Abstract
This paper evaluates the valorization potential of industrial hemp (Cannabis sativa L.) fibers produced on HM-contaminated soil as a safe feedstock for the textile industry. The chosen strategy was phytoattenuation, which combines the progressive soil quality improvement of contaminated land using phytoremediation techniques with the production of safe non-food biomass. A field experiment was set up with two hemp cultivars on a site contaminated with Cd, Pb, and Zn and on a nearby site containing clean soil as a control. Stem height and diameter were analyzed, as well as stem and fiber yield and the HM concentrations in the fibers, which were compared to legal safety standards and toxicity thresholds used in the textile industry. The hemp cultivar Carmagnola Selected (CS) had a significantly higher stem and bigger stem diameter compared to cultivar USO 31 on both sites. Stem yields showed a decrease of 30% and 50%, respectively, for both hemp cultivars grown on the contaminated site. However, the stem yield of CS growing on the contaminated site was similar to the stem yield of USO 31 growing on the control site, indicating that hemp cultivation on contaminated soil can be economically viable. Total and extractable Cd, Pb, and Zn fiber concentrations were far below the toxicity standards for textile production purposes. These results are promising in terms of the potential valorization of contaminated land with hemp cultivation and the development of a non-food value chain within a phytoattenuation strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concern about the environmental impact of textile production is increasing in Europe, and more attention is paid to its sustainable production (Sajin 2019). The textile industry is responsible for producing 8–10% of global CO2 emissions and for releasing volatile organic compounds and acid gasses into the air, causing respiratory diseases (Niinimäki et al. 2020). Also, microfibers from synthetic textiles are a major source of microplastics in the environment, and 35% of the microplastics in the oceans originate from the textile industry (Acharya et al. 2021). Cotton, one of the most popular natural fibers in the textile industry, has an important environmental footprint due to the large quantities of pesticides used for its cultivation, which covers 24% of the worldwide insecticide market (Ahirwar and Behera 2022), as well as due to high water and land use and pollution caused by transportation (Duque Schumacher et al. 2020). Thus, an economically viable and sustainable alternative to cotton (Gossypium hirsutum L.) and synthetic fibers must be introduced in the textile industry which can ensure a high, fast, and local production, to lower the ecological footprint of the industry.
One of the biomass crops that are getting increased attention worldwide in the textile industry is industrial hemp (Cannabis sativa L.). Hemp is an eco-friendly fast-growing crop that could present a more locally produced and sustainable alternative for synthetic fibers and the cotton industry due to its multiple advantages in cultivation (Piotrowski and Carus 2011). No pesticides are needed for hemp cultivation in comparison to cotton (Ahirwar and Behera 2022), and its fertilizer demand is low. The water needed for hemp cultivation is lower than one-third of that of cotton, and it requires only half the land to achieve a similar biomass production (Averink 2015). Moreover, hemp grows 2 times faster, has 3 times higher fiber yield, has between 2–3 times lower carbon emissions compared to cotton (Ahirwar and Behera 2022; Duque Schumacher et al. 2020), and has a positive effect on biodiversity when assessing for biodiversity friendliness, while cotton has a negative score (Piotrowski and Carus 2011). Although the current industrial hemp degumming process is not yet sustainable, reducing hemp’s advantage over cotton in overall production sustainability (Duque Schumacher et al. 2020), significant progress is being made toward sustainable degumming (Kalia et al. 2013; Kozłowski and Rózańska 2020; Nair et al. 2015). Moreover, the quality of hemp as a textile fabric, known to be quite stiff and ruff compared to cotton, has improved over the years thanks to adapted fiber treatment and spinning, making hemp fabric’s aesthetic value comparable to that of cotton (Vanderhoeven et al. 2020; Vandepitte et al. 2020). The remaining parts of the plant, mainly the shives, can be used in insulation panels in the eco-construction industry or as animal bedding in stables (Lühr et al. 2018; Musio et al. 2018; Paulitz et al. 2017), as well as for the production of bioenergy (Moscariello et al. 2021).
To develop a more sustainable value chain for textile production, keeping the value chain local is important. However, in Europe and especially densely populated West-European countries, such as Belgium, pressure on available land is constantly increasing (EEA 2019; Harvey and Pilgrim 2011). Among the solutions to decrease pressure on available land is the redevelopment of contaminated land. The European Environment Agency (2020) estimates that approximately 340,000 sites in Europe are contaminated, with several of these sites left unused. To lower the competition for land use and give a second life to these unused sites, biomass cultivation for the production of non-food bio-based resources can be a good candidate through phytomanagement and more specifically phytoattenuation (Edrisi and Abhilash 2016; Meers et al. 2005).
Phytoattenuation is the combined valorization and gradual soil remediation of contaminated land with the production of safe non-food biomass. This phytomanagement strategy focuses firstly on the production of high-yielding crops, accumulating only limited amounts of HMs, for safe biomass production, and secondarily on soil remediation (Linger et al. 2002a, b, c; Meers et al. 2010; Perlein et al. 2021; Shi et al. 2012; Thewys et al. 2010; Van Slycken et al. 2013). Phytoattenuation can be a solution to overcoming the economic constraints of phytoremediation projects. Industrial hemp is known to tolerate well HMs and thus could be grown on HM-contaminated land for the production of hemp biomass for non-food applications, such as the production of local hemp fibers for the textile industry (De Vos et al. 2022).
Previous research showed that HM uptake in hemp does not or only slightly affect the biomass yield, primarily in the leaf area, and does not affect fiber quality compared to hemp grown on non-contaminated land (Linger, et al. 2002a, b, c; Pietrini et al. 2019). However, knowledge is lacking on the uptake of HMs into the above-ground hemp plant parts, especially the total and extractable HM concentrations in the fibers, to ensure safe textile production. In our previous study (De Vos et al. 2022), the concentration in the different plant parts, including the fibers, was investigated and compared for 6 cultivars in a pot experiment. Two cultivars were selected based on their fiber quality and HM concentrations in the fibers for further investigation on a field scale. In this study, a field trial was carried out to evaluate the yielding capacity and HM uptake of these two hemp cultivars on a field scale and to confirm the safety of the fibers for use as feedstock for textile products and the potential of a phytoattenuation strategy with industrial hemp on HM contaminated land in favor of the textile industry.
Also, when cultivating hemp for textile fiber production, the hemp stems are retted after harvest. Retting is a process during which pectins, the cementing compounds that hold fibers together in the stem, are broken down by microorganisms. This facilitates the separation of the fibers from the shives in a further stage. Field retting is the easiest and most economically advantageous retting method, in which the hemp stems are spread out in the field after harvest for several weeks (Liu et al. 2015). However, when cultivating hemp on HM-contaminated soil, the question arises if HMs, accumulated into the fibers during hemp growth, go back to the soil when hemp stems are retted in the field. Indeed, it has been shown that HMs have a high affinity to bind with the pectin in the fibers (Krzesłowska 2011), which is broken down during the retting process. No study was found in the literature on this matter. For this reason, this study also investigated the potential influence of field retting on the HMs in the fibers and soil.
Material and methods
Site location
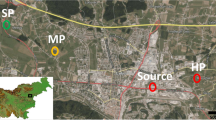
The field experiment took place in the North of France, on agricultural land nearby the former metallurgic industry of Metaleurop-Nord. The closure of this plant in 2003 left behind several economic, social, and environmental consequences for the region. Two experimental sites were used in this field experiment: one control site outside the contaminated zone of Metaleurop-Nord, at approximately 4.5 km from the former metallurgic site (50°23′26.2″N 3°01′10.2″E), and one contaminated site inside the contaminated zone of Metaleurop-Nord at approximately 2.5 km from the former industrial plant (50°24′59.1″N 3°02′03.3″E) (Fig. 5 in Appendix 1). The control site is considered non-contaminated, and the polluted site is moderately contaminated according to the legislation (Vlarebo 2008).
