Abstract
Immobilization and heterogenization of acidic/basic groups or organic tags on inorganic supports have found many important applications in recent years. In this investigation, a new hybrid organic–inorganic nanostructure was prepared. The structure of novel nanomaterial was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, thermogravimetric analysis, vibrating sample magnetometer, scanning electron microscopy and the energy-dispersive X-ray spectroscopy techniques. The spirocompounds, especially spiroperimidines and pyranopyrazoles, are important structures with diverse biological activities and many applications in industries. So, the prepared hybrid nanomaterial was used as an efficient catalyst in the one-pot, green and simple protocol for the synthesis of pyranopyrazole, spiropyranopyrazole and spiroperimidine derivatives. The structure of some compounds is characterized by FT-IR, 1H-NMR and 13C-NMR analyses. In addition, the proposed intermediates were synthesized and identified to prove the presented mechanism of the reaction. This hybrid nanomaterial is a recyclable and highly efficient heterogeneous catalyst and easily separated by an external magnet from the reaction media. The short reaction time, high efficiency, operational safety and use of environmentally benign solvent are some benefits of this procedure.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic nanoparticles have found many applications in various fields such as drug delivery, hyperthermia, magnetic resonance imaging (MRI), cell and stem cell separation, pharmacology, enzyme immobilization, magnetic fluids, sensor, pigments and catalysis area [1,2,3,4]. In recent years, magnetic nanoparticles have received considerable attention as the catalysts in organic chemistry because of exceptional properties including high surface area, thermal stability, low toxicity, strong activity, easy separation (by a magnet), the capability of surface modification, reusability and recyclability in the organic reaction for several runs without noticeable loss of catalytic activity [4,5,6,7,8,9,10]. The manganese ferrite (MnFe2O4), as a class of soft magnetic materials with distinct advantages like extraordinary saturation, low toxicity, excellent compatibility and superparamagnetic properties, has obtained particular interest [11].
Multicomponent reactions (MCRs) have attracted considerable attention in medicinal chemistry and modern organic synthesis because of time, energy and environmental conservation [12]. MCRs produce complex and diverse combinations of products and follow many principles of green chemistry, such as solvent-free or aqueous condition, atom economy, energy saving with short reaction times, waste diminish, high yields and selectivity [12,13,14]. Possible elimination of hazardous solvents and using solvent-free or aqueous conditions in MCRs have a notable impact on the protection of the environment [12,13,14].
Among the various classes of heterocyclic compounds, perimidines and pyranopyrazoles are important structures with diverse biological activities [15,16,17,18,19]. Furthermore, perimidine and pyranopyrazole derivatives have many applications in industries and agriculture, such as organic solar cells, plasma display panels, optical recording media, molecular switches, photochemical memory devices, insecticides, acaricides and herbicides [17, 20]. Spirocompounds with structural rigidity are bioactive materials like antimicrobial, hypoglycemic, antifungal, anti-inflammatory, anti-bacterial and anticancer [16, 21,22,23,24,25]. Due to their broad range of pharmacological activity and industrial applications, several methods have been reported for the synthesis of pyranopyrazole, spiropyranopyrazoles, spiroperimidines and other spirocompounds [26,27,28,29]. However, most of these methods experience limitations such as low yields, elevated temperatures, prolonged reaction time and unrecyclable catalysts. As a result, the development of green and straightforward methods for the synthesis of pyranopyrazole, spiropyranopyrazoles and spiroperimidines is necessary.
The heterogenization of homogeneous acidic and basic groups in catalytic reactions (such as covalently bound acidic or basic catalysts) typically allows for the easy separation and beneficial reuse of catalysts. The inorganic-supported catalysts, especially hybrid organic–inorganic magnetic nanomaterials, have been widely used in recent years that could be extremely useful in the catalysis area [6]. Hence, herein, we introduce a clean and efficient synthetic way for the preparation of pyranopyrazole, spiropyranopyrazoles and spiroperimidines gently using MNPs@SiO2-BTEAT-SO3H particles as a novel hybrid catalyst in mild and green conditions.
