Abstract
Different techniques have been used to alleviate metal toxicity in medicinal plants; accordingly, nanoparticles (NPs) have a noticeable interest in modulating oxidative stresses. Therefore, this work aimed to compare the impacts of silicon (Si), selenium (Se), and zinc (Zn) NPs on the growth, physiological status, and essential oil (EO) of sage (Salvia officinalis L.) treated with foliar application of Si, Se, and Zn NPs upon lead (Pb) and cadmium (Cd) stresses. The results showed that Se, Si, and Zn NPs decreased Pb accumulation by 35, 43, and 40%, and Cd concentration by 29, 39, and 36% in sage leaves. Shoot plant weight showed a noticeable reduction upon Cd (41%) and Pb (35%) stress; however, NPs, particularly Si and Zn improved plant weight under metal toxicity. Metal toxicity diminished relative water content (RWC) and chlorophyll, whereas NPs significantly enhanced these variables. The noticeable raises in malondialdehyde (MDA) and electrolyte leakage (EL) were observed in plants exposed to metal toxicity; however, they were alleviated with foliar application of NPs. The EO content and EO yield of sage plants decreased by the heavy metals but increased by the NPs. Accordingly, Se, Si, and Zn NPS elevated EO yield by 36, 37, and 43%, respectively, compared with non-NPs. The primary EO constituents were 1,8-cineole (9.42–13.41%), α-thujone (27.40–38.73%), β-thujone (10.11–12.94%), and camphor (11.31–16.45%). This study suggests that NPs, particularly Si and Zn, boosted plant growth by modulating Pb and Cd toxicity, which could be advantageous for cultivating this plant in areas with heavy metal–polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metalloids and trace metals can pollute soils through industrial emissions, metal waste, pesticides, coal combustion residues, mine tailings, leaded gasoline, paints, chemical fertilizers, wastewater irrigation, atmospheric deposition, and sewage sludge (Beduk et al. 2022). Any metallic chemical substance with a relatively high density that is poisonous and toxic even at low concentrations is considered a trace metal (Khosropour et al. 2022). Metals endanger the food chain and human health by contaminating food and water. Cadmium (Cd) is the most toxic trace metal to living organisms and humans, with biological activity in both terrestrial and aquatic organisms (Nasirzadeh et al. 2022). Agriculture and industrial development have resulted in a higher concentration of Cd in agricultural soils (Kicińska et al. 2022). Cadmium is easily absorbed by plant roots and transferred to aerial tissues due to its high mobility, causing primary problems in animals and humans (Nasirzadeh et al. 2022). It builds up in various organs of the human body, particularly the kidneys (Wang et al. 2021a). Because Cd is similar to calcium in terms of charge, ionic radius, and chemical behavior, it can easily transfer to the human body and be stored at a high level in various organs (Gutsch et al. 2018). Cadmium toxicity damages the liver and bones and can reduce Ca absorption in the body. Soil lead (Pb) concentrations greater than 30 ppm are toxic and inhibit biochemical processes and plant growth (Saleh et al. 2020). Plants exposed to Pb accumulate more reactive oxygen species (ROS), which causes cell death. Lead toxicity increases the production of free radicals, which increases lipid peroxidation and impairs plant nutrient balance (Baig et al. 2020).
Various methods have been developed to reduce metal toxicity in plants. Foliar nutrient application is an effective method for preventing trace metal accumulation in plant tissues. Nanoparticles (NPs) are a type of nutrient application that is highly resistant to metal stresses. Because of their small size and high active surface area, they can easily penetrate plant cells (Afshari et al. 2021). Among the nutrients, silicon (Si) is a quasi-essential element for plants, with a plentiful supply in soils, primarily in the form of insoluble silicates. As a soluble form of monosilicic acid, it can be absorbed by plants and stimulate physiological and biochemical processes. Zinc (Zn) is a trace element that is essential for plant growth and development and has an impact on plant physiology and development. Zinc also enhances important biochemical processes in plants such as photosynthesis and sugar transport (Aram et al. 2021). Furthermore, selenium (Se) is a beneficial micronutrient for humans and animals that acts as a cofactor and coenzyme in the human body’s immune system (Ikram et al. 2021). As a result, NPs play an important role in improving plant physiology in response to environmental stresses (Nasirzadeh et al. 2022).
