as adsorption desulfurization (ADS) (Yang et al. 2018), extraction desulfurization (EDS) (Jiang et al. 2016), bio-desulfurization (Boniek et al. 2015), ultrasound-assisted oxidation (Chen et al. 2010), and oxidative desulfurization (ODS) (Gu et al. 2017; Liu et al. 2020; Chen et al. 2010; Safa et al. 2017; Rezvani et al. 2018, Rezvani et al. 2020a; Rezvani and Fereyduni 2019; Rezvani and Imani 2021) have been considered and developed. Among them, oxidative desulfurization (ODS) should be the most promising method due to its high desulfurization efficiency [17–21]. In the ODS procedure, the sulfur-containing compounds are oxidized to corresponding sulfides or sulfones in the presence of active oxygen species. The oxidized products can be removed or extracted by a polar solvent. Oxidative desulfurization (ODS) is thoroughly studied and performed under atmospheric pressure at room temperature. So, the mild operating conditions of this method make it an economical technique for the removal of organosulfur compounds from fuels (Boniek et al. 2015). However, there is still demand for designing more efficient heterogeneous catalysts for use in the ODS process. Polyoxometalates (POMs) are inorganic clusters between oxygen and tens to hundreds of early transition metal atoms (e.g., M = V, Mo, W, Ti), and different types of heteroatoms (e.g., X = P, As, Si, Ge) can be found in their structure (Zhou et al. 2014). POMs have a large variety of compositions and sizes that find various applications in medicine, catalysis, and materials chemistry (Zhou et al. 2014). A specific number of electrons can be accepted or released by POMs without changing or decomposition in their structural frameworks, which makes them good candidates in electrocatalysis and redox chemistry (Ammam 2013). To overcome the self-aggregation, tedious separation, decreasing the catalytic activity of POMs, and requirement of sustainable processes, numerous researchers have considered the supporting of POMs on different solid materials to access heterogeneous catalysts (Ye and Wu 2016). Many effective methods have been developed to heterogeneity and immobilize the POMs, including encapsulation, intercalation, electrodeposition, impregnation, chemisorption, co-condensation sol–gel methods, hydrothermal, solvothermal, and the covalent grafting of POMs into the surface of various solid supports (Cherevan et al. 2020). Many research groups described the stabilization of POMs on different kinds of solid supports and their catalytic applications in organic synthesis, hydrolysis, photocatalysis, electrocatalysis, oxidation, and oxidative desulfurization that benefit from high selectivity and easy workup procedure (Jiang et al. 2016; Liu et al. 2020; Ye and Wu 2016; Craven et al. 2018; Rezvani and Fereyduni 2019; Cherevan et al. 2020; Rezvani et al. 2020b; Rezvani and Mirsadri 2020; Taghizadeh et al. 2020; Rezvani and Imani 2021). Magnetic nanoparticles (MNPs) do not have tedious separation procedures and are separated easily by a magnet. Besides, they have a high surface area for catalytic application (Injumpa et al. 2017). Coating the MNPs with a polymeric or inorganic matrix reduced their undesirable features such as aggregation and leaching under acidic conditions (Hozhabr Araghi and Entezari 2015). SiO2 has high chemical-thermal stability and can be modified by many kinds of functional groups. So, SiO2 is a suitable coating agent to increase the chemical and colloidal stability of MNPs (Gawande et al. 2013; Bodaghifard et al. 2018). As part of our ongoing endeavor to extend green organic reactions and efficient heterogeneous magnetic nanocatalysts (Bodaghifard 2019; Hamidinasab et al. 2020a; Bodaghifard and Shafi 2021), a new heterogeneous polyoxometalate-supported hybrid nanostructure was fabricated [MNPs@SiO2-DETA@POM]. In this work, POM was immobilized on NiFe2O4@SiO2 surface to act as an efficient heterogeneous nanocatalyst for the oxidative desulfurization process of diesel fuel. The thiophenic sulfur compounds (benzothiophene and dibenzothiophene) were dissolved in n-heptane to access a simulated fuel. Then, the catalytic proficiency of prepared hybrid nanomaterial [MNPs@SiO2-DETA@POM] on the ODS of typical and real gasoline was investigated. The prepared hybrid catalyst showed high catalytic activity in the oxidation of sulfur compounds. In addition, the catalyst could be reused easily due to its fast magnetic separation.