Experimental setup
The experimental design was set up the same way on both sites: an experimental parcel of 133.25 m2 was divided into 8 micro-parcels of 19.5 m2 with 0.5 m between each plot. Half of the parcels were sown with the hemp cultivar USO 31 (USO) and the other half with the hemp cultivar Carmagnola Selected (CS) in an alternating block design (Fig. 1).
Before sowing, the soil was prepared with 80 kg N/ha and 150 kg P/ha on the control site and 90 kg N/ha and 40 kg K2SO4/ha on the polluted site. The different fertilization on both sites is the result of different soil characteristics. Both hemp cultivars were sown on the 12th of June, with a sowing density of 50 kg/ha. The late sowing date is due to a very dry May month, with unsuited conditions for germination. The parcels were controlled regularly, scarecrows were placed to prevent seed loss to birds, and weeds were removed manually during the germination stage. One micro-parcel of USO 31 on the contaminated site was damaged by rodents during this stage and was lost for this experiment. Hot weather alternating with heavy rainfall occurred during the months of the growing stage. Hemp was harvested when it achieved 50% of flowering (Fig. 6a in Appendix 2), as this is recommended to obtain optimal fiber quality for textile production (Westerhuis et al. 2019). The hemp cultivar USO 31 was harvested on the 24th of August and the later cultivar Carmagnola Selected on the 10th of September with a manual electric hedge trimmer with a handle. Within each of the 8 micro-parcels, a surface of 3 m2 was demarcated for harvest (Fig. 1). The border of 1 m around this 3 m2 surface was not harvested due to potential border effects. A border of 2.5 m was left on the side where the machine started sowing to ensure correct sowing density, as the seeder needs 1 to 2 m to come to full sowing capacity.
Plant parameters: stem length and diameter, stem and fiber yield
Before harvest, the stem height and diameter of 10 plants per micro-parcel were measured. After harvest, the hemp was field retted for about 4 to 5 weeks and was turned over every 2 weeks, as recommended for hemp (Liu et al. 2015; Mazian et al. 2018) (Fig. 6b in Appendix 2). Per micro-parcel, 5 randomly chosen hemp plants were removed from the field directly after harvest for the analysis of HM concentrations in unretted hemp fibers. By the end of the retting period, the stems were gray, and fibers could easily be peeled off. Retted and unretted hemp stems were air-dried vertically in wooden boxes in a dry and ventilated barn and turned upside down once a week to prevent rotting until dry. To dry the stems completely, they were put inside a cupboard with integrated hot-air blowers during the 3 last days of the drying stage. The hemp bundles from each micro-parcel were weighed. The stems were passed through a lab-scale breaker unit (Worthmann Maschinenbau GmbH, Barbel-Harkebrügge, Germany) to separate the shives from the fibers (Fig. 6c in Appendix 2). Stem and fiber yields (kg/ha) were calculated by dividing respectively stem and fiber weight (kg) by the surface of the micro-parcel (ha).
Soil characterization
Before sowing, 3 soil samples were taken from each of the 8 micro-parcels on both the control and contaminated sites to characterize the soil and analyze its heavy metal content. An auger was used to collect soil samples at 0–30-cm depth. Before soil characterization, the soil samples were sieved to pass a 2-mm sieve and oven-dried at 105 °C for 24 h. Soil pH, EC, and CEC were measured following standard methods described by Van Ranst et al. (1999). The water holding capacity (WHC) of the soil was determined by adding excess water to 500 g of dry-weight soil. When water droplets stopped leaching from the pot, the soil was weighed again to obtain an estimation of the WHC. Organic matter (OM) content was measured through loss-on-ignition in a muffle furnace (LacCore 2013). The percentages of silt, clay, and sand particles were measured with a Bouyoucos Hydrometer (1962) to determine the soil texture. Total nitrogen was measured using the Kjeldahl digestion method.
Macro- and micro-nutrients, as well as total HM concentrations in soil, were determined by aqua regia digestion, inductively coupled plasma-optical emission spectroscopy (ICP-OES), and inductively coupled plasma mass spectrometry (ICP-MS). Aqua regia digestion was performed by weighing 1 g of soil per sample in an Erlenmeyer flask, where after 2.5 mL of deionized water, 2.5 mL of 65% HNO3, and 7.5 mL of 37% HCl were added. Erlenmeyer flasks were heated on a hot plate at 150 °C for 2.5 h after being covered with a watch glass and kept overnight for pre-digestion. After cooling down to room temperature, the digested soil solutions were passed through a Whatman filter paper (Cytiva, USA) and put into a 50-mL volumetric flask, pre-rinsed with 1% HNO3. Total concentrations of nutrients and HMs in the soil solutions were then determined with ICP-OES (Varian Vista MPX, USA) and ICP-MS (Thermo Scientific iCAP Q., USA).
To determine the phytoavailable HM fraction in the soil, a single CaCl2 extraction was performed by weighing 10 g of oven-dried soil in a glass beaker and adding 50 mL of 0.01 M CaCl2 previous to mixing the soil solution for 2 h on an orbital shaker (Meers et al. 2007). After passing the solution through an ashless white filter paper (MN 640 m), phytoavailable HM concentration was determined using ICP-OES. This phytoavailable HM fraction in the soil describes the available fraction of the heavy metals in the soil for plant uptake. It is presented as a percentage and was calculated as the following:
Cultivar choice
Two EU-registered hemp cultivars were chosen based on their flowering stage, yield, fiber quality, and suitability for cultivation in Central Europe, as well as based on their total HM concentrations in the authors’ former study (De Vos et al. 2022). Their characteristics are shown in Table 1.
Heavy metal concentrations in the fibers
The oven-dried fibers, previously rinsed with Milli-Q water to remove dust particles, were ground with a cross-beater mill (Gladiator BO 3567). Per sample, 0.4 g fiber was weighed in a microwave tube, 5 mL of 67–69% HNO3 was added, and closed microwave digestion was performed (ultraWAVE, Milestone Srl, Italy). After cooling down to room temperature, digested samples were transferred to centrifuge tubes and filled up to 50 mL with Milli-Q water. Whatman filter paper (Cytiva, USA) was used to filter the solutions before total HM concentration was analyzed with ICP-OES (Varian Vista MPX, USA) and ICP-MS (Thermo Scientific iCAP Q., USA).
The extractable HM concentrations were analyzed by exposing the rinsed and oven-dried retted fibers to an artificial acid sweat solution containing 0.5 g/L C6H9O2N3.HCl.H2O, 5 g/L NaCl, and 2.2 g/L NaH2PO4.H2O, according to ISO 105-E04:2013(E)), also used by OEKO-TEX to determine extractable HM concentrations and safety limits (OEKO-TEX 2022). One gram of fiber per sample was put into a beaker with 50 mL of sweat solution and covered with a cap. The fibers sat for 30 min in the solution, and the mixture was shaken regularly. Hereafter, the fibers were removed and squeezed out until no more solution was leaching. The solution was filtered with syringe filters (0.45 µm pore size) (Chromafil, Macherey–Nagel, Germany) and analyzed for Cd, Pb, and Zn concentration via ICP-MS (Thermo Scientific iCAP Q., USA).
