Abstract
Cadmium (Cd) is toxic to plants and animals, making it necessary to develop strategies that seek to reduce its introduction into food chains. Thus, the aim of this study was to investigate whether silicon (Si) and selenium (Se) reduce Cd concentrations in Pfaffia glomerata medicinal plant and attenuate the oxidative stress promoted by this metal. These plants were cultivated in hydroponics under the following treatments: control (nutrient solution), 2.5 μM Se, 2.5 mM Si, 50 μM Cd, 50 μM Cd + 2.5 μM Se, 50 μM Cd + 2.5 mM Si. After 14 days of exposure to treatments, leaves and roots were collected for the determination of dry weight of shoot and roots, Cd concentrations, chlorophyll and carotenoids content, and biochemical parameters (lipid peroxidation and guaiacol peroxidase and superoxide dismutase activities). The data were submitted to analysis of variance and means were compared with Scott-Knott test at 5% error probability. Roots of P. glomerata plants showed a significant reduction on dry weight accumulation when exposed to Cd. However, both Se and Si promoted a significant reduction of deleterious effects of Cd. The Cd concentrations in the tissues were reduced in the presence of Se or Si. Plants treated with Cd together with Se or Si presented higher pigment content than those with only Cd, thus showing a reduction in the negative effects caused by this element. In the treatments in which Se and Si were added in the growth medium together with Cd, an activation of superoxide dismutase and guaiacol peroxidase enzymes was observed in the roots and shoot, which may have contributed to lower lipid peroxidation. Thus, Se and Si reduce Cd concentrations and have potential to ameliorate Cd toxicity in P. glomerata plants, which can be used to increase productivity and quality of medicinal plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medicinal plants are used worldwide for the treatment of several diseases and are important raw material for the pharmaceutical industry for the production of phytopharmaceuticals and dietary supplements (Who 2002). However, medicinal plants grown in polluted areas, with metal mining, smelting operations and when agricultural expedients are used, including cadmium-containing fertilizers (Dayang and Fauziah 2013; Nakamura et al. 2017) can contain high levels of heavy metals in their tissues, among them the cadmium (Cd) (Gil et al. 2016). Poisonings associated with the presence of toxic metals in medicinal plants were reported in Europe, Asia, and in USA (Dayang and Fauziah 2013).
In Brazil, 76% of the medicinal plants analyzed in the work of Caldas and Machado (2004), among them Pfaffia glomerata, contained detectable levels of Cd. Samples of P. glomerata presented Cd concentrations between 0.1 to 0.3 μg g−1 DW, being that the recommended limit for medicinal plants is below 0.3 μg g−1 DW (Who 1999). In the world, human exposure to Cd from the diet and drinking water can reach up to 60% of provisional tolerable weekly intake (420 μg person−1 of Cd) (Who 1993).
Cadmium can cause damage to both animals and plants. In humans, Cd primarily affects the kidneys, causing tubular proteinuria (Whitfield et al. 2010), low bone-mineral density and osteoporosis (Alfven et al. 2004), and lung cancer (Navarro and Rohan 2007).
In plants, studies revealed that Cd accumulation can interfere in several metabolic processes, since changes in redox state of cell (Gill and Tuteja 2010) to negative effects on photosynthetic apparatus (Qian et al. 2010). Additionally, cadmium can cause changes in stomatal conductance, leaf transpiration (Souza et al. 2011; Dong et al. 2017); it interferes in minerals and water absorption (He et al. 2011), and it raises oxidative stress, leading to disturbance in composition and function of cell membranes (Gallego et al. 2012).
Thus, it is necessary to develop strategies that result in lower absorption of this element, optimizing the use of natural resources and the production of safe food, especially in the case of a medicinal plant as P. glomerata, which presents reasonable degree of metal tolerance. This species, known as Brazilian ginseng, belongs to family Amaranthaceae, and is of great commercial interest in production of herbal medicines and food supplements due properties of antitumor, antidiabetic, tonic, stimulating, and its effect against gastric and rheumatism disorders (Mendes and Carlini 2007; Gupta et al. 2013).
Among alternatives tried to solve the problems caused by Cd on growth plants is the use of alleviates, i.e., elements taken until then as beneficial that when used in low concentrations can alleviate the harmful effects of toxic metals. Studies have shown that interaction of Cd with essential and beneficial elements can reduce Cd accumulation and thus alleviate symptoms of its toxicity (Nazar et al. 2012; Gharaibeh et al. 2016).
Accordingly, silicon (Si) is a beneficial element to growth, development, yield and disease resistance in a wide variety of plant species (Ma et al. 2015; Dorneles et al. 2016). Another element considered beneficial is selenium (Se), and has also been proven that it can enhance plant tolerance to environmental stresses (Yao et al. 2013) and Cd toxicity (Thiruvengadam and Chung 2015).