Experimental
All chemical materials were purchased from reputable chemical companies (Merck, Across, Sigma-Aldrich) and were used without further purification. Urea-hydrogen peroxide was prepared in the laboratory. The real crude oil sample was supplied by the South Pars Company (Iran). The crystal structure was carried out by Philips XPERT X-ray powder diffraction (XRD) diffractometer (Cu-Kα radiation and λ = 0.15406) in the range of Bragg angle 10–80 using 0.05° as the step length. The FT-IR spectra were recorded by Unicom Galaxy Series. The surface morphology and elemental content of MNPs@SiO2-DETA@POM were investigated on a Hitachi S-4160. The thermal stability of MNPs@SiO2-DETA@POM was investigated by a thermogravimetric analyzer with model Mettler TA4000 System under an N2 atmosphere at a heating rate of 10°Cmin−1. The magnetization and hysteresis loop for the synthesized magnetic nanoparticles were measured at room temperature using a 7300 VSM system with a maximum field of 10 kOe. The content of total sulfur in gasoline and model fuel was measured by an X-ray sulfur analyzer (Tanaca spectrometer RX 360 SH) using X-ray fluorescence (XRF) technique.
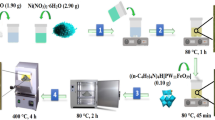
Preparation of nano-NiFe2O4@SiO2-DETA
The co-preparation method was applied to prepare the magnetic nickel-ferrite nanoparticles (Hamidinasab et al. 2020b). Hydrolysis of TEOS in a basic solution leads to the formation of a silica shell onto the NiFe2O4 nanoparticles surface (Ahadi et al. 2020). The reaction of nano-NiFe2O4@SiO2 with 3-chloropropyltrimethoxysilane produced the nano-NiFe2O4@SiO2-PrCl particles [32]. 0.5 g NiFe2O4@SiO2-PrCl was mixed with 25 mL dry toluene and sonicated for 30 min. Then 5.53 mmol (0.06 mL) of diethylenetriamine was added, and the suspension was heated at 80 °C for 24 h. The NiFe2O4@SiO2-DETA was separated by a magnet, eluted with toluene, and dried in an oven (80 °C).
Preparation of nano-NiFe2O4@SiO2-DETA@POM
The NiFe2O4@SiO2-DETA (0.3 g) was dispersed in deionized water (50 mL) for 30 min, then the solution of H4PMo11TiO40 (0.7 g in 20 mL deionized water) was appended to the suspension (30 °C). The suspension was mixed at room temperature for 24 h. The NiFe2O4@SiO2-DEA@POM was isolated by a magnet, rinsed with deionized water (3 × 10 mL), and dried in an oven (60 °C).
Batch ODS experiment of simulated gas oil and real gas oil
At first, 600 ppm of the sulfur content solution was prepared with the proper amount of model compounds (BT and DBT) in n-heptane (25 mL). Then 0.1 g of urea-hydrogen peroxide/acetic acid (1 g/4 mL), 0.1 g of NiFe2O4@SiO2-DETA@POM as a nanocatalyst, and 25 mL of the simulated or real fuel solution was added into the round bottom flask at 50 °C under ultrasound condition for 60 min. After completing the reaction, the solution was cooled to room temperature, and by using an external magnet the magnetic nanocatalyst was separated. Then, 4 mL of polar acetonitrile was poured to extract products of the oxidation process. A separation funnel was used to separate the immiscible mixture of n-heptane and water phase.
Result and discussion
Preparation and characterization of the hybrid nanostructure
Polyoxometalate-organic molecule tags were grafted onto the surface of nickel-ferrite nanoparticles to afford a new heterogeneous magnetic nanocatalyst. Scheme 1 shows the schematic steps for the preparation of MNPs@SiO2-DETA@POM nanostructure. FT-IR spectroscopy, FE-SEM, TEM, XRD patterns, EDS, EDS map scan, VSM, and TGA as standard techniques were used to characterize the prepared nanostructure.