Mint (Lamiaceae) is a family of aromatic and medicinal plants known for their high nutritional and pharmacological value. Sage (Salvia officinalis L.) is the most well-known species in this family, native to the Middle East and Mediterranean regions but now found all over the world (Ghorbani and Esmaeilizadeh 2017). Sage is anticancer, antinociceptive, antimicrobial, antimutagenic, antidementia, hypoglycemic, and hypolipidemic (Ghorbani and Esmaeilizadeh 2017; Jakovljevi et al. 2019). Furthermore, sage essential oil (EO) is economically significant due to key compounds such as thujone and 1,8-cineole (Kulak et al. 2020). As a result, finding methods to maintain its growth, EO quality, and EO quantity on contaminated soils is critical.
Recently, there is an interest in the use of NPs to alleviate the metal toxicity in medicinal plants and crops. Accordingly, the positive effects of Si (Siddiqui et al. 2020; El-Saadony et al. 2021; Memari-Tabrizi et al. 2021), Se (Sardar et al. 2022; Nasirzadeh et al. 2022; Babashpour-Asl et al. 2022), and Zn (Venkatachalam et al. 2017; Sharifan et al. 2020) have been addressed at heavy metal–induced toxicity. However, little information is reported on comparing these NPs in alleviating metal toxicity in medicinal plants. Therefore, the present study was conducted to discover Se, Si, and Zn NPs in alleviating Pb and Cd stress on metal accumulation, growth, lipid peroxidation, and EO quality and quantity of sage plants.
Materials and methods
Plan materials and experimental design
Sage (S. officinalis L.) seeds were purchased from the Pakan Bazr company in Iran. Soils from an unpolluted area in an agricultural field were chosen and sterilized in an oven. The experimental soil was a sandy loam with pH: 7.06, and EC: 0.97 dS m−1. N: 0.21%, P: 13.1 mg kg−1; K: 276 mg kg−1, total Cd: 0.12 mg kg1, Pb: 11.23 mg kg1.
The factorial experiment with soil metal stress and foliar application of NPs was conducted in a completely randomized design (CRD) in five replicates. To test metals toxicity, the soils were polluted by chloride cadmium (CdCl2) at 40 mg kg−1 soil for Cd stress and by lead nitrate (Pb NO3) at 400 mg kg−1 soil for Pb stress.
Metal concentration
To determine Cd and Pb concentrations in leaves and roots, 0.2 g of dry matter was mixed with 4 ml of 65% nitric acid (NO2) and kept at room temperature for 24 h. Following that, the sample was placed in a 90°C oven for 5 h to allow the NO2 to evaporate. After cooling, the sample was filtered through filter paper and the volume was increased to 10 ml with distilled water. The concentration of Cd and Pb was determined using ICP/MS (Khosropour et al. 2019).
Plant weight
The plants were completely cut from the bottom of the stem and dried in the shade. A digital scale with an accuracy of 0.001 g was used to measure the shoot weight of plants (Afshari et al. 2021).
Chlorophyll (Chl) assay
The Arnon (1949) instruction was used to measure leaf total Chl. For this, 0.2 g of fresh leaves were homogenized in 8 mL of 80% acetone. The mixture was centrifuged at 4 °C for 15 min (3000 rpm). A spectrophotometer was used to read supernatant at 645 and 663 nm.
Where A is the absorbance at respective wavelengths. V is the final volume of extract, and W is the fresh weight in g.
Relative water content (RWC) measurement
RWC was determined using fresh leaves in three steps. The fresh weight (FW) of the leaves was determined, and they were immersed in distilled water for 24 h to achieve the saturation weight (SW). The samples were then dried in an oven until the weight remained stable, at which point it was determined to be dry weight (DW) (Dhopte and Manuel 2002):
Malondialdehyde (MAD) measurement
To measure leaf MAD content, 0.5 g of fresh leaves was mixed with 4 mL of 20% w/v trichloroacetic acid (TCA) containing 0.5% thiobarbituric acid and then kept in a hot water bath (95 °C) for 25 min and centrifuged at 14,000 rpm for 30 min. The samples were placed in a hot water bath for 30 min and centrifuged at 10,000 rpm for 10 min. Samples were read at a wavelength of 532 nm (Heath and Packer 1968).
Electrolyte leakage measurement (EL)
To measure EL, 1 cm discs of fresh leaves were prepared and moved to Erlenmeyer’s containing 10 mL of twice-distilled water. The EC was measured by a gauge digital EC (EL1 μS/cm) after shaking for 20 h. After that, the solution was placed in an autoclave for 1 h at 120 °C and then its EC was determined (EL2 μS/cm) and calculated as follows (Sheppard et al. 1995):
Essential oil (EO) distillation
To obtain the EO, the aerial parts of the plants were harvested during the flowering stage and dried in the shade. To obtain the EO content (w/w), 80–100 g of dried samples were used in Clevenger type apparatus via hydro-distilling for 3 h. The EO yield was calculated based on dry plant weight in each pot (Sefidkon et al. 2006).