The roots of the plants are the organs most affected by the Cd, consequence of the greater contact with this element in the soil (Bonanno and Cirelli 2017; Borges et al. 2018). This is a food safety problem because P. glomerata roots are widely used in folk medicine and when grown on contaminated soils, they can accumulate this metal in this organ. In this sense, the use of strategies to reduce the Cd accumulation in medicinal plants is of great importance for human health. Thus, the aim of this study was to investigate whether Si and Se reduce Cd concentrations in P. glomerata medicinal plant and attenuate the oxidative stress promoted by this metal.
Materials and methods
Plant material and growth condition
Tests were developed in the Biochemical Plants Laboratory, Industrial Chemical Analysis and Environmental Laboratory (LAQIA) and greenhouse of the Federal University of Santa Maria. P. glomerata plants (GD access, belonging to the collection of medicinal plants from Federal University of Grande Dourados (UFGD)) were used in this experiment, which were obtained by in vitro culture from nodal segments (1.0-cm long without leaves). The species was chosen because it is widely used in popular medicine in Brazil and because it has economic importance, since tons of roots of this species are monthly destined to the national and international market. These plants were cultivated in MS medium (Murashige and Skoog 1962), supplemented with 6 g L−1 agar, 30 g L−1 sucrose, and 0.1 g L−1 myo-inositol.
On day 25 of in vitro growth, plants were transferred to plastic containers with a capacity of 17 L containing modified Hoagland solution (Hoagland and Arnon 1950), with the following composition (in mg L−1): 85.31 N; 7.54 P; 11.54 S; 97.64 Ca; 23.68 Mg; 104.75 K; 176.76 Cl; 0.27 B; 0.05 Mo; 0.01 Ni; 0.13 Zn; 0.03 Cu; 0.11 Mn, and 2.68 Fe (FeSO4/Na-EDTA). After the acclimation period (5 days), plants were transferred to hydroponics with the following treatments: control (nutrient solution), 2.5 μM Se, 2.5 mM Si, 50 μM Cd, 50 μM Cd + 2.5 μM Se, and 50 μM Cd + 2.5 mM Si. The insoluble salts Na2SiO3, Na2SeO3, and CdCl2 were used. The treatments were arranged in a randomized design with 3 replications for each treatment and 30 plants per replicate. The pH of the solution was adjusted daily and maintained at 4.5 (± 0.1). The experiment was conducted in a greenhouse with controlled temperature (25 °C ± 3), relative humidity of 80% and light intensity of 1081 μmol m−2 s−1. The plants were exposed to the different treatments for 14 days, when they presented visual symptoms of cadmium toxicity (mainly chlorosis in the leaves).
Dry weight
Shoot and roots of the plants were collected and immediately placed in paper bags and brought to the oven at 65 °C until constant weight, when the dry weight was determined in a precision scale.
Cadmium concentration in roots and shoot
Roots and shoot were dried at 60 °C to constant weight using oven drying with forced ventilation. The dry shoot and roots (500 mg) were ground and digested with 5 mL of HNO3 and 0.2 mL of H2O in sealed Teflon vessel, which was heated to 100 °C for 3 h in a digester block (Tecnal, TE-007D). The samples were then diluted to 50 mL with high purity water. Cadmium concentrations were determined using mass spectrometry with inductively coupled plasma (ICP-MS) using a Perkin-Elmer Sciex Elan instrument DRC II equipped with pneumatic nebulizers (Meinhard type A), nebulizer cyclonic chamber, and torch with a gun tube quartz of a 2.0-mm internal diameter. The plasma was generated from argon (99.998% purity).
The species ability to translocate Cd from the root to shoot was evaluated by the translocation index (Ti), as described in Bernardy (2015):
- [Cd]R:
-
Cd concentration in roots (μg g DW−1);
- [Cd]RS:
-
Cd concentration in roots + Cd concentrations in shoot (μg g DW−1).
Chlorophyll and carotenoids content
For the pigment content (chlorophylls a, b and carotenoids), leaf samples in disc form were removed using a standard paper punch with 6 mm of diameter. Immediately, the samples were placed in reaction tubes (Eppendorff) and frozen in a freezer at − 80 °C for later analysis. Chlorophylls a and b and carotenoids were extracted according to the method of Hiscox and Israelstam (1979) and estimated using the equation of Lichtenthaler (1987).
Lipid peroxidation
Roots and shoot (all leaves) were collected and immediately frozen in liquid nitrogen. Afterwards, plants were kept in a freezer at − 80 °C for biochemical analysis. Lipid peroxidation was determined by the method of El-Moshaty et al. (1993). Samples of leaves and roots (0.5 g) were used by malondialdehyde (MDA) quantification, a product from membrane lipids oxidation by reactive oxygen species (ROS). The lipid peroxidation was expressed as nmol MDA mg−1 of protein.
Antioxidant enzyme activity
The activity of guaiacol peroxidase enzyme was determined according to Zeraik et al. (2008) using guaiacol as substrate. The enzyme activity was estimated by guaiacol oxidation to tetraguaiacol by the increase in absorbance at 470 nm. The results were expressed as unit of enzyme per mg protein (U mg−1 of protein). The activity of superoxide dismutase (SOD) was estimated by spectrophotometry according to the method of Giannopolitis and Ries (1977). The photochemical blue formazan production from NBT was monitored by increasing absorbance at 560 nm. SOD activity was estimated as the enzyme amount required to inhibit the NBT photoreduction in 50% (Beauchamp and Fridovich 1971).