The FT-IR spectra of bare NiFe2O4 MNPs, NiFe2O4@SiO2 MNPs, NiFe2O4@SiO2-PrCl, NiFe2O4@SiO2-DETA, and NiFe2O4@SiO2-DETA@POM was recorded in the range of 400–4000 cm−1 (Fig. 1). Strong absorption bands around 422 and 591 cm−1, corresponding to stretching vibration of Ni–O and Fe–O sites, are seen in the curve (a) (Sen et al. 2015). The formation of a silica layer was confirmed by the appearance of the absorption at 468 cm−1, 959 cm−1, 801 cm−1, and 1086 cm−1, that related to symmetric and asymmetric stretching vibration bands of Si–O–Si groups (curve b). H–O–H molecules adsorbed on the silica surface show a weak twisting vibration band at 1628 cm−1. The weak aliphatic C–H symmetric and asymmetric stretching vibrations at 2930 and 2984 cm−1 confirmed the anchoring of alkyl groups (curves c to e). The NH bending and C–N stretching bands appeared at 1632 and 1384 cm−1 in curves d and e. The bands observed in the range of 3200 to 3500 cm−1 correspond to the stretching vibration of N–H and OH groups. The stretching vibration of Mo–O–Mo and Mo = O in curve e appeared at 992 and 1000 cm−1, respectively. The P–O vibration appears at 1080 cm−1, which overlaps with the Si–O band (curve e). Figure 2 shows the FT-IR spectra of NiFe2O4@SiO2-DEA (a), NiFe2O4@SiO2-DEA@POM (b), and heteropolyacid (HPA) which indicate POM (HPA) grafted successfully on the surface of NiFe2O4@SiO2-DETA particles.
Figure 3 shows X-ray patterns of NiFe2O4 nanoparticles and NiFe2O4@SiO2-DETA@POM particles. Many diffraction peaks at (111), (220), (311), (222), (400), (422), (511), (440), and (533) Miller planes appeared and confirmed that the crystal structure of NiFe2O4 nanoparticles is cubic (Nejati and Zabihi 2012; Kim et al. 2014), which comply with standard JCPDS file (no. 44–1485) (Sen et al. 2015). Figure 6b shows 5 specific peaks in 2θ = 12.7, 23.3, 25.7, 29.7, 38.9 that related to the MoO3 bonds in heteropolyacid structure. The Scherrer equation (D = Kλ/βcosθ) was applied for the estimation of the average crystallite size of NiFe2O4@SiO2-DETA@POM. λ is the x-ray Cu wavelength, β is the width of the x-ray peak on the 2θ axis, which is measured as the line broadening at half the maximum intensity, θ is the Bragg angle, and K is the so-called Scherrer constant. The average crystallite size as calculated from the width of the peak at 2θ = 35.8° (311), is 23 nm, which is smaller than the range determined using FE-SEM and TEM analyses (Figs. 4 and 5).
Figure 4 shows the FE-SEM image of the hybrid nanostructure. In Fig. 4a, the spherical and regular shape of NiFe2O4@SiO2-DETA@POM nanoparticles is visible. The histogram chart in Fig. 4b shows 45 nm mean diameter for the nanoparticles. The nanoparticles’ morphology, shape, and size were elucidated by transmission electron microscopy (Fig. 5). The TEM analysis revealed that NiFe2O4@SiO2-DETA@POM magnetic nanoparticles have almost spherical shapes, and average particles size was detected as 30–60 nm.
The energy dispersive X-ray spectroscopy (EDS) of NiFe2O4@SiO2-DETA@POM particles shows the existence of Fe, Ni, Si, P, Mo, N, and C atoms (Fig. 6). The EDS map scan indicates that the elements are well dispersed on the surface of the hybrid nanostructure (Fig. 7). These results corroborate the prosperous construction of NiFe2O4@SiO2-DETA@POM nanomaterial.
The magnetic properties of NiFe2O4 and NiFe2O4@SiO2-DETA@POM nanoparticles were elucidated using a vibrating sample magnetometer (VSM) at room temperature (Fig. 8a and b). The S-like magnetization curves, the coincidence of the hysteresis loop (Hc), the low remanence (Mr), and the coercivity confirm the superparamagnetic behaviors of these hybrid materials. The magnetization of the sample could be completely saturated at high fields. Ms of the sample was dropped from 21.2 to 10.3 emu g−1 due to the formation of core/shell nanostructure.
The stability of NiFe2O4@SiO2-DETA@POM nanocatalyst was evaluated using TGA/DTG technique (Fig. 9). The initial 2% weight loss from r.t. to about 190 °C is related to the elimination of water and solvent molecules that are physically adsorbed and hydroxyl groups located on the surface of nanostructure. The decomposition of the organic tags within the nanostructure makes the major weight loss (8%W) beyond 190 °C to nearly 800 °C. Therefore, the catalyst shows good thermal stability and can be used safely under heterogeneous conditions.