Results and discussion
Preparation of MNPs@SiO2-BTEAT-SO3H catalyst
The schematic preparation of MNPs@SiO2-BTEAT-SO3H particles is shown in Scheme 1. MnFe2O4 nanoparticles were prepared using the co-precipitation method [30]. Subsequently MnFe2O4 nanoparticles were coated with a silica layer via the Stöber procedure [30, 31]. Then, 3-aminopropyltriethoxysilane (APTS) was bound covalently to the OH groups on the MnFe2O4@SiO2 surface to gain MnFe2O4@SiO2-PrNH2 nanostructure. MnFe2O4@SiO2-PrNH2 nanoparticles in the presence of Et3N as a base were reacted with cyanuric chloride. MnFe2O4@SiO2-Pr-BTEAT particles were prepared from the reaction between MnFe2O4@SiO2-Pr-TDCl2 and triethanolamine. Ultimately, the reaction of MnFe2O4@SiO2-Pr-BTEAT with chlorosulfonic acid was performed, and the desired hybrid material (MNPs@SiO2-BTEAT-SO3H) was obtained.
Characterization of MNPs@SiO2-BTEAT-SO3H catalyst
The FT-IR spectroscopy
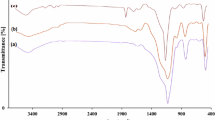
For all synthesized MNPs (Fig. 1), the characteristic band at 587 cm−1 is properly assigned to the Fe–O bond stretching vibration. Also, the H–O–H twisting vibration appeared at 1627 cm−1. The absorption bands at 459 cm−1 (rocking mode), 800 and 954 cm−1 (bending), and 1087 cm−1 (asymmetric stretching) are related to Si–O–Si groups and confirm the formation of SiO2 shell (Fig. 1b). The weak bands at 2974 and 2930 cm−1 are attributed to C–H stretching vibrations, establishing the covalent bonding of organic tags. The spectra of MNPs-TDCl2 and MNPs-BTEAT (Fig. 1d, e) show bands around 1450 to 1650 cm−1 that are typically corresponding to C=N, C=C, C–N and C–O bonds and confirm the heterocyclic ring attachment [27]. The appeared band around 700 cm−1 at the spectrum of MNPs-TDCl2 (Fig. 1d) is related to C–Cl stretching vibration. The elimination of this band in the spectrum of MNPs-BTEAT (Fig. 1e) promptly confirms the successful substitution of Cl groups with triethanolamine. Eventually, MNPs@SiO2-BTEAT-SO3H was characterized by the absorption band at 1201 cm−1, related to O=S=O moieties, that has overlapped with Si–O–Si stretching band [32] (Fig. 1f).
The XRD diffraction patterns
The XRD patterns for MnFe2O4 (a) and MNPs@SiO2-BTEAT-SO3H (b) are shown in Fig. 2. Diffraction peaks at 2θ = 30, 35, 43, 53, 57 and 63 can be assigned to the (200), (311), (400), (331), (422) and (333) miller planes of MnFe2O4, respectively. These data are granting to the standard MnFe2O4 sample (JCPDS card no. 1964-73) and instantly confirm the cubic spinel structure for MnFe2O4 nanoparticles. The broad peak at 2θ = 17–25 is related to the amorphous SiO2 shell on the surface of MnFe2O4 cores (Fig. 2b) [4]. The average size for MNPs@SiO2-BTEAT-SO3H is calculated to be about 29 nm [25].
The TGA-DTA and DTG analyses
The stability and functionalization of the MNPs@SiO2-BTEAT-SO3H catalyst were evaluated by the TGA, DTG and DTA analyses (Fig. 3). TGA curve outlined three steps of weight loss for the MNPs@SiO2-BTEAT-SO3H catalyst at 25–800 °C. The first step shows 3% weight loss at T < 200 °C that is related to the removal of physically absorbed solvent and surface hydroxyl groups. The second step shows 12% weight loss at 200–590 °C range, attributed to the decomposition of organic moieties in the hybrid nanomaterial. The weight loss (11%) at T > 600 °C shows the destruction of the silica layer and particle deformation. This mass loss shows that 0.13 mmol of H+ was loaded on 1 gr MNPs@SiO2-BTEAT-SO3H catalyst. These results assure the stability of the MNPs@SiO2-BTEAT-SO3H particles at below 200 °C that is in agreement with back titration results and establish that the organic groups are successfully loaded on the surface of MnFe2O4 nanoparticles. DTG analysis shows that MNPs@SiO2-BTEAT-SO3H nanoparticles were deformed at 580 °C. Moreover, the DTA diagram (blue curve) revealed that this gradual process was endothermic.