Protein determination
The Bradford method (Bradford 1976) was used to determine the total protein concentration.
Statistical analysis
For statistical analysis, data were submitted to analysis of variance and means were compared with Scott-Knott test at 5% error probability using the Sisvar application (Ferreira 2011). Graphics program SigmaPlot 12.5.
Results and discussion
Using the “Visual MINTEQ” software assistance, it was possible to calculate the availability of Si, Se, and Cd. The Si and Se have approximately 100% availability, even when applied with the Cd. The Cd presents 73.3% availability as CdCl2 and 22.79% as CdCl2.2H2O, both readily absorbed forms, thus totaling 96.09% Cd available (data not shown). In this way, it is possible to affirm that any effect of both beneficial elements occurs in plant and not in solution.
Dry weight
Cadmium is one of the most toxic heavy metals and has high mobility in the environment (Tang et al. 2015), being absorbed by the roots and transported to the shoot of many plant species (He et al. 2011). However, in this work, Cd promoted reduction only on root dry weight (Table 1), compared to the control. The root growth inhibition may be because the root system is the first organ to be exposed to Cd, resulting in greater growth inhibition. Cadmium also damages the cell membrane integrity (Filek et al. 2008), and it may also inhibits the nutrients absorption (Zembala et al. 2010), causing a reduction in biomass accumulation.
The root dry weight of plants treated with Si and Se with Cd was statistically equal to the plants of control treatment. Thus, through this parameter, it was observed that Se and Si were able to reduce the Cd toxicity. Selenium and Si enter the food chain through plants and especially through cultivated and medicinal plants, which are part of the diet of both primary and secondary consumers (Longchamp et al. 2015). Selenium plays an important role in the detoxification of oxidative stress induced by Cd (Khan et al. 2015) and can alleviate deleterious effects of various environmental stresses including heavy metals, drought, ultraviolet radiation, and salinity (Liu et al. 2015a). According to Hossain et al. (2007), the benefits promoted by Si in plant metabolism may be indirect (higher resistance to disease) or direct (improved nutrition). According to the same authors, Si stimulates growth due to increase the cell wall extensibility. Moreover, the beneficial effect of Se and Si on growth parameters of P. glomerata plants can be related to lower Cd absorption or the formation of metal-silicate within the plant.
Cadmium concentration in roots and shoot
Cadmium (Cd) levels were higher in plants exposed only Cd, being that the roots had 15 times more Cd accumulation than the shoot (Fig. 1). This response is likely due to Cd retention in the roots, since its accumulation occurs mainly in the vacuole or cell wall of these tissues, which consequently leads to transport limitation in the shoot (Milner and Kochian 2008; Dong et al. 2017).
According to Grant et al. (1998), the higher accumulation of Cd in the roots seems to be a result of Cd binding to negatively charged cell walls of the root system at the expense of increased absorption, and subsequent transfer to shoot. Through these results, it is possible to observe that the metal is slightly movable in the plant, given the expressive difference of Cd found in the two compartments of the plant. This result raises concern, since the main part of the P. glomerata plant used in folk medicine is the root. Salt et al. (1995) reported that mustard plants (Brassica junceae) and alpine pennycress (Thlaspi caerulescens) exposed to Cd in nutrient solution accumulate substantial amounts of Cd in the roots and shoot, in addition to higher metal accumulation in the roots.
The addition of Se and Si in the nutrient solution reduced Cd concentration in the roots and shoot. This decrease was most evident in the roots, which is about 53 and 50% (for Se and Si, respectively) compared with the treatment with only Cd. The addition of Si reduced the Cd concentration in the symplast of root cells by reducing the amount of Cd absorbed, which is more mobile in the cellular wall, thus softening the Cd toxicity in the cytoplasm (Da Cunha and Do Nascimento 2009; Ye et al. 2012). The possible mechanism for the inhibition of Cd transport by the plant induced by Si can be related to two aspects. The first is that the deposition of Si in the lignin of the cell wall promotes a greater connection of metallic ions to the cell wall, thus reducing the translocation of this metal from the root to the shoot. The second is the complex formation or co-precipitation of toxic metals with Si within the plant. These complexes are translocated and accumulated in the vacuole in undiscovered forms (Neumann and Zur Nieden 2001; Fan et al. 2016).
Selenium is co-transported with Cd ions by same carrier, which results in a decreased amount of Cd ions in active sites of transmembrane transporter (Zembala et al. 2010). Both Cd and Se bind to thiol groups of cysteine, an amino acid found in certain proteins. Thus, the competition for particular binding sites in proteins may explain the reduced Cd absorption and the protective effect of Se against Cd toxicity (Lin et al. 2012). Additionally, previous studies showed that Se in rice and tobacco seedlings enabled a reduction of Cd accumulation due to a decrease in absorption (Lin et al. 2012; Liu et al. 2015b).