The bioconcentration factor (BCF) was calculated for the total aboveground plant biomass using total (BCFtot) and extractable (BCFextr) HM concentrations in the soil. The BCF indicates the plants’ ability for HM uptake from the soil into the plant tissues (Mishra and Pandey 2018). It is calculated for the different plant parts as follows:
Statistical analysis and calculations
Statistical data analysis was performed using MS Excel and SPSS Statistics 28. Before comparing means, the homogeneity of variance was tested. When the data were parametric, the data of the yields and HM concentrations in the soil and the hemp fibers were subjected to independent samples T-test to determine significant differences (p < 0.05) between means when comparing sites as well as cultivars. When the data were non-parametric, the 2 independent samples test was performed. The two-sided p was used to interpret the significance.
Results and discussion
Soil characterization
A summary of the physical and chemical properties of the soils used in this study is shown in Table 2. The OM of productive agricultural soils is generally between 3 and 6%, which corresponds with the OM of both experimental soils. Nevertheless, the OM of the contaminated site is 2 × higher compared to the OM of the control site. According to the USDA texture diagram (USDA 2017), the texture of the control and contaminated soils are, respectively, loam and clay. A loamy soil texture is considered a well-suited texture for hemp, while clay soil is less optimal (Harper et al. 2018). The CEC is extremely low for both sites, as, in loamy soils, CEC values lie in general between 5 and 15 meq/100 g, while in clay soils, they exceed 30 meq/100 g (Culman et al. 2019).
Total metal and metalloid analysis of the soil (Table 3) showed that all concentrations were significantly higher in the contaminated soil compared to the control soil (p < 0.05), but only Pb, Cd, and Zn concentrations exceeded legal sanitation thresholds (mg/kg) for agricultural soils according to the Flemish legislation (Vlarebo 2008). Other elements stayed below background concentrations. Extractable HM concentrations showed different results when comparing both sites. The control site, when compared to the contaminated site, had higher extractable Al, Fe, and Mn concentrations (p < 0.05), similar extractable Co, Cr, Cu, Ni, and Zn concentrations (p ≥ 0.05), and lower extractable Pb and Cd concentrations (p < 0.05). Extractable Pb and Cd exceeded legal sanitation thresholds for the HM concentration in groundwater.
The phytoavailable HM fraction (%) in the soil is related to the extractable HM fraction and was small compared to the total HM concentration in both soils, ranging in decreasing order of phytoavailability for the control soil as follows: Cd (4.3%) > Co (0.79%) > Cu (0.76%) > Ni (0.20%) > Pb (0.18%) > Zn (0.13%) > Cr (0.093%) > Fe (0.011%) > Al (0.011%). The contaminated soil followed a similar pattern, and the phytoavailability in it was similar to or lower than in the control site and ranged as follows: Cd (2.1%) > Co (0.76%) > Cu (0.46%) > Ni (0.16%) > Pb (0.11%) > Zn (0.062%) > Cr (0.087%) > Fe (0.0058%) > Al (0.0055%). The lower phytoavailability on the contaminated site should be attributed to the phenomena of “soil aging,” which results in the lower availability of HMs over time through interaction and complexation with soil particles (Lock and Janssen 2003).
Yields
Two hemp cultivars USO 31 and Carmagnola Selected (CS) were cultivated under field conditions in control and contaminated soil to better understand the impact of contamination on yields and the HM concentration in the hemp fibers. The stem height, stem diameter at harvest, stem yield, fiber yield, and fiber content are presented in Table 4.
Stem height and diameter were significantly higher on the control site compared to the contaminated site (p < 0.05) for both hemp cultivars, which also resulted in higher stem and fiber yield on the control site. Nevertheless, the fiber content of both cultivars was quite similar for both the control and the contaminated site. The presence of high concentrations of HMs on the contaminated site could play a role in the lower stem height, diameter, and yield of the hemp plants. Pietrini et al. (2019) reported no significant differences in stem height and stem diameter between the control and the moderately contaminated site; however, the HM concentrations in the soil they used were half the value of the HM concentrations in this study. Guidi Nissim et al. (2018) observed no inhibition of hemp growth on moderately contaminated soils, with Pb and Zn concentrations similar to this study, but with around 50% lower Cd concentrations. Nevertheless, growth inhibition of around 50% was noticed by Shi et al. (2012) for a Cd concentration of 25 mg/kg in the soil, which is 5 × higher compared to the Cd concentration of the contaminated soil in this study. Higher Cd concentrations in this study could thus negatively impact the hemp height and stem diameter. Luyckx et al. (2021) showed a significant reduction in xylem vessel diameter when hemp was grown on Cd-contaminated land. This can affect the stem height and diameter and eventual stem yield. The study showed no significant reduction in xylem vessel diameter when growing on Zn-contaminated soil. Nevertheless, other studies have shown that hemp can grow on soil with much higher contamination levels (17 mg/kg) without negative effects (Linger et al. 2005). Soil characteristics also play an important role in the difference in stem height and stem diameter (Tang et al. 2016). The 2 sites were located only 2 km away from each other to have a control and a contaminated site with different contamination levels but similar soil characteristics. However, soil characteristics were still not identical (Table 2). The soil texture on the control site was loamy, which is optimal for hemp growth, while clayish soil was present on the contaminated site, which is less optimal since excess soil wetness can lead to plant rotting (Roseberg et al. 2019).
When comparing hemp cultivars, CS had a significantly higher stem height and bigger stem diameter compared to USO 31 on both sites. This is the consequence of differences in hemp genotypes, i.e., USO 31 is an early cultivar, resulting in a shorter growth stage until flowering, while CS is a late cultivar with a longer growth stage and is known for high biomass yields (Table 1). As plants are harvested at flowering, late cultivars which have a longer growth period in the field will tend to produce more stem mass (Struik et al. 2000; Tang et al. 2016; Vandepitte et al. 2020).
The cultivation in the contaminated site had a greater impact on cultivar CS compared to USO 31 (Table 4). Stem yields were 36% and 52% higher on the control site respectively for USO 31 and CS compared to the contaminated site. For both cultivars, stem yields on the control site were very similar to the stem yields of hemp grown on non-contaminated agricultural soil in the study of Vandepitte et al (2020). This shows that the cultivars grew as expected on the control soil. Even though stem yields on the contaminated soil were significantly lower, CS grown on the contaminated site still had stem yields similar to USO 31 stem yields on non-contaminated soil, as well as to 2 other hemp cultivars grown on non-contaminated soil from the study of Vandepitte et al. (2020). This shows that still a sufficient stem yield can be obtained on contaminated soil for hemp biomass production through phytomanagement. Moreover, in the context of the valorization of contaminated sites, this is a good opportunity for contaminated land that would be left unused due to the contamination, otherwise, with interesting yields and sufficient fiber content when growing industrial hemp.
When looking at the fiber yields, 32% and 54% higher yields (p < 0.05) were observed on the control site for, respectively, USO 31 and CS. Fiber yields from the control site were lower compared to the fiber yields from the study of Vandepitte et al. (2020) for both USO 31 and CS. This can be because the hemp fibers of this study were separated from the shives manually, inducing more fiber loss, while in the study of Vandepitte et al. (2020), hemp scutching took place on an industrial flax line. For both hemp cultivars, the fiber content (retted fiber weight/retted stem weight) did not differ significantly between the control and contaminated site (Table 4). The fiber content of USO 31 was significantly higher compared to CS on both sites, which was expected as the early cultivar USO 31 is known for its higher fiber content compared to the late cultivar CS (Vanderhoeven et al. 2020; Troch et al. 2020).