Essential oil (EO) profile
Gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) were used to analyze the EOs. The oil samples were dehydrated using dry sodium sulfate. After injecting the EOs into the GC and determining the best column temperature program, the EOs were diluted with dichloromethane and injected into the GC-MS, where the retention index, mass spectra, and related chromatograms were determined, as well as the quantitative and qualitative amounts of the EOs’ active ingredients. Varian 3400 GC-MS system equipment with AOC-5000 auto-injector and DB-5 fused silica capillary column (30 m 0.25 mm i.d.; film thicknesses 0.25 m) was used to analyze the EO composition. Helium served as the carrier gas.
Identification of essential oil (EO) compounds
For the identification of components, retention time (RT) and mass spectra of the compounds were compared with those of authentic compounds or with data available from the Wiley library and the literature (Adams 2007). A mixture of n-alkanes (C8-C25) was used for further identification and calculation of retention indices (RI) of the components.
Statistical analysis
Data were analyzed using SAS software version 9.2 and the mean of data was compared using Duncan multiple range test at 0.05 probability level. Agglomerative Hierarchical Clustering (AHC) and principal component analysis (PCA) were carried out by XLSTAT.
Results
Pb and Cd concentrations in leaves and roots
When plants were exposed to Pb, the concentration of Pb in their leaves and roots increased significantly. The highest Pb amount obtained in Pb-exposed plants without foliar NP application was 8.83 mg g−1 DW. Nonetheless, NPs reduced Pb accumulation in sage roots and shoots. In Pb-exposed plants, Se, Si, and Zn NPs reduced Pb accumulation by 23, 30, and 22% for roots and 35, 43, and 40% for shoots, respectively, when compared to the control. Cadmium toxicity followed a similar pattern, with remarkable decreases in its accumulations in sage tissues following NPs. Silicon NPs reported the greatest reduction in shoot Cd (39%), followed by Zn NPs (36%), and Se NPS (29%). As a result, NPs may inhibit the accumulation of Cd and Pb in sage tissues, particularly in the leaves (Table 1).
Plant weight
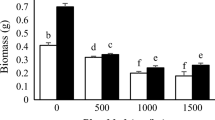
Heavy metals (Pb and Cd) significantly (P0.05) decreased plant weight, but foliar-applied NPs modulated oxidative stresses by increasing plant weight. When compared to non-polluted soils, Cd and Pb stresses reduced shoot weight by 41 and 35% (Fig. 1a), and root weight by 50 and 33% (Fig. 1b), respectively. However, NPs played a significant role in increasing plant weight. In comparison to non-sprayed plants, Se, Si, and Zn NPs caused 25, 40, and 44% increases in shoot weight (Fig. 1a) and 11, 25, and 16% increases in root weight (Fig. 1b), respectively. Therefore, Cd was more toxic than Pb, and Si and Zn NPs were more effective in increasing sage plant weight.
Relative water content (RWC) and chlorophyll content
Plants exposed to Pb and Cd stress had noticeable reductions in RWC, whereas NPs had remarkable effects on improving RWC. In this regard, Cd and Pb-exposed plants experienced 28 and 22% decreases in RWC, respectively, compared with the control. In contrast, Se, Si, and Zn NPs enhanced RWC in Cd-stressed plants by 18, 23, and 22%, respectively, when compared with non-NPs application (Fig. 2a). Total chlorophyll followed the RWC trend after the treatments in this study. Compared with the non-polluted treatment, remarkable reductions of total chlorophyll were observed in Cd (36%) and Pb (29%) contaminated soils without foliar-applied NPS. However, NPs especially Si NPs improved total chlorophyll in Cd (37%) and Pb (31%) exposed plants (Fig. 2b). Totally, Cd and Pb toxicity led to decreased RWC and chlorophyll but NPs, particularly Si NPs, improved these variables.