Oxidation desulfurization of simulated fuels
After the characterization of NiFe2O4@SiO2-DETA@POM nanostructure, its effect on the removal of sulfur contaminants was investigated on the model fuel. In this work, during the oxidation-desulfurization (ODS) process, the urea-H2O2 was used as a safe oxidant accompanying acetic acid to in situ forming of active per-acid. This oxidation system can efficiently transform organic sulfurs into sulfoxide and sulfone compounds without producing residual products. Benzothiophene (BT) and dibenzothiophene (DBT) as sources of sulfur solution (600 ppm) were mixed with urea-hydrogen peroxide/acetic acid and NiFe2O4@SiO2-DETA@POM as a catalyst then the oxidation reaction was carried out at 50 °C under ultrasound condition. Extraction of oxidized compounds was done using a polar liquid (acetonitrile) to obtain a sample with low sulfur content. The following equation was afforded the percentage of sulfur removal (∆S%):
where Si represents the initial concentration and Sf is the final concentration of sulfur compounds (ppm), respectively. The XRF technique was applied to analyze the sulfur content. At a controlled temperature and time, the amount of nanocatalyst and oxidizer was optimized, and following that, the temperature and time were optimized.
The effect of nanocatalyst and oxidant dosage on the ODS efficiency
At first, different amounts of the NiFe2O4@SiO2-DETA@POM catalyst and urea-hydrogen peroxide/acetic acid as oxidant were determined for the ODS of HSCs present in model fuels with the concentration of 600 ppm at 30 °C in 30 min (Table 1). The effect of varying amounts of NiFe2O4@SiO2-DETA@POM catalyst (0–0.20 g) on desulfurization efficiency was investigated in the presence of constant amounts of urea-hydrogen peroxide/acetic (1 g) and acetic acid (1 mL). According to Table 1, entry 1, in the blank test (in the absence of a catalyst), only 10% of the BT and 13% of the DBT within 30 min were eliminated. As shown in Table 1, entry 3, for the optimal amount of nanocatalyst (0.1 g), 43% of the BT and 45% of the DBT removal were observed. Nevertheless, by increasing the amount of nanocatalyst to 0.2 g, no change in the sulfur removal was observed. The effect of various parameters on the ODS process is indicated in Table 1. Urea-hydrogen peroxide in the presence of an organic acid such as acetic acid was selected as an oxidizing agent. The results in Table 1, entry 10, confirm that the best sulfur removal percentage has occurred in the presence of 1 g of urea-hydrogen peroxide with 4 mL of acetic acid for DBT and BT compounds. By increasing the amount of the urea-hydrogen peroxide and acetic acid in sulfur removal, desulfurization efficiency increased rapidly from 0 g to 1 g for urea-hydrogen peroxide and 4 mL for acetic acid, and after that, no change has been observed. The literature survey revealed that in real gasoline, inorganic acids cannot dissolve; therefore, the sulfur removal percentage is lower than in organic acids (Rezvani et al. 2020b).
The effect of reaction temperature and time on the ODS efficiency
The two other influencing factors on the ODS efficiency of heterocyclic sulfur compounds (HSCs) are temperature and reaction time. The influence of temperature at 25, 30, 40, 50, and 60 °C and time 0 to 60 min on the desulfurization of sulfur compounds is demonstrated in Fig. 10a. As shown, the sulfur removal efficiency in HSCs increases with increasing the temperature from 25 to 50 °C in 30 min. However, increasing the temperature from 50 to 60 °C did not affect the oxidation efficiency. As shown in Fig. 10b, the ODS reaction was done at the optimum reaction temperature (50 °C) at various times. The best result occurred in 60 min for the desulfurization of HSCs. The oxidation reactivity was in the order of DBT > BT at the same temperature. The sulfur atom in BT has a lower electron density compared with DBT. So, the increase of the aromatic л-electron density has a positive effect on the oxidation efficiency of HSCs.