Heavy metal uptake in the fibers
Total heavy metal concentration
Total Cd, Pb, and Zn concentrations in the fibers of both hemp cultivars were measured on the control and contaminated site, as shown in Fig. 2.
Total Cd, Pb, and Zn concentrations were significantly higher (p > 0.001) in the fibers produced on the contaminated site compared to the control site for both hemp cultivars. The increase in concentrations in the contaminated fibers compared to the control fibers was similar for the two cultivars, of around 1.5 × for Zn and 2.5 × for Cd and Pb. Angelova et al. (2004) analyzed the HM concentrations in hemp fibers grown on contaminated soil with similar concentrations to those in the present study for Pb, 2.5 × higher for Cd, and 1.5 × higher for Zn. The HM concentration in the fibers was also approximately 2 × to 3 × higher for Cd and 1.5 × higher for Zn compared to the present study, showing a similar rate of HM uptake in the fibers. However, for Pb, results differed with fiber concentrations 3 × to 6 × higher in the study of Angelova, while soil concentrations were similar. This indicates that HM uptake does not only depend on the HM concentration in the soil but also depends on soil characteristics and plant genotype, as well as climatic conditions (Husain et al. 2019; Tang et al. 2016).
Looking at the potential safe use of the hemp fibers coming from a contaminated site for textile production, the HM concentrations in the fibers were assessed against the OEKO-TEX toxicity control values, which is a worldwide used label in the textile industry to guarantee qualitative and safe products to the customer. Total Pb and Cd concentrations were far below the toxicity limits (40 mg/kg Cd and 90 mg/kg Pb), while no toxicity limit for total Zn concentration is included in the OEKO-TEX standards. Sungur and Gülmez (2015) analyzed the concentrations of HMs in dyed textile fabrics from different textile plants. In cotton fibers, Cd concentrations were around 61 × and 19 × higher compared to the Cd concentrations in the fibers of respectively USO 31 and CS. Pb concentrations were around 12 × and 2.5 × higher compared to Pb concentrations in, respectively, USO 31 and CS fibers. The analysis of different synthetic fabrics also showed far higher Cd and Pb concentrations compared to the concentrations in the fibers of this study, while all fabrics in the study of Sungur and Gülmez (2015) met the OEKO-TEX safety standards. These results show that the dyes are the major factor for increased concentrations of HMs in the fibers and fabrics and that the contamination in the fibers itself seems to be low compared to the added HM concentrations through dyes. As a consequence, these results are promising for the production of dyed fabrics made from fibers grown on contaminated land, meeting the OEKO-TEX standards. However, natural and sustainable dyes should always be preferred.
Extractable heavy metal concentration
Besides total HM concentration, the extractable HM concentration is essential from a safety point of view to evaluate the risks and potential safe use of the fibers for textile production. While part of the HMs stays bound in the fiber, the extractable part of the HMs can come in contact with human skin through sweating and can end up in the water when textile products are washed. Legal thresholds, as well as OEKO-TEX thresholds, exist for extractable HMs in textile products. Therefore, extractable Cd, Pb, and Zn concentrations in the fibers of both hemp cultivars were measured on the control and contaminated sites, as shown in Fig. 3.
Similar patterns in extractable Cd, Pb, and Zn concentrations were found for USO 31 when comparing sites. For the 3 elements, the extractable concentration in the fibers from the control site was slightly higher compared to the fibers from the contaminated site, even though the total HM concentrations were always higher in the fibers from the contaminated site (Fig. 2). For the CS cultivar, the opposite trend was shown for the 3 elements, i.e., the fibers from the control site had lower extractable concentrations than the ones from the contaminated site, and this difference was significant for the 3 elements (p < 0.05). However, even if differences were occasionally significantly different, the extractable concentrations were overall quite similar between the fibers from control and contaminated sites.
When looking at the difference between hemp cultivars on the same site, extractable Cd, Pb, and Zn concentrations in the fibers from the control site were overall higher in USO 31 compared to CS. On the contaminated site, the opposite trend was shown except for Pb. Nevertheless, even though significant, the differences in HM concentrations between the cultivars on the same site were very small. The extracted fraction of the total HM concentration was 38% for Cd, 27% for Pb, and 29% for Zn in the USO 31 control fibers and, respectively, 12%, 10%, and 18% for Cd, Pb, and Zn in the contaminated fibers. For the CS cultivar, 6%, 3%, and 11% of, respectively, total Cd, Pb, and Zn concentrations were extracted from the control fibers and 7%, 2%, and 7% of, respectively, total Cd, Pb, and Zn concentration was extracted from the contaminated CS fibers. These results show that Cd and Zn seem to become mobile more easily when subjected to a sweat or washing solution compared to Pb. This could mean that Cd and Zn are more weakly bonded to the fibers compared to Pb or that Pb is accumulated in lesser accessible fiber parts. According to Fritz (2007) and Krzesłowska (2011), Pb2+ cations are more strongly bonded to pectin, a component of the hemp fiber, compared to Zn2+ and Cd2+ cations.
When assessing the extractable Cd, Pb, and Zn concentrations in the hemp fibers against the OEKO-TEX standards, the values were far below the toxicity limits for Cd, Pb, and Zn (0.1 mg/kg, 1.0 mg/kg, and 750 mg/kg). The extractable concentrations were also compared to legal standards from Regulation (EC) No 1907/2006 (appendix 12) of the European REACH legislation (EC 2018). These legal standards demand Pb and Cd concentrations below 1 mg/kg after extraction, which were also met in this study, while Zn is not included in the legislative limits as a toxic element in textile products. It should be noted that the discussed OEKO-TEX standards apply to adult clothing products and that for baby textile products the extractable Pb toxicity limits are lower (0.2 mg/kg). Thorough control or even avoidance of use is advised when using the fibers for baby textile products since extractable Pb concentrations of the hemp fibers in the present study exceeded this limit. This was only the case for USO 31, which has been shown to have a higher affinity to Pb uptake compared to CS (De Vos et al. 2022). However, it should be noted that the extractable Pb concentrations in the control fibers were higher (p < 0.05) compared to that in the contaminated fibers and that thus careful control of the Pb concentrations in textile fibers should occur in all fibers and not only in those coming from a contaminated site. Sungur and Gülmez (2015) analyzed the extractable fraction of HMs in different dyed fabrics through an artificial sweat solution, very similar to the one used in this study. Extractable Pb concentrations in dyed cotton and acrylic fabrics were around 8 × and 10 × times higher compared to Pb concentrations in, respectively, USO 31 and CS fibers of this study, while Cd and Zn concentrations were not analyzed. These results show that, as with the total HM concentrations, the extractable HM concentrations from the dyes are much more important and the extractable concentration of HMs coming from the contamination on-site could be considered negligible compared to that of HMs in dyes. Again, natural dyes should be chosen to lower the HM concentrations to a minimum in textile products.
Influence of field retting on the HM concentrations in the hemp fibers
Cd, Pb, and Zn concentrations were analyzed in the hemp fibers right before harvest and after field retting. The HM concentrations in unretted and retted USO 31 and CS fibers are presented in Table 5.