Malondealdehyde (MDA) and electrolyte leakage (EL)
Oxidative stress caused by Cd and Pb increased MDA and EL in sage plants, whereas NPS decreased these variables. The MDA and EL levels were highest in plants exposed to Cd toxicity without foliar NPs. In non-sprayed plants, Cd and Pb stress increased MDA by 46 and 34% (Fig. 3a) and EL by 30 and 19% (Fig. 3b), respectively. The most intriguing results were obtained for NPs by decreasing MDA and EL. For example, Se, Si, and Zn led to 14, 22, and 20% declines in MDA (Fig. 3a) and 16, 17, and 18% reductions in EL (Fig. 3b) compared with non-foliar application of NPs in plants with Cd toxicity.
Essential oil (EO) content and EO yield
The EO content and EO yield of sage plants decreased by the heavy metals but improved by the NPs. Upon non-foliar application, 20 and 15% reductions in EO content were respectively observed in Cd and Pb-exposed plants in comparison to the control. In contrast, NPs elevated EO content, with 15, 31, and 24% increases for Se, Si, and Zn NPs, respectively, relative to non-foliar NPs in plants experiencing Cd stress (Fig. 4a). The EO yield followed the pattern of EO content upon heavy metals and NPs. In non-NP plants, 2.1 and 1.8-fold increases were observed in Cd and Pb-exposed plants, respectively. In non-stressful plants, Se, Si, and Zn NPS elevated EO yield by 36, 37, and 43%, respectively, compared with non-NPs (Fig. 4b).
Essential oil (EO) profile
The GC/MS analysis revealed 25 compounds, which accounted for more than 98% of the total EO profile. The main EO constituents were 1,8-cineole, α-thujone, β-thujone, and camphor with different amounts of heavy metals and NPs. The Cd and Pb toxicity led to elevated 1,8-cineole, ranging from 9.42% in Se NPs without heavy metal stress to 13.41% in Pb stress and Si NPs. The amount of α-thujone was obtained in a range of 27.40 to 38.73%, with increasing upon the NPs. Like α-thujone, β-thujone increased when plants were sprayed with NPs. it differed from 10.12% without heavy metals toxicity and NPs (control) to 12.94% in Cd stress and Zn application. Camphor mainly increased with NPs as Se, Si, and Zn NPs increased it by 20, 12, and 21%, respectively, compared with control. Relative to sesquiterpene, the monoterpenes described the major amount of EO compositions in sage plants (Table 2).
Multivariate analysis
According to PCA eigenvalues, F1 justified α-thujone and camphore, while F2 explained 1,8 cineole and α–thujone. Additionally, 1,8-cineole negatively correlated with α–thujone and camphore (Fig. 5a). Based on AHC, three distinct clusters were identified with cluster 1: T1 (Non-toxicity + Non-NPs), T5 (Pb + Non-NPs), T6 (Pb + Se), T9 (Cd + Non-NPs), and T10 (Cd + Se), cluster 2: T2 (Non-toxicity +Se) and T3 (Non-toxicity + Si), and T4 (Non-toxicity + Zn), and cluster 3: T7 (Pb + Si), T8 (Pb + Zn), T11 (Cd + Si), and T12 (Cd + Zn) (Fig. 5b).
Principal component analysis (PCA) a and Agglomerative Hierarchical Clustering (AHC) b for main essential oil components of sage plants. T1: Non-toxicity + Non-NPs, T2: Non-toxicity +Se, T3: Non-toxicity + Si, T4: Non-toxicity + Zn, T5: Pb + Non-NPs, T6: Pb + Se, T7: Pb + Si, T8: Pb + Zn, T9: Cd + Non-NPs, T10: Cd + Se, T11: Cd + Si, T12: Cd + Zn
Discussion
The current study demonstrated that when plants were exposed to heavy metals, Pb and Cd accumulation increased in the tissues. The accumulation of Pb and Cd in roots was higher than in shoots, as previously reported by Nasirzadeh et al. (2022) on wheat and Babashpour-Asl et al. (2022) on coriander plants. Cadmium, due to its high mobility, can easily transfer from plant roots to aerial parts (Ismael et al. 2019), posing a threat to the food chain (Liu et al. 2020). As a result, techniques for inhibiting heavy metal uptake by plants must be developed. The NPs inhibited the accumulation of Pb and Cd in the roots and, in particular, the shoots of sage plants. Accordingly, deleterious effects of Cd and Pb due to increased ROS in plant tissues have been reported by Hussain et al. (2020). Foliar application of NPs can alter the Cd and Pb transporters by influencing metal accumulation in plant tissues (Hussain et al. 2020; Babashpour-Asl et al. 2022). Because of their small size and high active surface, nanoparticles can easily enter plant cells and improve tolerance to environmental stresses (Afshari et al. 2021; Memari-Tabrizi et al. 2021). It has been reported that Si can regulate the expression of genes involved in metal accumulation in plant tissues. For example, the down-regulated gene expression related to Cd transport in the shoot (OsLCT1 and OsNramp5) has been addressed (Cui et al. 2017). Furthermore, metal accumulation is affected by the quantity and type of NPs. Hussain et al. (2020) demonstrated that Si decreased Cd and Pb accumulation in grains, whereas Se increased Cd and decreased Pb amounts in rice grains. Babashpour-Asl et al. (2022) demonstrated significant decreases in Cd accumulation for shoot and root of Cd-exposed coriander plants, which is consistent with the current study’s findings. Therefore, different results in inhibiting or accelerating metal accumulations in plant tissues can be observed depending on the type and dosage of NPs.