ODS process of real gas oil
Gas oil and its products have different sulfur compounds, such as complex and linear compounds. In real samples, the variety and the high content of sulfur compounds make the desulfurization process more complicated. To evaluate the desulfurization process, a real sample of gas oil containing 600 ppm of sulfur was supplied by South Pars Company (Iran). Optimized reaction conditions (0.1 g of nano-NiFe2O4@SiO2-DETA@POM and 1 g/4 mL of urea hydrogen peroxide/acetic acid at 50 °C) were employed for ODS of a real gas oil sample. The excellent result of 96% removal of sulfur from the real gas oil was obtained in 60 min.
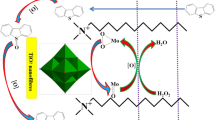
The proposed mechanism of the ODS process
A mechanism for the ODS process of HSCs in the presence of NiFe2O4@SiO2-DETA@POM nanocatalyst has been proposed in Scheme 2. The ODS process of the BT and DBT was done in n‐heptane as a nonpolar oil phase. After that, the nanocatalyst and oxidant were added, and the oxidation reactions were performed in the nonpolar/catalyst interface phase. During the ODS process, the urea-H2O2 reacts with acetic acid (CH3COOH) to in situ produce per-acetic acid (CH3CO3H). As one of the most considerable oxidizing systems, the catalytic sulfur oxidation mechanism is based on the peroxo-metal systems. The role of the metal atom (Mo) in the NiFe2O4@SiO2-DETA@POM nanocatalyst is to form peroxo-metal species that can convert organic sulfur to sulfones without forming residual products. The sulfur oxidation occurs via the electrophilic mechanism, so these electrophilic intermediate complex, MO2, were formed by the reaction of the peroxide oxygen in CH3CO3H with terminal metal‐oxygen groups (M = Ot) in the structure of sandwich‐type POM.
Catalyst recovery and reusability
The reusability of nanocatalysts is a main parameter in evaluating their performance. To study the recoverability of the NiFe2O4@SiO2-DETA@POM nanocatalyst for green chemistry view and commercial applications, at the end of the desulfurization process of real fuel, the nanocatalyst was separated by an external magnet, rinsed with ethanol, dried at 80 °C, and used again in the desulfurization process. The recycled nanocatalyst revealed the same catalytic performance without significant loss of activity after five runs (Fig. 11a). After the last run, the structural stability of the hybrid nanostructure was confirmed by the FT-IR spectrum (Fig. 11b), XRD patterns (Fig. 11c), and FE-SEM image (Fig. 11d).
A comparison of the performance of the prepared hybrid nanostructure (NiFe2O4@SiO2-DETA@POM) and CH3COOH/UHP oxidant system with other reported catalysts for the oxidative desulfurization of simulated fuels in literature is presented in Table 2. The presented data proves that NiFe2O4@SiO2-DETA@POM is a suitable and efficient hybrid catalyst for the oxidative desulfurization of simulated and real fuels.
Conclusions
This research work was focused on the synthesis of magnetic hybrid nanomaterial (NiFe2O4@SiO2-DETA@POM) as a new polyoxometalate-supported nanocatalyst in the presence of urea-H2O2/acetic acid as oxidant system to catalyze the oxidation-desulfurization reaction of the simulated and real gas oils. The sulfur-containing compounds were removed from simulated and real gas oils with high yields (96–98%). Moreover, this hybrid nanomaterial showed good reusability after five oxidation runs with only a slight deterioration in its activity. Several key characteristics such as high performance, oxidant safety, easy work-up, eco-friendly process, and simple separation of catalyst by an external magnet provide new insights into the applications of polyoxometalate-decorated magnetic nanoparticles in the effective removal of organic sulfur from gas oil. Finally, we suggest the effectiveness of this nanostructure by variation in heteropolyacid structure or magnetic nanoparticles could be investigated in future works.
Data availability
There is no additional data.