From the estimate of Cd translocation is observed that about 50% of this element is transloated to shoot (Fig. 1c). In plants exposed to Cd (Cd, Se + Cd and Si + Cd treatments), there was no significant difference between treatments for Cd translocation rate. Therefore, Se and Si only influence the Cd absorption, not avoiding the translocation of this element to shoot. Once Cd is in plant root cells, they can be translocated to plant leaves (Rossi et al. 2018). The translocation from roots to shoot is greatly affected by the vacuolar compartmentation of Cd-phytochelatin complexes as well as the transporters involved in xylem loading of Cd (Shute and Macfie 2006; Li et al. 2017). Besides, chelated Cd is also more effectively sequestered in the vacuole of plant root cells, reducing the loading efficiency to xylem tissues and therefore less efficient translocation to shoot (Meng et al. 2017; Rossi et al. 2018).
Silicon taken up by the roots is supposedly translocated to the shoot along with the transpiration steam and then concentrated and finally physically gelled in rapidly transpiring organs. Nutrient ions present in the soil also sometimes affect Si uptake (Hasanuzzaman et al. 2014). For example, excess nitrogen is reported to decrease the Si content and number of silica bodies in rice (Ishizuka and Tanaka 1950). Different metabolic inhibitors like pyruvate and acetate were found to disturb Si uptake (Ma and Takahashi 2002). Some environmental factors like low temperature sometimes inhibit transportation (Mitani and Ma 2005).
Selenium translocation from roots to stems in plants is strongly dependent of the chemical form of Se added to the culture media (Terry et al. 2000). In general, plants exposed to Se shown a high translocation index as consequence of selenate using the sulfate path through the plants (Eapen and D’Souza 2005; Pedrero et al. 2007). Selenate and sulfate share the same transport pathway in plants, over expression of high-affinity sulfate transporters in root tissue can lead to substantial absorption of selenate (Shibagaki et al. 2002; Liu et al. 2015a). To reduce the damage, plant roots translocate excessive Se to the shoot through a series of internal metabolic processes, thereby alleviating the stress (Liu et al. 2015c).
The P. glomerata plants used in this work exposed to Cd accumulated high levels of Cd in the roots, even in the presence of the beneficial elements. However, both beneficial elements were able to reduce the Cd accumulation in roots by approximately 50% compared to plants treated with Cd alone. Given that the Cd concentration used in this experiment was often higher than that found even in contaminated soils, it is possible to observe the beneficial capacity of both Si and Se to reduce the Cd content in P. glomerata plants. In addition, it is possible that higher concentrations of the beneficial elements employed herein could further reduce the Cd concentration in the P. glomerata tissues under these growth conditions.
Therefore, if Si and Se have reduced cadmium concentrations in this situation (when Cd was supplied in high concentrations), they may also reduce in a situation where Cd is present in lower concentrations or in areas contaminated with this metal.
Content pigments (chlorophylls a, b, total and carotenoid)
The highest content of photosynthetic pigments was observed in the control treatment, whereas for the other treatments there was a decrease in the content of these pigments, mainly in the plants only exposed to Cd (Fig. 2). The decrease in chlorophyll content associated with stress caused by Cd may be due to the inhibition of enzymes responsible for biosynthesis of chlorophyll (Qian et al. 2009). The reduction promoted by cadmium in the content of photosynthetic pigments was not accompanied by reduction in the production of shoot biomass (Table 1). This indicates that somehow the plants exposed to cadmium compensated the photosynthetic activity in the presence of this metal, avoiding damages in the production of shoot biomass.
Plants treated with Cd together with Se or Si presented higher pigment concentration than those with only Cd, thus showing a reduction in the negative effects caused by this element (Fig. 2). However, in plants exposed only Se or Si was observed a decrease in pigments content compared to control. The dual effects of Se on non-stressed plants have been established, and these effects are related the regulation of the uptake and redistribution of some essential elements, mainly Fe (Feng et al. 2013). This essential element acts as a co-factor in many enzymes, such as SOD, POD, CAT, and enzymes involved in the chlorophyll biosynthesis pathway (Feng et al. 2013). Guerrero et al. (2014) observed in wheat plants that Se has dual effects; at low concentrations, it acts as growth stimulant, whereas at high concentrations, it reduces root elongation and biomass production and alters uptake and translocation of several essential nutrients. In barley, Se applications caused a decrease in the chlorophyll content. The reduced chlorophyll accumulation in leaves could be explained by the inhibition of chlorophyll biosynthesis (Akbulut and Çakir 2010). The Si can cause the same response. The Si concentration also influenced to pigment content in cells, which is likely a result of its ability to partition cations such as Mn, Fe, and even Mg (Farooq and Dietz 2015), which are involved in structure of tetrapyrrole molecules (Hu et al. 2017).