Heavy metal toxicity reduced plant weight, but NPs modulated the corresponding stress. Heavy metals cause plants to produce more ROS by altering antioxidant activity (Khosropour et al. 2022). As a result, they inhibit the activity of Calvin-cycle enzymes, disrupt ionic exchange, and demolish chloroplast, ultimately reducing plant biomass (Handa et al. 2018). Nanoparticles, on the other hand, increased plant weight by improving growth conditions. In comparison to Se, Si and Zn NPs improved plant weight more effectively. Zinc and Si play important roles in the activation of chlorophyll enzymes, which affects plant growth and weight (Mahmoud et al. 2019). Similarly, the positive role of Si NPs (Ali et al. 2019; El-Saadony et al. 2021), Se NPs (Wang et al. 2021a, 2021b; Nasirzadeh et al. 2022), and Zn NPs (Hussain et al. 2018; Rizwan et al. 2019a) have been addressed on plant growth.
Heavy metals lowered chlorophyll content, but nanoparticles continued to increase photosynthesis content. They have a detrimental effect on stomatal size and density in epidermal cells, which slows transpiration and multiplies intercellular CO2 content in leaves (Khosropour et al. 2022; Sardar et al. 2022). The declines in metal-exposed plants might be attributed to the increased oxidation of photosynthesis pigments (Hussain et al. 2021). As a consequence, reductions in chlorophyll content have been disclosed in plants exposed to Pb and Cd, including Coriandrum sativum L. (Sardar et al. 2022), Triticum aestivum L. (Ozfidan-Konakci et al. 2018), and Persicaria hydropiper L. (Hussain et al. 2021). The NPs, particularly Zn and Si, modulated the photosynthesis rate by boosting the enzymatic activity of the Calvin cycle. The NPs used in this research were less than 50 nm in size, enabling them to penetrate plant cells and influence biochemical processes such as photosynthesis (Babashpour et al. 2022). Similarly, the foliar application of Si (Ali et al. 2019; Memari-Tabrizi et al. 2021; El-Saadony et al. 2021), Se (Sardar et al. 2022; Qi et al. 2021), and Zn (Bashir et al. 2021; Hussain et al. 2021) NPs on heavy metal toxicity have been addressed.
The RWC, like chlorophyll, is diminished by heavy metals and increased by NPs. Relative water content is a useful indicator for determining plant water status, which can affect biochemical processes in plant cells (Khosropour et al. 2022). To control the water status in plant cells, plants change their stomatal opening and closing in response to metal toxicity. Conversely, the NPs improved growth conditions by raising the RWC. Memari-Tabrizi et al. (2021) demonstrated improved RWC with Si NPs on coriander plants exposed to Cd toxicity. As a direct consequence, NPs, particularly Si and Zn, had a positive effect on chlorophyll content and RWC in sage plants grown in Cd and Pb-polluted soils.
Plant stress is primarily determined by cell membrane indicators such as MDA and EL. The MDA and EL levels increased in response to stress, but NP levels decreased. Increased MDA and EL are signs of plant damage, similar to proteins and lipids, that occur primarily in stressful situations by elevating ROS (Rizwan et al. 2019b; Huang et al. 2019). Furthermore, this study found that Cd was more harmful than Pb because it accumulated more MDA and EL in sage plants. Metal nanoparticles have a positive effect on antioxidant capacity, resulting in lower MDA and EL (Fatemi et al. 2021). Adrees et al. (2021) mentioned that Zn NPs altered MDA in wheat grown on Cd-exposed soils. Furthermore, when rice plants were sprayed with Si NPs, Rizwan et al. (2019b) evidenced 28 and 44% decreases in EL and MDA, respectively. Theodore, the current study found that NPs could indeed minimize MDA and EL in sage plants exposed to Cd and Pb stress.