References
Ammam M (2013) Polyoxometalates: formation, structures, principal properties, main deposition methods and application in sensing. J Mater Chem A 1:6291–6312. https://doi.org/10.1039/C3TA01663C
Ahadi N, Bodaghifard MA, Mobinikhaledi A (2020) Preparation and characterization of a novel organic–inorganic hybrid nanostructure: application in synthesis of spirocompounds. Res Chem Intermed 46:3277–3294
Bodaghifard MA, Hamidinasab M, Ahadi N (2018) Recent advances in the preparation and application of organic– inorganic hybrid magnetic nanocatalysts on multicomponent reactions. Curr Org Chem 22:234–267. https://doi.org/10.2174/1385272821666170705144854
Bodaghifard MA, Shafi S (2021) Ionic liquid-immobilized hybrid nanomaterial: an efficient catalyst in the synthesis of benzimidazoles and benzothiazoles via anomeric-based oxidation. J Iran Chem Soc 18:677–687. https://doi.org/10.1007/S13738-020-02055-1
Boniek D, Figueiredo D, Dos Santos AFB, De Resende Stoianoff MA (2015) Biodesulfurization: a mini review about the immediate search for the future technology. Clean Technol Environ Policy 17:29–37. https://doi.org/10.1007/S10098-014-0812-X
Bodaghifard MA (2019) Palladium-melamine complex anchored on magnetic nanoparticles: a novel promoter for CC cross coupling reaction. J Organomet Chem 886:57–64. https://doi.org/10.1016/j.jorganchem.2019.02.010
Chen TC, Shen YH, Lee WJ et al (2010) The study of ultrasound-assisted oxidative desulfurization process applied to the utilization of pyrolysis oil from waste tires. J Clean Prod 18:1850–1858. https://doi.org/10.1016/J.JCLEPRO.2010.07.019
Cherevan AS, Nandan SP, Roger I et al (2020) Polyoxometalates on functional substrates: concepts, synergies, and future perspectives. Adv Sci 7:1903511. https://doi.org/10.1002/ADVS.201903511
Craven M, Xiao D, Kunstmann-Olsen C et al (2018) Oxidative desulfurization of diesel fuel catalyzed by polyoxometalate immobilized on phosphazene-functionalized silica. Appl Catal B 231:82–91. https://doi.org/10.1016/j.apcatb.2018.03.005
Gawande MB, Branco PS, Varma RS (2013) Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev 42:3371–3393. https://doi.org/10.1039/C3CS35480F
Gu Q, Wen G, Ding Y et al (2017) Reduced graphene oxide: a metal-free catalyst for aerobic oxidative desulfurization. Green Chem 19:1175–1181. https://doi.org/10.1039/C6GC02894B
Hamidinasab M, Bodaghifard MA, Mobinikhaledi A (2020a) Green synthesis of 1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile derivatives using a new bifunctional base–ionic liquid hybrid magnetic nanocatalyst. Appl Organomet Chem 34. https://doi.org/10.1002/AOC.5386
Hamidinasab M, Bodaghifard MA, Mobinikhaledi A (2020b) Synthesis of new, vital and pharmacologically important bis phthalazine-triones using an efficient magnetic nanocatalyst and their HF and NBO investigation. J Mol Struct 1200:127091. https://doi.org/10.1016/J.MOLSTRUC.2019.127091
HozhabrAraghi S, Entezari MH (2015) Amino-functionalized silica magnetite nanoparticles for the simultaneous removal of pollutants from aqueous solution. Appl Surf Sci 333:68–77. https://doi.org/10.1016/j.apsusc.2015.01.211
Injumpa W, Ritprajak P, Insin N (2017) Size-dependent cytotoxicity and inflammatory responses of PEGylated silica-iron oxide nanocomposite size series. J Magn Magn Mater 427:60–66. https://doi.org/10.1016/j.jmmm.2016.11.015
Jiang W, Jia H, Fan X et al (2019) Ionic liquid immobilized on magnetic mesoporous microspheres with rough surface: application as recyclable amphiphilic catalysts for oxidative desulfurization. Appl Surf Sci 484:1027–1034. https://doi.org/10.1016/j.apsusc.2019.03.341
Jiang W, Li H, Wang C et al (2016) Synthesis of ionic-liquid-based deep eutectic solvents for extractive desulfurization of fuel. Energy Fuels 30:8164–8170. https://doi.org/10.1021/ACS.ENERGYFUELS.6B01976/SUPPL_FILE/EF6B01976_SI_001.PDF
Kim HS, Kim D, Kwak BS et al (2014) Synthesis of magnetically separable core at shell structured NiFe2O4 at TiO2 nanomaterial and its use for photocatalytic hydrogen production by methanol/water splitting. Chem Eng J 243:272–279. https://doi.org/10.1016/j.cej.2013.12.046