For hemp cultivar USO 31, Cd and Zn concentrations overall did not differ between unretted and retted fibers on both sites. Pb concentrations were significantly lower in unretted compared to retted fibers in the control site, while the opposite was true for the contaminated site. Nevertheless, HM concentrations could be considered similar in unretted and retted USO 31 fibers due to the small differences observed, even when statistical significance was found. The small decrease in Pb concentration in the contaminated site could be due to the degradation of pectin in the fibers during the retting process. It has been shown that pectin can bind divalent and trivalent metal ions as a defense mechanism against HM stress and that Pb2+ has a higher binding affinity than Cd and Zn (Krzesłowska 2011). For the CS fibers, all 3 elements showed significantly higher concentrations in the retted fibers compared to the unretted fibers, on both the control and contaminated site. With retting, the HM concentrations in the fibers increased 4 × , 7 × , and 3 × , respectively, for Cd, Pb, and Zn, on the control site and 7 × , 10 × , and 3 × , respectively, for Cd, Pb, and Zn, on the contaminated site.
The reason that CS fibers showed a significant increase in HM concentrations upon retting while USO 31 fibers did not can be due to the difference in climatic conditions of the respective retting periods. The hemp cultivar CS was harvested 17 days later than USO 31 and was retted for 5 weeks, while USO 31 was retted for 4 weeks. The retting period of USO 31 was associated with low rainfall, while the retting period of CS included frequent rains, which resulted in more extensively retted CS fibers compared to the USO 31 fibers. The degree of retting can be evaluated by the ease of separating the fibers from the woody core and by the change in color of the fibers. A higher degree of retting results usually in darker fibers (Manian et al. 2021) (Fig. 4).
Since retting degrades pectin in the hemp fiber and the study of Krzesłowska (2011) has shown the affinity of HMs to bind with pectin, a reduction in HM concentrations due to pectin removal could be expected. However, the results in this study showed that field retting on HM-contaminated soil overall increased the HM concentrations in the fibers. During pectin removal through retting, the hemp fiber bundles and fibers, bound together by pectin, are separated, and fibers are less enclosed in the hemp stem, thereby being more in contact with external influences, such as soil moisture. Hemp fibers have a hydrophilic nature and thus tend to take up moisture. The cellulose in the fibers contains free hydroxyl groups which easily form hydrogen bonds with water molecules (Shahzad 2012). Fibers are also known to be excellent sorbents for HMs in wastewater treatment (Morin-Crini et al. 2019). Studies have shown that functional groups such as carboxylic, carbonyl, and hydroxyl groups present in hemp fiber components (cellulose, hemicelluloses, lignin, and extractives) have a high affinity for binding metal ions (Mongioví et al. 2021; Tofan et al. 2010). Thus, it could be that the hemp fibers took up some soil humidity containing HMs while retting on the ground. Since the fibers were well washed and dried before analysis, the argument of the increase in HM concentration due to soil dust on the fibers or difference in moisture content can be rejected. Also, the hypothesis that the increase in HM concentrations would be the result of the degradation of lignin, containing no HMs in this hypothesis, can be rejected since hemp fibers contain approximately 1–17% of pectin (Liu et al. 2017a, b), resulting thus in a maximal decrease in biomass by 17%, which corresponds to an increase in HM concentration with maximal multiplication factor 1.2, while HM concentrations multiplied at least 3 × up to 10 × . As a consequence, these high increases in HM concentrations cannot be due only to weight loss due to lignin removal.
Field retting seems thus to be less suitable when aiming for the lowest HM content in the fibers. Moreover, field retting is very difficult to control since it is dependent on the climate, resulting in difficulties to maintain the same fiber quality over the years (Troch et al. 2020), which is important for textile production. Thus, other retting methods than field retting, such as enzymatic retting (Dreyer et al. 2002; Liu et al. 2017a) or chemical retting methods (Hurren et al. 2012), should be investigated in any circumstances but especially when cultivating hemp on non-contaminated soil.
Bioconcentration factor
The bioconcentration factor (BCF) was calculated for the fibers with total and extractable Cd, Pb, and Zn concentrations as presented in Table 6.
The BCFtot was far below 1 for the 3 elements, indicating that hemp does not tend to accumulate these HMs in its fibers. Previous literature has shown that hemp is not a candidate for phytoextraction when calculating the BCF with the total above-ground biomass (Usman et al. 2019). The results on BCFtot in this study are in line with the findings on BCFtot in the hemp cultivars on a greenhouse scale in the authors’ previous study (De Vos et al. 2022), indicating that the study at the greenhouse level is reliable. Hemp fibers tend to accumulate Cd and Zn the most easily, while Pb is taken up to a lesser extent in the fibers, as shown in Table 6. Pietrini et al. (2019) showed that Cannabis sativa L. tends to accumulate Pb mainly in the roots, with minimal translocation to the above-ground biomass, which explains the relatively low BCFtot for Pb in the fibers in the present study. For hemp cultivar USO 31, the BCFtot was higher on the control site compared to the contaminated site for Cd and Zn. The lower BCFtot on the contaminated site can be attributed to the phenomenon of soil ageing, i.e., that a fraction of the HMs present in the soil forms complexes with soil particles over time, rendering this fraction non-available for plant uptake. However, for Zn, the difference was not significant (p > 0.05). For Pb, the BCFtot was significantly higher on the contaminated site compared to the control site. This can be linked to the high affinity for cultivar USO 31 to take up Pb in its above-ground biomass (De Vos et al. 2022). For the CS cultivar, the BCFtot was similar on control and contaminated soil for all 3 elements (p < 0.05), thus the more HMs are concentrated in the soil, the more HMs are taken up in the fibers of the plant.
When looking at the BCFextr, different results than for the BCFtot are found. All BCFextr, except for Cd for contaminated CS fibers, were above 1, indicating that hemp fibers have a certain affinity to take up these HMs, with accumulation rising in the following order: Cd < Pb < Zn. Cd tends to be less bioavailable to plants compared to Pb and Zn but also tends to be less complexed with soil particles during soil ageing, resulting in a greater bioavailable fraction compared to Pb and Zn over time. These results were similar to the study of Hadi et al. (2014), showing that the extractable HM concentrations tend to be comparable to spiked HM concentrations that are still fully bioavailable to plants, and that soil ageing significantly lowers the bioavailability of HMs to plants (Roca et al. 2017). Moreover, Di Candilo et al. (2004) showed that increasing spiked concentrations of Cd results in increased BCF in upper plant parts in hemp, while for Pb, the BCF declines when spiked concentrations in the soil increase. This can indicate that, apart from soil ageing, different plant uptake mechanisms for Cd and Pb also play a role in the HM uptake in the plant. Finally, since Zn is an essential element, it is likely to be taken up in greater amounts compared to Cd and Pb, which are non-essential elements to the plant (Nordløkken et al. 2015).
BCFextr was similar on control soil and contaminated soil for Cd and Zn for hemp cultivar USO 31, indicating that the ratio between the total HM concentration in the fibers and the available HM concentration in the soil is constant. In other words, the more HMs are available in the soil, the more HMs will be concentrated in the hemp fibers. For Pb, the BCFextr was higher in contaminated soil compared to the control site, supporting the fact that USO 31 has shown an affinity for the uptake of Pb in its above-ground biomass, including the fibers (De Vos et al. 2022). For hemp cultivar CS, The BCFextr was similar on control and contaminated sites for Pb. The BCFextr for Cd was lower in the contaminated soil compared to the control soil, indicating that only a limited amount of the phytoavailable Cd is taken up by the plant into the fibers. For Zn, it was the way around, as the BCFextr was higher on the contaminated site (p > 0.05), showing that HM uptake in the fibers increased.