Pb and Cd toxicity lowered EO content and yield. Because EO yield is calculated using plant weight and EO content, changes in plant weight have a noticeable impact on EO yield. Plants exposed to metal toxicity reduce their secondary metabolism, including the biochemical pathways of EO content. Memari-Tabrizi et al. (2021) on summer savory plants and Babashpour-Asl et al. (2022) on coriander plants addressed the declines in EO content and yield under 20 mg Cd kg−1 soil. Cadmium had a greater effect on EO than Pb, as did other variables. Cadmium can affect all stages of plant growth, from seed germination to development. Cd alters phytohormones like auxin and abscisic acid during seed germination. Cadmium inhibits mitotic division of meristematic cells, causing root length and weight to decrease and root diameter to increase. It also disrupts the balance of nutrients and water for plants, affecting the production of secondary metabolites such as EO (Haider et al. 2021). However, when sage plants were exposed to Cd and Pb toxicity, the NPs, particularly Si and Zn, increased EO yield. Specific functions of Si and Zn in plants include improved water absorption, nutrient uptake, gas exchange, and phytohormone adjustment (El Moukhtari et al. 2021). Improvements in EO yield have previously been documented for Si (El-Saadony et al. 2021; Mukarram et al. 2021), Se (Babashpour-Asl et al. 2022), and Zn NPs (Esmaielpour et al. 2020; Shahhoseini et al. 2020).
The main EO profile consisted of 1,8-cineole, α-thujone, β-thujone, and camphor, which had previously been reported in various studies (Rioba et al. 2015; Kulak et al. 2020). 1,8 cineole is a major herbal product with high antioxidant and anti-inflammatory properties, as well as a noticeable role in respiratory and heart decreases (Cai et al. 2021). It differed significantly depending on the treatments, with metal toxicity declining and NPs growing. Thujone is a monoterpene ketone found primarily in Artemisia absinthium, Salvia officinalis, Tanacetum vulgare, and Thuja occidentalis. This compound has anticancer, antidiabetic, and immune-boosting properties (Zámboriné Németh and Nguyen 2020). Cadmium and Pb stress reduced α-thujone and β-thujone levels, whereas NPs increased these chemicals. Heavy metals, on the other hand, reduced camphore, whereas NPs elevated it. As a consequence, variations in the main EO compositions of sage plants might well be likely as a result of these treatments, which may be beneficial for pharmacological and medicinal purposes.
Conclusions
The current study discovered that nanoparticles have varying effects on metal toxicity. Silicon and zinc nanoparticles are the most effective at reducing heavy metal oxidative stress. Metal accumulation in sage plant tissues can be reduced by using nanoparticles. Changes in essential oil yield and profile can be observed when plants are sprayed with nanoparticles and exposed to metal toxicity, according to the essential oil profile. As a result, the current study suggested applying foliar silicon to lessen the toxicity of lead and cadmium in sage plants. This is important for growing the plant in areas with heavy metal-polluted soils, and it can also be advantageous for the food and human health industries.
Data availability
The data are available on request.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream: Allured publishing corporation 456:544–545
Adrees M, Khan ZS, Hafeez M, Rizwan M, Hussain K, Asrar M, Ali S (2021) Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol Environ Saf 208:111627
Afshari M, Pazok A, Sadeghipour O (2021) Foliar-applied silicon and its nanoparticles stimulate physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum Sativum L.) essential oil under different irrigation regimes. Silicon 13:4177–4188
Ali S, Rizwan M, Hussain A, ur Rehman MZ, Ali B, Yousaf B, Ahmad P (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem 140:1–8
Aram S, Weisany W, Daliri MS, Mirkalaie SAAM (2021) Phenology, physiology, and fatty acid profile of canola (Brassica napus L.) under agronomic management practices (Direct Seeding and Transplanting) and zinc foliar application. J Soil Sci Plant Nutr 21(2):1735–1744
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–8
Babashpour-Asl M, Farajzadeh-Memari-Tabrizi E, Yousefpour-Dokhanieh A (2022) Foliar-applied selenium nanoparticles alleviate cadmium stress through changes in physio-biochemical status and essential oil profile of coriander (Coriandrum sativum L.) leaves. Environ Sci Pollut Res 29(53):80021–80031