Magnetic properties (VSM analysis)
The magnetization curve of MnFe2O4 and MNPs@SiO2-BTEAT-SO3H is shown in Fig. 4. The magnetization hysteresis loops for samples (a) and (b) are S-like and show superparamagnetic properties for them. The saturation magnetization (Ms) is 33 Oe for MnFe2O4 (a) and 22 Oe for MNPs@SiO2-BTEAT-SO3H (b). Data show that Ms for MNPs@SiO2-BTEAT-SO3H is lower than the MnFe2O4 core. These direct results can amply confirm the successful preparation of MNPs@SiO2-BTEAT-SO3H structure.
The SEM, EDS and EDS map scan analyses
The size and morphology of MNPs@SiO2-BTEAT-SO3H particles were considered employing the FE-SEM analysis (Fig. 5a). The results show an average diameter of 20–35 nm and nearly spherical shape of nanoparticles that are in agreement with the obtained results from XRD patterns. The energy-dispersive X-ray spectroscopy (EDS) result, obtained from FE-SEM analysis, is shown in Fig. 5b. This analysis indicates clearly the presence of S, N, O, Si, Fe and Mn elements. The higher intensity of the Si peak compared with Mn and Fe peaks indicates that the MnFe2O4 nanoparticles were coated by SiO2 shell. Also, besides the column chart for quantitative results obtained from EDS analysis is shown in Fig. 5c. Based on the column chart, W% for S/N is 0.70 that is in agreement with the proposed structure of nanoparticles (S/N = 0.66). EDS map scanning spectra in Fig. 6 show the dispersion–aggregation phenomenon of Si, O, C, N, S, Fe, Mn and integration of the outer surface of MNPs@SiO2-BTEAT-SO3H particles. The different color spots over the dark background confirm the location of the relative elements on the external surface of MNPs@SiO2-BTEAT-SO3H particles. The blue, purple, red, white, orange, golden, green and integration spots in Fig. 6 corresponded to carbon (C), iron (Fe), manganese (Mn), nitrogen (N), sulfur (S), oxygen (O), silicon (Si) and integration, respectively.
The catalytic activity of MNPs@SiO2-BTEAT-SO3H
The catalytic activity of MNPs@SiO2-BTEAT-SO3H was carefully investigated in the synthesis of spiroperimidines, pyranopyrazoles and spiropyranopyrazoles (Schemes 2, 3). Primitively, the catalytic activity of MNPs@SiO2-BTEAT-SO3H was examined in the synthesis of spiroperimidine derivatives. The reaction of cyclohexanone (1 mmol) and naphthalene-1,8-diamine (1 mmol) as a model reaction was performed in various conditions (Table 1). The results show the most productive efficiency gained in the presence of 20 mg MNPs@SiO2-BTEAT-SO3H as a catalyst and EtOH/H2O as solvent at reflux condition (Table1, Entry 6). Also, the presence of a catalyst is necessary for reaction performance (Table1, Entry 5). Finally, the model reaction was performed in the presence of other MNPs (MnFe2O4, MnFe2O4@SiO2, MnFe2O4@SiO2-PrNH2, MnFe2O4@SiO2-Pr-TDCl2 and MnFe2O4@SiO2-Pr-BTEAT) as catalysts and the results are shown in Table 1 (Entries 9–13). After optimizing the conditions, the synthesis of spiroperimidine derivatives was carried out and high yields (73–94%) of the desired products obtained (Scheme 2, Table 2). All the products were carefully characterized by possible comparison of physical constants, elemental analysis, FT-IR, 1H-NMR and 13C-NMR spectroscopy (SI). In continuation, this work was compared with other reported procedures for the synthesis of spiroperimidine derivatives (Table 3). The results confirm MNPs@SiO2-BTEAT-SO3H is a suitable and efficient catalyst in the synthesis of spiroperimidines with respect to time and yield.