The increase in pigment content of plants grown under Cd + Se treatment was possibly due to Se being able to alleviate the oxidative stress induced by Cd in chloroplasts, mainly through the elimination of reactive oxygen species, which can decrease chlorophyll content (Djanaguiraman et al. 2010). The same increase can be observed in the Cd + Si treatment. It can be explained by the effect of Si in the increment of epidermal cell rigidity (Sattar et al. 2013), increasing lignification. Consequently, a mechanical barrier is formed (Meharg and Meharg 2015), decreasing the Cd input, and leading to less damage to pigments.
Beneficial effects of Se and Si were observed in the other species exposed to different Cd concentrations. Pedrero et al. (2008) observed that Se caused a significant increase in chlorophyll concentration for cabbage plants (Brassica oleracea) cultivated with Cd. Moreover, Mohsenzadeh et al. (2012) observed that the addition of Si during the cultivation of maize plants (Zea mays) promoted a significant increase in the total content of chlorophylls and carotenoids.
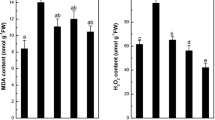
Lipid peroxidation
Free radicals generated in excess caused by growing of plants in Cd contaminated soil can accumulate in cells, which leads to lipid peroxidation of biomembranes. The final product of this process is malondialdehyde (MDA), which is used as an indicator of lipid peroxidation (Muradoglu et al. 2015). In the present experiment, higher MDA content in the shoot and roots was observed for plants grown with Cd, with the highest level in the shoot (Fig. 3), despite the roots presented a higher level of Cd. The highest Cd concentration in the roots rather than in shoot might be a natural protective response of plants to Cd toxicity. The highest proportion Cd concentration is observed in the cell wall both root and shoot (Wu et al. 2016) not causing severe stress to cellular metabolism. However, the higher MDA content found in shoot tissue may be related to the relatively high amount of metabolically active cells.
Selenium and Si significantly reduced damage caused by Cd in membrane lipids, in which MDA values in plants exposed to Se and Si were significantly lower than in plants exposed only to Cd in the growth medium. For the roots, Si had a more significant mitigating effect than Se for MDA content.
In the shoot, lower concentration of MDA for plants grown only with Si was observed compared to the control treatment. In relation to the roots, the lowest concentration was observed for the control treatment and treatment containing Si and Cd (Fig. 3). Cd does not appear to generate free radicals directly, but it can increase lipid peroxidation contributing to the cellular damage process (Lin et al. 2017). In this context, Si may be acting more expressively in the alleviation of the phytotoxicity confined by Cd, since Si stimulates antioxidant activity (Dorneles et al. 2016). Furthermore, the MDA content may indicate that exposure to Cd causes oxidative damage, and selenium has the potential to alleviate such effects by reducing MDA levels and increasing unsaturation of fatty acids (Barrientos et al. 2012).
Activity of antioxidant enzymes
During stress caused by heavy metals, highly reactive intermediate forms of oxygen (H2O2, hydroxyl, and superoxide radicals) are formed (Sharma et al. 2012). Cadmium is a non-redox metal, and therefore cannot produce reactive oxygen species (ROS) directly; however, it may interfere with the antioxidant system of plants (Lin et al. 2017). In order to eliminate ROS, which can promote oxidative damage, plants have several antioxidant enzymes. Among these enzymes, superoxide dismutase (SOD) is the first involved in the detoxification process, since it converts superoxide radicals to H2O2 (hydrogen peroxide) at a very fast rate (Gratão et al. 2005). Another antioxidant enzyme is guaiacol peroxidase (POD), which fights free radicals mainly in the cell wall (Polidoros and Scandalios 1999). Thus, the increase in the level of these antioxidant enzymes in plants can prevent oxidative damage and improve tolerance to oxidative stress (Ekmekçi et al. 2008).
The combined action of SOD and POD is fundamental for attenuating the effects of oxidative stress caused by Cd, since its roles in cellular metabolism are complementary (Wu et al. 2017). The SOD and POD activities were reduced in the plants grown with Cd in both the shoot and roots (Fig. 4). This response probably occurred because Cd induces lipoxygenase production, resulting in the inhibition of antioxidant enzymes, such as SOD and CAT (Somashekaraiah et al. 1992). However, in the presence of Se or Si together with Cd, there was an increase in SOD and POD activity in both parts of the plants. For treatment containing Cd + Si, the SOD activity in the roots was similar to that observed for the control treatment (Fig. 4b). Thus, it is possible to affirm that the presence of Se and Si was beneficial for reducing Cd toxicity, since there was an incentive in SOD activity. The highest activity of enzyme was observed in the shoot of the plants, whereas the lowest was observed in the roots (Fig. 4a, b).
Effect of selenium (2.5 μM) or silicon (2.5 mM) on superoxide dismutase (SOD) of shoot (a) and roots (b) and guaiacol peroxidase (POD) activity of shoot (c) and roots (d) on of Pfaffia glomerata plants exposed to cadmium (50 μM). Different letters indicate a significant difference among treatments by the Scott-Knott test
Selenium has the ability to increase the activity of antioxidant enzymes that are part of protective mechanisms, which may alleviate oxidative stress (Liu et al. 2015b). The negative charge of Si complexes in the cell wall can lead to Cd binding and thereby, inhibit cadmium transport in apoplast and plant xylem (Ma et al. 2015).