Baig MA, Qamar S, Ali AA, Ahmad J, Qureshi MI (2020) Heavy metal toxicity and tolerance in crop plants. In: Contaminants in agriculture. Springer, Cham, pp 201–216
Bashir A, ur Rehman MZ, Hussaini KM, Adrees M, Qayyum MF, Sayal AU, Alyemeni MN (2021) Combined use of zinc nanoparticles and co-composted biochar enhanced wheat growth and decreased Cd concentration in grains under Cd and drought stress: a field study. Environ Technol Innov 23:101518
Beduk F, Aydin S, Aydin ME, Bahadir M (2022) Consequences of heavy metals in water and wastewater for the environment and human health. In: Water and Wastewater Management. Springer, Cham, pp 221–228
Cai ZM, Peng JQ, Chen Y, Tao L, Zhang YY, Fu LY, Shen XC (2021) 1, 8-Cineole: a review of source, biological activities, and application. J Asian Nat Prod Res 23:938–954. https://doi.org/10.1080/10286020.2020.1839432
Cui J, Liu T, Li F, Yi J, Liu C, Yu H (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ Pollut 228:363–369
Dhopte AM, Manuel LM (2002) Principles and techniques for plant scientists, 1st edn. Updesh Purohit for Agrobios (India), Odhpur
El Moukhtari A, Lamsaadi N, Oubenali A, Mouradi M, Savoure A, Farissi M (2021) Exogenous silicon application promotes tolerance of legumes and their N2 fixing symbiosis to salt stress. Silicon 14:6517–6534
El-Saadony MT, Desoky ESM, Saad AM, Eid RS, Selem E, Elrys AS (2021) Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J Environ Sci 106:1–14
Esmaielpour B, Shiekhalipour M, TORABI GM (2020) Effects of zinc nanoparticles on growth, some physiological characteristics, and essential oil yield of Dracocephalum moldavica L. under salinity stress conditions. Iranian J Med Arom Plant Res 36:867–884
Fatemi H, Esmaiel Pour B, Rizwan M (2021) Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ Sci Pollut Res 28:1417–1425
Ghorbani A, Esmaeilizadeh M (2017) Pharmacological properties of Salvia officinalis and its components. J Trad Complem Med 7:433–440. https://doi.org/10.1016/j.jtcme.2016.12.014
Gutsch A, Zouaghi S, Renaut J, Cuypers A, Hausman JF, Sergeant K (2018) Changes in the proteome of Medicago sativa leaves in response to long-term cadmium exposure using a cell-wall targeted approach. Int J Molecul Sci 19:2498
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887
Handa N, Kohli SK, Kaur R, Sharma A, Kumar V, Thukral AK, Bhardwaj R (2018) Role of compatible solutes in enhancing antioxidative defense in plants exposed to metal toxicity. Plants under metal and metalloid stress: responses, tolerance and remediation, pp 207–228
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Huang B, Chen YE, Zhao YQ, Ding CB, Liao JQ, Hu C, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677
Hussain A, Ali S, Rizwan M, ur Rehman MZ, Javed MR, Imran M, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environment Pollut 242:1518–1526
Hussain B, Lin Q, Hamid Y, Sanaullah M, Di L, Khan MB, Yan X (2020) Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci Total Environ 712:136497
Hussain F, Hadi F, Rongliang Q (2021) Effects of zinc oxide nanoparticles on antioxidants, chlorophyll contents, and proline in Persicaria hydropiper L. and its potential for Pb phytoremediation. Environ Sci Pollut Res 28:34697–34713
Ikram M, Javed B, Raja NI (2021) Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int J Nanomed 16:249
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11:255–277
Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H, Banjari I (2019) Bioactive profile of various Salvia officinalis L. preparations. Plants 8:55
Khosropour E, Attarod P, Shirvany A, Pypker TG, Bayramzadeh V, Hakimi L, Moeinaddini M (2019) Response of Platanus orientalis leaves to urban pollution by heavy metals. J For Res 30:1437–1445
Khosropour E, Weisany W, Tahir NAR, Hakimi L (2022) Vermicompost and biochar can alleviate cadmium stress through minimizing its uptake and optimizing biochemical properties in Berberis integerrima bunge. Environ Sci Pollut Res 29:17476–17486
Kicińska A, Pomykała R, Izquierdo-Diaz M (2022) Changes in soil pH and mobility of heavy metals in contaminated soils. Europ J Soil Sci 73:e13203
Kulak M, Gul F, Sekeroglu N (2020) Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind Crop Prod 145:112078
Liu N, Huang X, Sun L, Li S, Chen Y, Cao X, Rinnan R (2020) Screening stably low cadmium and moderately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 241:125065. https://doi.org/10.1016/j.chemosphere.2019.125065