At the second step, the catalytic activity of MNPs@SiO2-BTEAT-SO3H was studied in the model reaction of 4-chlorobenzaldehyde, malononitrile, ethyl acetoacetate and hydrazine hydrate to afford the desired pyranopyrazole product (Table 4). The reaction was performed in the presence of varying amounts of catalyst, various solvents and different temperatures (Table 4). The results show the best efficiency gained in the presence of 15 mg MNPs@SiO2-BTEAT-SO3H as a catalyst and H2O as solvent at room temperature (Table 4, Entry 4). Moreover, the presence of a catalyst is necessary for reaction performance, and the catalytic activity of MNPs@SiO2-BTEAT-SO3H is superior in comparison with other catalysts (Table 4, Entries 2, 8–12).

After optimizing the reaction, various carbonyl compounds were used in the reaction and the desired products were obtained in high yields within short times (Scheme 3, Table 5). Initially, various aromatic aldehydes were used as starting carbonyl compounds and the equivalent products were produced in excellent yields (Table 5, Entries 1–7). The presented results confirm that the reactions with aromatic aldehydes bearing electron-withdrawing substituents are performed in shorter times than aldehydes including electron-donating substituents. At the second step, various ketones including indoline-2,3-dione derivatives, 1H-indene-1,2,3-trione and 11H-indeno[1,2-b]quinoxalin-11-one were inserted in the designed reaction and the desired valuable spiroproducts were acquired in good yields (Table 5, Entries 8–17). To the best of our knowledge, the product 11g, 13a and 14 are synthesized for the first time. All the products were carefully characterized by possible comparison of physical constants, elemental analysis, FT-IR, 1H-NMR and 13C-NMR spectroscopy (SI). The comparison of MNPs@SiO2-BTEAT-SO3H catalyst with other reported catalysts in the model reaction is provided in Table 6. The MNPs@SiO2-BTEAT-SO3H displays good or higher performance in comparison with other catalysts.
Reusability of recyclability the MNPs@SiO2-BTEAT-SO3H catalyst
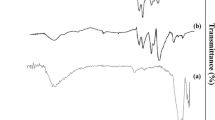
In the green synthetic processes, the reusability and recyclability of catalysts are essential. Therefore, the reusability of MNPs@SiO2-BTEAT-SO3H was evaluated in the model reactions. After completion, the solvent was evaporated, the precipitate was dissolved in ethanol and the catalyst was separated by a magnet. The recovered catalyst was reused in the ensuing reaction without further activation. As shown in Fig. 7a, the catalyst could be recycled without any change in catalytic activity in five runs. The FT-IR spectra (Fig. 7b) and XRD patterns (Fig. 7c) of fresh and reused catalysts are compared. The reused catalyst receives no noticeable change in structure. Also, Fig. 7d indicates an easy separation of the hybrid catalyst by an external magnet (a) and re-dispersion of MNPs@SiO2-BTEAT-SO3H after removal of the magnet (b). These results confirm that the MNPs@SiO2-BTEAT-SO3H has excellent stability and performance in organic reactions.
Plausible mechanism for the synthesis of products in the presence of MNPs@SiO2-BTEAT-SO3H as a catalyst
A plausible pathway for the synthesis of spiroperimidine derivatives in the presence of the MNPs@SiO2-BTEAT-SO3H catalyst is shown in Scheme 4. Initially, the carbonyl group is typically activated by MNPs@SiO2-BTEAT-SO3H catalyst, and a nucleophilic attack of the 1,8-diaminonaphthalene on the activated carbonyl group produces intermediate A. The imine bond attacked by NH2, and intermediate B performed. At last, the possible removal of H+ afforded the desired spiroperimidine [27].
A proposed mechanism for the synthesis of pyranopyrazole derivatives in the presence of MNPs@SiO2-BTEAT-SO3H is described in Scheme 5. Initially, a condensation of hydrazine hydrate and ethyl acetoacetate produces the 3-methyl-1H-pyrazol-5-ol (intermediate A). The Knoevenagel condensation between malononitrile and activated carbonyl compound produced the arylidenemalononitrile (intermediate B). Subsequently, the Michael addition takes place between intermediate A and intermediate B and the successive tautomerization resulted in the pyranopyrazole formation [25, 45]. For consolidation, the intermediates A and B were synthesized and identified (SI). The typical reaction of as-prepared intermediates A and B successfully produced the desired product.