In the present experiment, in the shoot of the plants, the POD activity was higher in the control treatment and lower for the treatment containing only Cd (Fig. 4c, d), In the roots, the POD activity in the Se + Cd and Si + Cd treatments was similar to the control treatment. The plants cultivated with Se exhibited higher POD activity in comparison with the plants cultivated with Si. This response was observed for both the shoot and roots (Fig. 4c, d). As for SOD, the presence of Se and Si was beneficial in reducing the toxicity of Cd, especially in the shoot. Selenium can increase the activity of antioxidant enzymes and protect cells and tissues against oxidative damage caused by stress (Liu et al. 2015c). In addition, the antioxidant enzyme activity in silicon-treated plants seems to create conditions of tolerance to this type of stress (Rehman et al. 2016), while assuring cell wall preservation (Ali et al. 2016). In this manner, lower concentrations of Cd lead to reduced toxic effects of this element.
Van Assche and Clijsters (1990) observed that the increase in POD activity in plants is possibly correlated with Cd tissue levels and the degree of growth inhibition. The reduction in POD and SOD activity observed in the present experiment in the presence of Cd indicates that the damages to the plants were likely a result of the toxicity of Cd. The reduction of POD and SOD activity (Fig. 4) shows that the enzymes are unable to remove H2O2 and O2−, respectively. Cadmium may have generated damage on the antioxidant system of the plants, which affected the response of these enzymes. All of these changes in the antioxidant activities may be due to the differences among plant species, time of exposure, silicon and/or selenium concentrations, and experimental conditions (Wu et al. 2017).
Conclusion
Based on the data obtained in this work, it is possible to conclude that both selenium and silicon are effective in reducing cadmium toxicity in Pfaffia glomerata plants. Furthermore, Se and Si has great ability to reduce the accumulated Cd content in the roots of these plants, which is important due to the use of roots of these plants in pharmacology and folk medicine. The data from this experiment may be basis for future research on a joint action of these elements in plants, which could be an effective alternative in reduction of stress caused by toxic elements.
References
Akbulut M, Çakir S (2010) The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings. Plant Physiol Biochem 48:160–166
Alfven T, Elinder CG, Carlsson MD, Grubb A, Hellstrom L, Persson B (2004) Low level cadmium exposure and osteoporosis. J Bone Miner Res 15:1579–1586
Ali I, Liu B, Farooq MA, Islam F, Azizullah A, Yu C, Su W, Gan Y (2016) Toxicological effects of bisphenol A on growth and antioxidant defence system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol Environ Saf 124:277–284
Barrientos EY, Flores CR, Wrobel K, Wrobel K (2012) Impact of cadmium and selenium exposure on trace elements, fatty acids and oxidative stress in Lepidium sativum. J Mex Chem Soc 56:3–9
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 8:276–287
Bernardy K (2015) Effect of zinc in biochemical and physiological parameters of Pfaffia glomerata (Spreng.) Pedersen [Dissertation]. Santa Maria (RS): Federal University of Santa Maria, Brazil
Bonanno G, Cirelli GL (2017) Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol Environ Saf 143:92–101
Borges KLR, Salvato F, Alcântara BK, Nalin RS, Piotto FA, Azevedo RA (2018) Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 1:1–14
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Caldas ED, Machado LL (2004) Cadmium, mercury and lead in medicinal herbs in Brazil. Food Chem Toxicol 42:599–603
Da Cunha KPV, Do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
Dayang SN, Fauziah IC (2013) Soil factors influencing heavy metal concentrations in medicinal plants. J Trop Agric Sci 36:161–178
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defence system. J Plant Physiol Biochem 48:999–1007
Dong Q, Xu PX, Wang ZL (2017) Differential cadmium distribution and translocation in roots and shoots related to hyper-tolerance between tall fescue and Kentucky bluegrass. Front Plant Sci 8:113–120
Dorneles AOS, Pereira AS, Rossato LV, Possebom G, Sasso VM, Bernardy K, Sandri RQ, Nicoloso FT, Ferreira PAA, Tabaldi LA (2016) Silicon reduces aluminum content in tissues and ameliorates its toxic effects on potato plant growth. R Ciênc Rural 46:506–512
Eapen S, D'Souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23:97–114
Ekmekçi Y, Tanyolaç D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611
El-Moshaty EFIB, Pike SM, Novacky AJ, Sehgal OP (1993) Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ring spot virus or southern bean mosaic virus. Physiol Mol Plant Pathol 43:109–119
Fan X, Wen X, Huang F, Cai Y, Cai K (2016) Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38:1–9
Farooq MA, Dietz KJ (2015) Silicon as versatile player in plant and human biology: overlooked and poorly understood. Front Plant Sci 6:1–14