Cannabis sativa L. is generally not presented in the literature as a hyperaccumulator but as a high biomass crop that tends to tolerate HM contamination in soil and as a suitable candidate for phytoremediation when coupled with biomass production for economic valorization (Citterio et al. 2003; Linger et al. 2002a, b, c; Rheay et al. 2021). This tolerance to HMs is achieved through a plant-specific defense mechanism (Citterio et al. 2003; Husain et al. 2019; Kos et al. 2003; Linger et al. 2002a, b, c) and is the consequence of 2 genes detected in Cannabis sativa L., which are expressed under HM stress to protect the plant cells against oxidative damage (Ahmad et al. 2016). When comparing the BCF of Cannabis sativa L. with a well-studied hyperaccumulator Thlaspi caerulescens, the phytoextraction potential of the latter was over 16 times higher for Cd uptake (Citterio et al. 2003; Linger et al. 2002a, b, c).
This study shows that conclusions about the phytoremediation potential of industrial hemp will differ depending on the use of BCFtot or BCFextr. In general, the BCFtot is used when assessing the affinity of a crop toward HM uptake (Delplanque et al. 2013; Hosman et al. 2017; Paulauskas and Kasiulienė, 2019; Shi et al. 2012; Thijs et al. 2018; Usman et al. 2019; Xiao et al. 2015). However, since only the available fraction of the HMs present in the soil can be taken up by plants, the authors believe that the BCFextr would be better suited as a measure to compare different crops and plants for their phytoremediation potential, especially when comparing plants growing under different soil conditions. In that case, this study shows that Cannabis sativa L. has only a very limited tendency to extract Cd from the soil into its fibers, a low tendency to extract Pb, and does extract Zn into its fibers. Since Zn is an essential element, it seems to be taken up in fixed amounts. Finally, when comparing plants growing in different soil conditions, BCFextr can show the differences in HM uptake between plant species or soil organisms linked to the specific HM uptake mechanism of these certain species. This cannot be done with BCFtot since it does not take into account HM interactions with the soil related to soil properties and soil ageing processes.
Conclusion
In this study, the growth of industrial hemp on land contaminated by Cd, Pb, and Zn was assessed, as well as the HM accumulation in the hemp fibers. Contrary to several previous studies, stem yields showed a significant decrease on the contaminated site when compared to the control site. However, the yield was still sufficient to make this cultivation attractive for the valorization of a contaminated unproductive site. Also, yields are influenced by the soil characteristics, e.g., soil structure or nutritional state, and therefore, the lower yields found might not have been directly influenced by the soil contamination. CS had the highest stem and fiber yield on the contaminated site, while USO 31 had the highest fiber content. Higher stem height and bigger stem diameter were observed for CS compared to USO 31 on both sites. Looking at the contamination levels, significantly higher total Cd, Pb, and Zn concentrations were observed in the contaminated fibers compared to the control fibers of both hemp cultivars, while for the extractable HM concentrations, the differences were small, and extractable HM concentrations could be considered as similar in control and contaminated fibers. Both total and extractable Cd and Pb concentrations in the fibers were far below the toxicity limits from the OEKO-TEX label, which suggests the safe use of the fibers for textile production. The BCFtot. was below 1, indicating that hemp fibers do not tend to accumulate HMs, while the BCFextr. showed that hemp tends to take up the bioavailable fraction of HMs in the soil in the following order: Cd < Pb < Zn. Finally, field retting can result in significantly higher hemp fiber concentrations, as was seen in CS fibers. When cultivating hemp for textile production, other retting methods should be preferred to prevent additional HM uptake in the fibers. The results of this study are very promising for coupling the valorization of contaminated soils with the use of hemp fibers as a safe feedstock for the textile industry.
Data availability
All data are true and valid and can be available.
References
Acharya S, Rumi SS, Hu Y, Abidi N (2021) Microfibers from synthetic textiles as a major source of microplastics in the environment: a review. Text Res J 91(17–18):2136–2156. https://doi.org/10.1177/0040517521991244
Ahirwar M, Behera BK (2022) Development of hemp-blended cotton fabrics and analysis on handle behavior, low-stress mechanical and aesthetic properties. The J Text Ins 113:5, 934-942. https://doi.org/10.1080/00405000.2021.1909799
Ahmad R, Tehsin Z, Malik ST, Asad SA, Shahzad M, Bilal M, Shah MM, Khan SA (2016) Phytoremediation potential of hemp (Cannabis sativa L.): identification and characterization of heavy metals responsive genes. Clean - Soil, Air, Water 44(2):195–201. https://doi.org/10.1002/clen.201500117
Angelova V, Ivanova R, Delibaltova V, Ivanov K (2004) Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind Crops Prod 19(3):197–205. https://doi.org/10.1016/J.INDCROP.2003.10.001
Averink J (2015) Global water footprint of industrial hemp textile. University of Twente, Enschede. Retrieved on 28 November 2021 from http://essay.utwente.nl/68219/
Citterio S, Santagostino A, Fumagalli P, Prato N, Ranalli P, Sgorbati S (2003) Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 256(2):243–252. https://doi.org/10.1023/A:1026113905129
Culman S, Mann M, Brown C (2019) Calculating cation exchange capacity, base saturation, and calcium saturation. Agriculture and natural resources. Retrieved on the 12th April 2022 from https://ohioline.osu.edu/factsheet/anr-81
De Vos B, Souza MF, Michels E, Meers E (2022) Industrial hemp (Cannabis sativa L) in a phytoattenuation strategy: remediation potential of a Cd, Pb and Zn contaminated soil and valorization potential of the fibers for textile production. Ind Crops Prod 178:114592. https://doi.org/10.1016/J.INDCROP.2022.114592
Delplanque M, Collet S, Del Gratta F, Schnuriger B, Gaucher R, Robinson B, Bert V (2013) Combustion of Salix used for phytoextraction: the fate of metals and viability of the processes. Biomass Bioenerg 49:160–170. https://doi.org/10.1016/j.biombioe.2012.12.026
Di Candilo M, Ranalli P, Dal Re L (2004) Heavy metal tolerance and uptake of Cd, Pb and Tl by hemp. Adv Hortic Sci 18(3):138–144
Dreyer J, Müssig J, Koschke N, Ibenthal W-D, Harig H (2002) Comparison of enzymatically separated hemp and nettle fibre to chemically separated and steam exploded hemp fibre. J Ind Hemp 7(1):43–59. https://doi.org/10.1300/J237v07n01\_05
Duque Schumacher AG, Pequito S, Pazour J (2020) Industrial hemp fiber: a sustainable and economical alternative to cotton. J Clean Prod 268:122180. https://doi.org/10.1016/j.jclepro.2020.122180
Edrisi SA, Abhilash PC (2016) Exploring marginal and degraded lands for biomass and bioenergy production: an Indian scenario. Renew Sustain Energy Rev 54:1537–1551. https://doi.org/10.1016/j.rser.2015.10.050