Conclusion
A new hybrid inorganic–organic nanocatalyst (MNPs@SiO2-BTEAT-SO3H) as a recyclable and highly efficient heterogeneous catalyst was prepared. The FT-IR, XRD, VSM, EDS, EDS map and SEM analysis techniques have been confirmed the successful preparation of hybrid nanostructure and its excellent stability. The application of MNPs@SiO2-BTEAT-SO3H in the organic synthesis confirms its efficiency as a suitable heterogeneous catalyst in the synthesis of spiroperimidines, pyranopyrazole and spiropyranopyrazole derivatives. The MNPs@SiO2-BTEAT-SO3H has high acidity. In addition, we think that the long organic tags grafting and triazine ring moiety resulted in an intense performance of MNPs@SiO2-BTEAT-SO3H in organic solvents and aqueous medium. The advantages of this work are high yields, short reaction times, use of a green solvent, easy workup procedure, catalyst reusability without significant diminishing in its efficiency and simple separation of hybrid nanocatalyst by applying an external magnet.
References
K. Li, Y. Fan, Y. He, L. Zeng, X. Han, Y. Yan, Sci. Rep. 7, 1 (2017)
A.A. Alqadami, M. Naushad, Z.A. Alothman, A.A. Ghfar, Appl. Mater. 9, 36026 (2017)
A.A. Alqadami, M. Naushad, M.A. Abdalla, T. Ahamad, Z.A. Alothman, S.M. Alshehri, A.A. Ghfar, J. Clean. Prod. 156, 426 (2017)
Z. Rashid, H. Naeimi, A.-H. Zarnani, M. Nazari, M.-R. Nejadmoghaddam, R. Ghahremanzadeh, RSC Adv. 6, 36840 (2016)
D. Wang, D. Astruc, Chem. Rev. 114, 6949 (2014)
M.A. Bodaghifard, M. Hamidinasab, N. Ahadi, Curr. Org. Chem. 22, 234 (2018)
H. Tombuloglu, G. Tombuloglu, Y. Slimani, I. Ercan, H. Sozeri, A. Baykal, Environ. Pollut. 243, 872 (2018)
T. Kang, F. Li, S. Baik, W. Shao, D. Ling, T. Hyeon, Biomaterials 136, 98 (2017)
M. Jacintha, P. Neeraja, M. Sivakumar, K. Chinnaraj, J. Supercond. Novel. Magn. 30, 237 (2017)
F. Bonyasi, M. Hekmati, H. Veisi, J. Colloid, Interface Sci. 496, 177 (2017)
A.K. Rathi, R. Zboril, R.S. Varma, M.B. Gawande, Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation (ACS Publications, Washington, 2016), pp. 39–78
F. Chen, J. Zheng, M. Huang, Y. Li, Res. Chem. Intermed. 41, 5545 (2015)
H. Sachdeva, S. Khaturia, MOJ. Biorg. Org. Chem. 1, 239 (2017)
P.N. Sudhan, M. Ghashang, S.S. Mansoor, Beni Seuf. Univ. J. Appl. ScI. 5, 340 (2016)
F. Behbahani, F.M. Golchin, J. Taibah. Univ. Sci. 11, 85 (2017)
R. Kumar, N. Yadav, R. Lavilla, D. Blasi, J. Quintana, J.M. Brea, M.I. Loza, J. Mestres, M. Bhandari, R. Arora, Mol. Divers. 21, 533 (2017)
A. Moshtaghi Zonouz, D. Moghani, Synth. Commun. 46, 220 (2016)
V.V. Patil, G.S. Shankarling, Catal. Commun. 57, 138 (2014)
N.A. Harry, R.M. Cherian, S. Radhika, G. Anilkumar, Tetrahedron Lett. 60, 150946 (2019)
B.B.F. Mirjalili, M. Imani, J. Chin. Chem. Soc Taip. 66, 1542 (2019)