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência Agrotec 35:1039–1042
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Gallego SM, Penaa LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosalesa EP, Zawoznika MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gharaibeh MA, Albalasmeh AA, Marschner B, Saleem Y (2016) Cadmium uptake and translocation of tomato in response to simulated irrigation water containing elevated concentrations of cadmium and zinc in clayey soil. Water Air Soil Pollut 227:1–13
Giannopolitis CN, Ries SK (1977) Purification and quantitative relationship with water-soluble protein in seedlings. J Plant Physiol 48:315–318
Gil F, Hernández AF, Martín-Domingo MC (2016) Toxic contamination of nutraceuticals and food ingredients. Nutraceutical 58:825–837
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. J Plant Physiol Biochem 48:909–930
Grant CA, Buckley WT, Bailey LD, Selles F (1998) Cadmium accumulation in crops. J Plant Sci 78:1–17
Gratão PL, Polle Al PJ, Peter JL, Azevedo RA (2005) Making the life of heavy metal stressed plants a little easier. Plant Biol 32:481–494
Guerrero B, Llugany M, Palacios O, Valiente M (2014) Dual effects of different selenium species on wheat. Plant Physiol Biochem 83:300–307
Gupta DK, Huang HG, Nicoloso FT, Schetinger MR, Farias JG, Li TQ, Razafindrabe BHN, Aryal N, Inouhe M (2013) Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology 22:1403–1412
Hasanuzzaman M, Nahar K, Fujita M (2014) Silicon and selenium: two vital trace elements that confer abiotic stress tolerance to plants. P. Ahmad (ed): Emerging technologies and management of crop stress tolerance, 1(16): 377–422
He J, Qin J, Long L, Ma Y, Li H, Li K, Jiang X, Liu T, Polle A, Liang Z, Luo ZB (2011) Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus x canescens. Physiol Plant 143:50–63
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. J Bot 57:1132–1334
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Berkeley, CA: Agric. Exp. Stn., Univ. of California. (Circ. 347)
Hossain MT, Soga K, Wakabayashi K, Kamisaka S, Fujii S, Yamamoto R, Hoson T (2007) Modification of chemical properties of cell walls by silicon and its role in regulation of the cell wall extensibility in oat leaves. J Plant Physiol 164:385–393
Hu X, Page MT, Sumida A, Tanaka A, Terry MJ, Tanaka R (2017) The iron–sulfur cluster biosynthesis protein SUFB is required for chlorophyll synthesis, but not phytochrome signaling. The Plant J 89:1184–1194
Ishizuka Y, Tanaka A (1950) Studies on the nitrogen, phosphorus and potassium metabolism of the rice plant. 1. The influence of the nitrogen concentration in the culture solution on the growth of the rice plant, especially on the amount and the form of nitrogen in the plant. J Sci Soil Manure 21:23–28
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Li LZ, Tu C, Peijnenburg WJGM, Luo YM (2017) Characteristics of cadmium uptake and membrane transport in roots of intact wheat (Triticum aestivum L.) seedings. Environ Pollut 221:351–358
Lichtenthaler HK (1987) Chlorophylls and carotenoids pigments of photosynthetic. Biol Membranes Methods Enzym 148:350–382
Lin L, Zhou W, Dai H, Cao F, Zhang G, Wu F (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mat 235:343–351
Lin H, Fang C, Li Y, Lin W, He J, Lin R, Lin W (2017) Cadmium-stress mitigation through gene expression of rice and silicon addition. Plant Growth Regul 81:91–101
Liu X, Zhao Z, Hu C, Zhao X, Guo Z (2015a) Effect of sulphate on selenium uptake and translocation in rape (Brassica napus L.) supplied with selenate or selenite. Plant Soil 1–10
Liu W, Shang S, Feng X, Zhang G, Wu F (2015b) Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ Toxicol Chem 34:92–99
Liu W, Feng X, Shang S, Zhang G, Wu F (2015c) Selenium reduces cadmium accumulation and alleviates cadmium-induced quality degradation in tobacco. Plant Soil Environ 61:444–450
Longchamp M, Castrec-Rouelle M, Biron P, Bariac T (2015) Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem 182:128–135
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier, Amsterdam
Ma J, Cai H, He C, Zhang W, Wang L (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206:1063–1074
Meharg C, Meharg AA (2015) Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ Exp Bot 120:8–17
Mendes FR, Carlini EA (2007) Brazilian plants as possible adaptogens: an ethnopharmacological survey of books edited in Brazil. J Ethnopharmacol 109:493–500
Meng D, Xu P, Dong Q, Wang S, Wang Z (2017) Comparison of foliar and root application of potassium dihydrogen phosphate in regulating cadmium translocation and accumulation in tall fescue (Festuca arundinacea). Water Air Soil Pollut 228:1–8
Milner MJ, Kochian LV (2008) Investigating heavy metal hyper accumulation using Thlaspi caerulescens as a model system. Annals of Bot 102:3–13
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Biol 56:1255–1261
Mohsenzadeh S, Shahrtash M, Da Silva JAT (2012) Silicon improves growth and alleviates toxicity of cadmium in maize seedlings. Plant Stress 6: 39–43