European Commission (2018) COMMISSION REGULATION (EU) 2018/1513 of 10 October 2018. Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards certain substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR), category 1A or 1B. Retrieved on 10 January 2023 from https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1513&from=EN
European Environment Agency (2019) EEA Signals 2019-Land and soil in Europe. In: European Environment Agency. Retrieved on 25 November 2021 from https://doi.org/10.2800/779710
European Environment Agency (EEA) (2020) Soil contamination widespread in Europe. Retrieved on the 19th of April 2022 from https://www.eea.europa.eu/highlights/soil-contamination-widespread-in-europe
Fritz E (2007) Measurement of cation exchange capacity (CEC) of Plant cell walls by X-ray microanalysis (EDX) in the transmission electron microscope. Microsc Microanal 13(4):233–244. https://doi.org/10.1017/S1431927607070420
Guidi Nissim W, Palm E, Mancuso S, Azzarello E (2018) Trace element phytoextraction from contaminated soil: a case study under Mediterranean climate. Environ Sci Pollut Res 25(9):9114–9131. https://doi.org/10.1007/s11356-018-1197-x
Hadi F, Hussain F, Hussain M, Ahmad A, Ur Rahman S, Ali N (2014) Phytoextraction of Pb and Cd; the effect of urea and EDTA on Cannabis sativa growth under metals stress. Int J Agron Agric Res 5(3):30–39
Harper J, Collins A, Kime L, Roth G (2018) Industrial hemp production. Pennsylvania State University. Retrieved on 21 November 2021 from https://extension.psu.edu/industrial-hemp-production
Harvey M, Pilgrim S (2011) The new competition for land: food, energy, and climate change. Food Policy 36(SUPPL. 1):S40–S51. https://doi.org/10.1016/j.foodpol.2010.11.009
Hosman ME, Soliman El-Feky S, Elshahawy MI, Shaker EM (2017) Mechanism of phytoremediation potential of flax (Linum usitatissimum L.) to Pb, Cd and Zn. Pelagia Res Libr Asian J Plant Sci Res 7(4):30–40. Retrieved on 2 February 2022 from www.pelagiaresearchlibrary.com
Hurren CJ, Wang X, Dennis HGS, Clarke AFK (2012) Evaluation of bast fibre retting systems on hemp. January 2012, 1–9. Retrieved on 4 February 2022 from https://www.researchgate.net/publication/237746650_Evaluation_of_bast_fibre_retting_systems_on_hemp
Husain R, Weeden H, Bogush D, Deguchi M, Soliman M, Potlakayala S, Katam R, Goldman S, Rudrabhatla S (2019) Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS One 14(8):1–14. https://doi.org/10.1371/journal.pone.0221570
Kalia S, Thakur K, Celli A, Kiechel MA, Schauer CL (2013) Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: a review. J Environ Chem Eng 1(3):97–112. https://doi.org/10.1016/j.jece.2013.04.009
Kos, B., Grčman H, Leštan D (2003) Phytoextraction of lead, zinc and cadmium from soil by selected plants. Plant, Soil Environ 49(12):548–553. https://doi.org/10.17221/4192-pse
Kozłowski, RM, Rózańska W (2020) Enzymatic treatment of natural fibres. Handb. Nat. Fibres Vol. 2 Process. Appl 227–244. https://doi.org/10.1016/B978-0-12-818782-1.00007-9
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33(1):35–51. https://doi.org/10.1007/s11738-010-0581-z
LacCore (2013) Loss-on-ignition standard operating procedure, pp 1–5. Retrieved on 11 February 2022 from https://studylib.net/doc/8647267/loss-on-ignition--loi-
Linger P, Müssig J, Fischer H, Kobert J (2002a) Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 16:33–42. https://doi.org/10.1016/S0926-6690(02)00005-5
Linger P, Müssig J, Fischer H, Kobert J (2002b) Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 16(1):33–42. https://doi.org/10.1016/S0926-6690(02)00005-5
Linger P, Müssig J, Fischer H, Kobert J (2002c) Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 16(1):33–42. https://doi.org/10.1016/S0926-6690(02)00005-5
Linger P, Ostwald A, Haensler J (2005) Cannabis sativa L. growing on heavy metal contaminated soil: growth, cadmium uptake and photosynthesis. Biol Plant 49(4):567–576. https://doi.org/10.1007/s10535-005-0051-4
Liu M, Fernando D, Daniel G, Madsen B, Meyer AS, Ale MT, Thygesen A (2015) Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind Crops Prod 69:29–39. https://doi.org/10.1016/j.indcrop.2015.02.010
Liu M, Ale MT, Kołaczkowski B, Fernando D, Daniel G, Meyer AS, Thygesen A (2017a) Comparison of traditional field retting and Phlebia radiata Cel 26 retting of hemp fibres for fibre-reinforced composites. AMB Express 7(1):1–15. https://doi.org/10.1186/s13568-017-0355-8
Liu M, Thygesen A, Summerscales J, Meyer AS (2017b) Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: a review. Ind Crops Prod 108(February):660–683. https://doi.org/10.1016/j.indcrop.2017.07.027
Lock K, Janssen CR (2003) Influence of aging on metal availability in soils. In G. W. Ware (Ed.), Reviews of Environmental Contamination and Toxicology (pp. 1–21). Springer New York. https://doi.org/10.1007/0-387-21728-2_1
Lühr C, Pecenka R, Budde J, Hoffmann T, Gusovius H-J (2018) Comparative investigations of fibreboards resulting from selected hemp varieties. Ind Crops Prod 118:81–94. https://doi.org/10.1016/J.INDCROP.2018.03.031
Luyckx M, Hausman JF, Isenborghs A, Guerriero G, Lutts S (2021) Impact of cadmium and zinc on proteins and cell wall-related gene expression in young stems of hemp (Cannabis sativa L) and influence of exogenous silicon. Environ Exp Bot 183(December 2020):104363. https://doi.org/10.1016/j.envexpbot.2020.104363
Manian AP, Cordin M, Pham T (2021) Extraction of cellulose fibers from flax and hemp: a review. Cellulose 28(13):8275–8294. https://doi.org/10.1007/s10570-021-04051-x
Mazian B, Bergeret A, Benezet JC, Malhautier L (2018) Influence of field retting duration on the biochemical, microstructural, thermal and mechanical properties of hemp fibres harvested at the beginning of flowering. Ind Crops Prod 116:170–181. https://doi.org/10.1016/j.indcrop.2018.02.062
Meers E, Ruttens A, Hopgood M, Lesage E, Tack FMG (2005) Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 61(4):561–572. https://doi.org/10.1016/j.chemosphere.2005.02.026
Meers E, Du Laing G, Unamuno V, Ruttens A, Vangronsveld J, Tack FMG, Verloo MG (2007) Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma 141(3–4):247–259. https://doi.org/10.1016/J.GEODERMA.2007.06.002
Meers E, Van Slycken S, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, Witters N, Thewys T, Tack FMG (2010) The use of bio-energy crops (Zea mays) for “phytoattenuation” of heavy metals on moderately contaminated soils: a field experiment. Chemosphere 78(1):35–41. https://doi.org/10.1016/j.chemosphere.2009.08.015
Mishra T, Pandey VC (2018) Phytoremediation of red mud deposits through natural succession. Market Opportunities in Sustainable Phytoremediation. Elsevier Inc., In Phytomanagement of Polluted Sites. https://doi.org/10.1016/B978-0-12-813912-7.00016-8
Mongioví C, Morin-Crini N, Lacalamita D, Bradu C, Raschetti M, Placet V, Ribeiro ARL, Ivanovska A, Kostić M, Crini G (2021) Biosorbents from plant fibers of hemp and flax for metal removal: comparison of their biosorption properties. Molecules 26(14):4199. https://doi.org/10.3390/molecules26144199