Q. Niu, J. Xi, L. Li, L. Li, C. Pan, M. Lan, L. Rong, Tetrahedron Lett. 60, 151181 (2019)
Z. Wang, L. Gao, Z. Xu, Z. Ling, Y. Qin, L. Rong, S.-J. Tu, Tetrahedron 73, 385 (2017)
H. Naeimi, S. Lahouti, RSC Adv. 7, 2555 (2017)
B. Maleki, N. Nasiri, R. Tayebee, A. Khojastehnezhad, H.A. Akhlaghi, RSC Adv. 6, 79128 (2016)
G.M. Ziarani, M. Rahimifard, F. Nouri, A. Badiei, J. Serb. Chem. Soc. 80, 1265 (2015)
M. Abdi, S. Rostamizadeh, N. Zekri, Polycycl. Aromat. Compd. 39, 413 (2019)
M.A. Bodaghifard, S. Asadbegi, Z. Bahrami, J. Iran. Chem. Soc. 14, 365 (2017)
H. Kefayati, S.J. Bazargard, P. Vejdansefat, S. Shariati, A.M. Kohankar, Dyes Pigm. 125, 309 (2016)
A. Javid, A. Khojastehnezhad, H. Eshghi, F. Moeinpour, F.F. Bamoharram, J. Ebrahimi, Org. Prep. Proced. Int. 48, 377 (2016)
N. Ahadi, M.A. Bodaghifard, A. Mobinikhaledi, Appl. Organomet. Chem. 33, e4738 (2019)
J.-Q. Zhu, X.-J. Zhang, S.-W. Wang, G.-S. Wang, P.-G. Yin, RSC Adv. 6, 88104 (2016)
J. Li, H. Zhao, X. Hou, W. Fa, J. Cai, Micro. Nano Lett. 12, 53 (2017)
M.A. Bodaghifard, N. Ahadi, Iran. J. Catal. 6, 377 (2016)
V.D. Patil, K.P. Patil, N.R. Sutar, P.V. Gidh, Chem. Int. 3, 195 (2017)
A.R. Moosavi-Zare, M.A. Zolfigol, R. Salehi-Moratab, E. Noroozizadeh, J. Mol. Catal. A. Chem. 415, 144 (2016)
R.-Y. Guo, Z.-M. An, L.-P. Mo, S.-T. Yang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, Tetrahedron 69, 9931 (2013)
K.G. Patel, N.M. Misra, R.H. Vekariya, R.R. Shettigar, Res. Chem. Intermed. 44, 289 (2018)
A.S. Waghmare, S.S. Pandit, D.M. Suryawanshi, Comb. Chem. High. Throughput Screen. 21, 254 (2018)
M. Fatahpour, F.N. Sadeh, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, J. Iran. Chem. Soc. 14, 1945 (2017)
T. Liu, C.B. Li, Y.Q. Yu, D.Z. Xu, ChemistrySelect 2, 2917 (2017)
S.F. Hojati, A. Amiri, S. Mohamadi, N. MoeiniEghbali, Res. Chem. Intermed. 44, 2275 (2018)
P. Farokhian, M. Mamaghani, N.O. Mahmoodi, K. Tabatabaeian, J. Iran. Chem. Soc. 15, 11 (2018)
A.R. Moosavi-Zare, H. Goudarziafshar, K. Saki, Appl. Organomet. Chem. 32, e3968 (2018)
Y.A. Tayade, S.A. Padvi, Y.B. Wagh, D.S. Dalal, Tetrahedron Lett. 56, 2441 (2015)
A. Upadhyay, L.K. Sharma, V.K. Singh, R. Dubey, N. Kumar, R.K.P. Singh, Tetrahedron Lett. 58, 1245 (2017)
Acknowledgements
This study was supported by the Research Council of Arak University (Grant No. 94Ah.Ph.D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no other conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahadi, N., Bodaghifard, M.A. & Mobinikhaledi, A. Preparation and characterization of a novel organic–inorganic hybrid nanostructure: application in synthesis of spirocompounds. Res Chem Intermed 46, 3277–3294 (2020). https://doi.org/10.1007/s11164-020-04130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04130-x