Mahmoud AWM, Abdeldaym EA, Abdelaziz SM, El-Sawy MB, Mottaleb SA (2019) Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 10:19
Memari-Tabrizi EF, Yousefpour-Dokhanieh A, Babashpour-Asl M (2021) Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.). Plant Physiol Biochem 165:71–79
Mukarram M, Khan MMA, Corpas FJ (2021) Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J Hazard Material 412:125254
Nasirzadeh L, Kvarnheden A, Sorkhilaleloo B, Hervan EM, Fatehi F (2022) Foliar-applied selenium nanoparticles can alleviate soil-cadmium stress through physio-chemical and stomatal changes to optimize yield, antioxidant capacity, and fatty acid profile of wheat (Triticum aestivum L.). J Soil Sci Plant Nutr 22(2):2469–2480
Ozfidan-Konakci C, Yildiztugay E, Bahtiyar, M Kucukoduk M (2018) The humic acid-induced changes in the water status,chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol Environ Saf 155:66–75. https://doi.org/10.1016/j.ecoenv.2018.02.071
Qi WY, Li Q, Chen H, Liu J, Xing SF, Xu M, Wang SG (2021) Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J Hazard Material 417:125900
Rioba NB, Itulya FM, Saidi M, Dudai N, Bernstein N (2015) Effects of nitrogen, phosphorus and irrigation frequency on essential oil content and composition of sage (Salvia officinalis L.). J Applied Res Med Arom Plant 2:21–29
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Waris AA (2019a) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Rizwan M, Ali S, Malik S, Adrees M, Qayyum MF, Alamri SA, Ahmad P (2019b) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41:1–12
Saleh SR, Kandeel MM, Ghareeb D, Ghoneim TM, Talha NI, Alaoui-Sossé B, Abdel-Daim MM (2020) Wheat biological responses to stress caused by cadmium, nickel and lead. Sci Total Environ 706:136013
Sardar R, Ahmed S, Shah AA, Yasin NA (2022) Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 287:132332
Sefidkon F, Abbasi K, Khaniki GB (2006) Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem 99:19–23
Shahhoseini R, Azizi M, Asili J, Moshtaghi N, Samiei L (2020) Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of Feverfew (Tanacetum parthenium (L.) Schultz Bip.). Acta Physiol Plant 42:1–18
Sharifan H, Moore J, Ma X (2020) Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol Environ Saf 191:110177
Sheppard LJ, Franssen I, Cape JN (1995) Frost hardiness of Norway spruce treated with acid mist. Evaluation of the electrolyte leakage rate technique. Environ Exp Bot 35:139–149
Siddiqui H, Ahmed KBM, Sami F, Hayat S (2020) Silicon nanoparticles and plants: current knowledge and future perspectives. Sustainable agriculture reviews 41: Nanotechnology for plant growth and development, pp 129–142
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER. Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnO NPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochemi 110:59-69.
Wang C, Cheng T, Liu H, Zhou F, Zhang J. Zhang M, Cao T (2021a) Nano-selenium controlled cadmium accumulation and improved photosynthesis in indica rice cultivated in lead and cadmium combined paddy soils. J Environ Sci 103:336–346
Wang M, Chen Z, Song W, Hong D, Huang L, Li Y (2021b) A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bullut Environ Contamin Toxicol 106:65–74
Zámboriné Németh É, Thi Nguyen H (2020) Thujone, a widely debated volatile compound: What do we know about it? Phytochem Rev 19:405–423
Author information
Authors and Affiliations
Contributions
The data were prepared by Mitra Bakhtiari, Fereshteh Raeisi Sadati, and Seyede Yalda Raeisi; the initial draft was prepared by Mitra Bakhtiari and revised by Fereshteh Raeisi Sadati and Seyede Yalda Raeisi Sadati.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for this work.
Consent to participate
All authors agreed to submit the manuscript to Environmental Science and Pollution Research.
Consent for publication
All authors approved the final manuscript to publish in Environmental Science and Pollution Research.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakhtiari, M., Raeisi Sadati, F. & Raeisi Sadati, S.Y. Foliar application of silicon, selenium, and zinc nanoparticles can modulate lead and cadmium toxicity in sage (Salvia officinalis L.) plants by optimizing growth and biochemical status. Environ Sci Pollut Res 30, 54223–54233 (2023). https://doi.org/10.1007/s11356-023-25959-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25959-w