Liu Y, Zuo P, Wang R et al (2020) Covalent immobilization of Dawson polyoxometalates on hairy particles and its catalytic properties for the oxidation desulfurization of tetrahydrothiophene. J Clean Prod 274:122774. https://doi.org/10.1016/j.jclepro.2020.122774
Nejati K, Zabihi R (2012) Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem Cent J 6:1–6. https://doi.org/10.1186/1752-153X-6-23/TABLES/2
Rezvani MA, Fereyduni M (2019) Synthesis of organic−inorganic hybrid nanocomposite polyoxometalate/metal oxide/CS polymer (PMnW11@TiO2@CS): nanocatalyst for oxidative desulfurization of real fuel. ChemistrySelect 4:11467–11474. https://doi.org/10.1002/SLCT.201902654
Rezvani MA, Hadi M, Mirsadri SA (2020a) Synthesis of new nanocomposite based on nanoceramic and mono substituted polyoxometalate, PMo11Cd@MnFe2O4, with superior catalytic activity for oxidative desulfurization of real fuel. Appl Organomet Chem 34:e5882. https://doi.org/10.1002/AOC.5882
Rezvani MA, Shaterian M, Aghmasheh M (2020b) Catalytic oxidative desulphurization of gasoline using amphiphilic polyoxometalate@polymer nanocomposite as an efficient, reusable, and green organic–inorganic hybrid catalyst. Environ Technol 41:1219–1231. https://doi.org/10.1080/09593330.2018.1526217
Rezvani MA, Imani A (2021) Ultra-deep oxidative desulfurization of real fuels by sandwich-type polyoxometalate immobilized on copper ferrite nanoparticles, Fe6W18O70⊂ CuFe2O4, as an efficient heterogeneous nanocatalyst. J Environ Chem Eng 9. https://doi.org/10.1016/j.jece.2020.105009
Rezvani MA, Mirsadri SA (2020) Synthesis and characterization of new hybrid inorganic–organic polymer nanocomposite as efficient catalyst for oxidative desulfurization of real fuel. Appl Organomet Chem 34:e5585. https://doi.org/10.1002/AOC.5585
Rezvani MA, Shaterian M, Akbarzadeh F, Khandan S (2018) Deep oxidative desulfurization of gasoline induced by PMoCu@MgCu2O4-PVA composite as a high-performance heterogeneous nanocatalyst. Chem Eng J 333:537–544. https://doi.org/10.1016/J.CEJ.2017.09.184
Safa M, Mokhtarani B, Mortaheb HR et al (2017) Oxidative desulfurization of diesel fuel using a brønsted acidic ionic liquid supported on silica gel. Energy Fuels 31:10196–10205. https://doi.org/10.1021/ACS.ENERGYFUELS.6B03505
Sen R, Jain P, Patidar R et al (2015) Synthesis and characterization of nickel ferrite (NiFe2O4) nanoparticles prepared by sol- gel method. Mater Today: Proceedings 2:3750–3757. https://doi.org/10.1016/j.matpr.2015.07.165
Taghizadeh M, Mehrvarz E, Taghipour A (2020) Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: a critical review. Rev Chem Eng 36:831–858. https://doi.org/10.1515/REVCE-2018-0058/XML
Yang E, Yao C, Liu Y et al (2018) Bamboo-derived porous biochar for efficient adsorption removal of dibenzothiophene from model fuel. Fuel 211:121–129. https://doi.org/10.1016/j.fuel.2017.07.099
Ye JJ, De Wu C (2016) Immobilization of polyoxometalates in crystalline solids for highly efficient heterogeneous catalysis. Dalton Trans 45:10101–10112. https://doi.org/10.1039/C6DT01378C
Zhou Y, Chen G, Long Z, Wang J (2014) Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-phase organic transformations. RSC Adv 4:42092–42113. https://doi.org/10.1039/C4RA05175K
Funding
The authors acknowledge the financial support of this work by the Research Council of Arak University.
Author information
Authors and Affiliations
Contributions
M.A Bodaghifard conceived, planned, and supervised the project. P. Bayat carried out the experiments and analyses. M. Hamidinasab advised the project and wrote the manuscript with support from M.A. Bodaghifard. All authors discussed the results and contributed to the final manuscript. M.A. Bodaghifard revised the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bodaghifard, M.A., Hamidinasab, M. & Bayat, P. Deep oxidative desulfurization of simulated and real gas oils by NiFe2O4@SiO2-DETA@POM as a retrievable hybrid nanocatalyst. Environ Sci Pollut Res 30, 57821–57832 (2023). https://doi.org/10.1007/s11356-023-26614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26614-0