Muradoglu F, Gundogdu M, Ercisli S, Encu T, Balta F, Jaafar HZE, Zia-Ul-Haq M (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48:11–15
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Nakamura CS, Hodge FS, Valentine JL, Robbins WA (2017) Heavy metal contamination in Thelesperma megapotamicum. J Toxicol Environ Health Sci 9:14–22
Navarro SSA, Rohan TE (2007) Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control 18:7–27
Nazar R, Iqbal N, Mashood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. J Plant Sci 3:1476–1489
Neumann D, Zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692
Pedrero Z, Elvira D, Cámara C, Madrid Y (2007) Selenium transformation studies during Broccoli (Brassica oleracea) growing process by liquid chromatography–inductively coupled plasma mass spectrometry (LC–ICP-MS). Anal Chim Acta 596:251–256
Pedrero Z, Madrid Y, Hartikainen H, Cámara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56:266–271
Polidoros AN, Scandalios JG (1999) Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.). Physiol Plant 106:112–120
Qian H, Li J, Sun L, Chen W, Sheng GD, Liu W, Fu Z (2009) Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat Toxicol 94:56–61
Qian H, Li J, Pan X, Jiang H, Sun L, Fu Z (2010) Photoperiod and temperature influence cadmium’s effects on photosynthesis-related gene transcription in Chlorella vulgaris. Ecotoxicol Environ Saf 73:1202–1206
Rehman B, Yusuf M, Khan TA, Fariduddin Q, Hayat S, Ahmad A (2016) Silicon elicited varied physiological and biochemical responses in Indian mustard (Brassica juncea): a concentration dependent study. J Plant Sci 16:1–10
Rossi L, Sharifan H, Zhang W, Schwab AP, Ma X (2018) Mutual effects and in-planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ Sci Nano (1):1–8
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sattar A, Mumtaz C, Shahzad MAB, Wahid A (2013) Optimization of source and rate of soil applied silicon for improving the growth of wheat. Pak J Agric Sci 50:63–68
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 217037:1–26
Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1; 2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29:475–486
Shute T, Macfie SM (2006) Cadmium and zinc accumulation in soybean: a threat to food safety? Sci Total Environ 371:63–73
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85:85–89
Souza VL, De Almeida AA, Lima SG, De M, Cascardo JC, Da C, Silva D, Mangabeira PA, Gomes FP (2011) Morphophysiological responses and programmed cell death induced by cadmium in Genipa americana L. (Rubiaceae). Biometals 24:59–71
Tang H, Liu Y, Gong X, Zeng G, Zheng B, Wang D, Zeng X (2015) Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ Sci Pollut Res 22:9999–10008
Terry N, Zayed MA, Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Mol Biol 51:401–432
Thiruvengadam M, Chung IM (2015) Selenium, putrescine, and cadmium influence health-promoting phytochemicals and molecular-level effects on turnip (Brassica rapa ssp. rapa). Food Chem 173:185–193
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants (review). Plant Cell Environ 13:195–206
Whitfield JB, Dy V, Mcquilty R, Zhu G, Heath AC, Montgomery GW, Martin NG (2010) Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium, and zinc in erythrocytes. Environ Health Perspect 118:776–782
WHO—World Health Organization resource (1993) Guidelines for drinking-water Quality: recommendations, second ed. Geneva 1:1–94
WHO—World Health Organization resource (1999) Monographs on selected medicinal plants. Geneva 1: 1–295
WHO—World Health Organization resource (2002) Drug information: herbal medicines, Geneva 16: 1–91
Wu Z, Wang F, Liu S, Du Y, Li F, Du R, Wen D, Zhao J (2016) Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 131:173–180
Wu Z, Liu S, Zhao J, Wang F, Du Y, Zou S, Li H, Wen D, Huang Y (2017) Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 133:1–11
Yao X, Jianzhou C, Xueli H, Binbin L, Jingmin L, Zhaowei Y (2013) Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol Environ Saf 92:320–326
Ye J, Yan C, Liu J, Lu H, Liu T, Song Z (2012) Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata (S., L.) Yong. Environ Pollut 162:369–373
Zembala M, Filek M, Walas S, Mrowiec H, Kornaś A, Miszalski Z, Hartikainen H (2010) Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 329:457–468
Zeraik AE, Souza FS, Fatibello-Filho O (2008) Development of a spot test for peroxidase activity monitoring during a purification procedure. Quím Nova 31:731–734
Acknowledgments
The authors thank the Coordenação e Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Fundação de Amparo à Pesquisa de Estado do Rio Grande do Sul for the research fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Pereira, A.S., Dorneles, A.O.S., Bernardy, K. et al. Selenium and silicon reduce cadmium uptake and mitigate cadmium toxicity in Pfaffia glomerata (Spreng.) Pedersen plants by activation antioxidant enzyme system. Environ Sci Pollut Res 25, 18548–18558 (2018). https://doi.org/10.1007/s11356-018-2005-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2005-3