Morin-Crini N, Loiacono S, Placet V, Torri G, Bradu C, Kostić M, Cosentino C, Chanet G, Martel B, Lichtfouse E, Crini G (2019) Hemp-based adsorbents for sequestration of metals: a review. Environ Chem Lett 17(1):393–408. https://doi.org/10.1007/s10311-018-0812-x
Moscariello C, Matassa S, Esposito G, Papirio S (2021) From residue to resource: the multifaceted environmental and bioeconomy potential of industrial hemp (Cannabis sativa L.). Resources, Conservation and Recycling 175:105864. https://doi.org/10.1016/j.resconrec.2021.105864
Musio S, Müssig J, Amaducci S (2018) Optimizing hemp fiber production for high performance composite applications. Front Plant Sci 9(November):1–14. https://doi.org/10.3389/fpls.2018.01702
Nair GR, Lyew D, Yaylayan V, Raghavan V (2015) Application of microwave energy in degumming of hemp stems for the processing of fibres. Biosyst Eng 131:23–31. https://doi.org/10.1016/j.biosystemseng.2014.12.012
Niinimäki K, Peters G, Dahlbo H, Perry P, Rissanen T, Gwilt A (2020) The environmental price of fast fashion. Nat Rev Earth Environ 1(4):189–200. https://doi.org/10.1038/s43017-020-0039-9
Nordløkken M, Berg T, Flaten TP, Steinnes E (2015) Essential and non-essential elements in natural vegetation in southern Norway: contribution from different sources. Sci Total Environ 502:391–399. https://doi.org/10.1016/J.SCITOTENV.2014.09.038
OEKO-TEX (2022) Annex 6 : Limit values table. Retrieved on 2 February 2022 from https://www.oeko-tex.com/importedmedia/downloadfiles/STANDARD_100_by_OEKO-TEX_R__-_Limit_Values_and_Individual_Substances_According_to_Appendices_6___7_en.pdf
Paulauskas PV, Kasiulienė A (2019) Innovative Approach to Contaminated Soil Phytoremediation : Heavy Metal Phytoextraction Using Energy Crops 47(2):44–47
Paulitz J, Sigmund I, Kosan B, Meister F (2017) Lyocell fibers for textile processing derived from organically grown hemp. Procedia Eng 200:260–268. https://doi.org/10.1016/J.PROENG.2017.07.037
Perlein A, Bert V, Desannaux O, de Souza MF, Papin A, Gaucher R, Zdanevitch I, Meers E (2021) The use of sorghum in a phytoattenuation strategy: a field experiment on a TE-contaminated site. Appl Sci 11(8):3471. https://doi.org/10.3390/app11083471
Pietrini F, Passatore L, Patti V, Francocci F, Giovannozzi A, Zacchini M (2019) Morpho-physiological and metal accumulation responses of hemp plants (Cannabis Sativa L.) grown on soil from an agro-industrial contaminated area. Water 11(4):808. https://doi.org/10.3390/w11040808
Piotrowski BS, Carus M (2011) Ecological benefits of hemp and flax cultivation and products 2. Effects on soil and crop rotations. Small 68(73):1–6
Rheay HT, Omondi EC, Brewer CE (2021) Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. In GCB Bioenergy (Vol. 13, Issue 4, pp. 525–536). Blackwell Publishing Ltd. https://doi.org/10.1111/gcbb.12782
Roca N, Rodríguez-bocanegra J, Bech J (2017) Using Bioavailable Soil Fraction to Assess the Bioconcentration Factor of Plants in Phytoremediation of Mine Soils 19(January):10123
Roseberg RJ, Jeliazkov VD, Angima SD (2019) Soil, seedbed preparation and seeding for hemp. Oregon State University. EM 9239, August 2019. Retrieved on the 12th of April 2022 from https://catalog.extension.oregonstate.edu/em9239/html
Sajin N (2019) Environmental impact of the textile and clothing industry. What consumers need to know. Eur Parliam Res Serv PE 633.143(January) 1–10
Shahzad A (2012) Effects of water absorption on mechanical properties of hemp fiber composites. Polym Compos 33(1):120–128. https://doi.org/10.1002/pc.21254
Shi G, Liu C, Cui M, Ma Y, Cai Q (2012) Cadmium tolerance and bioaccumulation of 18 hemp accessions. Appl Biochem Biotechnol 168(1):163–173. https://doi.org/10.1007/s12010-011-9382-0
Struik PC, Amaducci S, Bullard MJ, Stutterheim NC, Venturi G, Cromack HTH (2000) Agronomy of fibre hemp (Cannahis satira L) in Europe. Ind Crops Prod 11(2–3):107–118. https://doi.org/10.1016/S0926-6690(99)00048-5
Sungur Ş, Gülmez F (2015) Determination of metal contents of various fibers used in textile industry by MP-AES. J Spectrosc 2015. https://doi.org/10.1155/2015/640271
Tang K, Struik PC, Yin X, Thouminot C, Bjelková M, Stramkale V, Amaducci S (2016) Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind Crops Prod 87:33–44. https://doi.org/10.1016/J.INDCROP.2016.04.026
Thewys T, Witters N, Van Slycken S, Ruttens A, Meers E, Tack F (2010) Economic viability of phytoremediation of a cadmium contaminated agricultural area using energy maize: part I: effect on the farmer’s income. Int J Phytoremediation 12(7):650–662. https://doi.org/10.1080/15226514.2010.493187
Thijs S, Witters N, Janssen J, Ruttens A, Weyens N, Herzig R, Mench M, Van Slycken S, Meers E, Meiresonne L, Vangronsveld J (2018) Tobacco, sunflower and high biomass src clones show potential for trace metal phytoextraction on a moderately contaminated field site in Belgium. Front Plant Sci 871(December):1–16. https://doi.org/10.3389/fpls.2018.01879
Tofan L, Paduraru C, Volf I, Balan C (2010) Removal of lead (II) from aqueous solution by sorption on natural hemp fibers. Lucr Ştiinţifice 53(1):150–152
Troch V, Vandepitte K, Vanderhoeven M, Vermeire S, Godefroid F, Vanderborght W, Latré J, Nollet J, Van Steenkiste D, De Raeve A (2020) De revival van hennep als low-impact textielvezel (HOGENT). Retrieved on 6 February 2022 from https://onderzoek.hogent.be/sites/onderzoek/assets/File/HOGENT%20Onderzoeksresultaten%20%E2%80%98Eigen%20Kweek%E2%80%99%20en%20%E2%80%98Hemp4All%27.pdf
USDA (2017) Soil Survey Manual Agriculture. Handbook 18. USDA, Nat Resour Conserv Serv 18(18):483. Retrieve on 5 December 2021 from https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/ref/?cid=nrcs142p2_054262
Usman K, Al-Ghouti MA, Abu-Dieyeh MH (2019) The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-42029-9
Van Ranst E, Verloo M, Demeyer A, Pauwels JM (1999) Manual for the soil chemistry and fertility laboratory: analytical methods for soils and plants equipment, and management of consumables. Ghent University, Faculty Agricultural and Applied Biological Sciences, Ghent, pp 243
Van Slycken S, Witters N, Meers E, Peene A, Michels E, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, Wierinck I, Van Dael M, Van Passel S, Tack FMG (2013) Safe use of metal-contaminated agricultural land by cultivation of energy maize (Zea mays). Environ Pollut 178:375–380. https://doi.org/10.1016/J.ENVPOL.2013.03.032
Vandepitte K, Vasile S, Vermeire S, Vanderhoeven M, Van der Borght W, Latré J, De Raeve A, Troch V (2020) Hemp (Cannabis sativa L.) for high-value textile applications: the effective long fiber yield and quality of different hemp varieties, processed using industrial flax equipment. Ind Crops Prod 158(September):112969. https://doi.org/10.1016/j.indcrop.2020.112969
Vanderhoeven M, Vermeire S, Troch V, Vasile S, Latré J, Nollet J, ADR (2020) 28 Multi-stage yield and quality improvement of hemp fibres for clothing applications - prerequisite for revival of hemp cultivation. Retrieved on 6 February 2022 from https://fashioninstitute.mmu.ac.uk/assets/uploads/2019/07/28-Multi-stage-yield-and-quality-improvement-of-hemp-fibres-for-clothing-applications-prerequisite-for-revival-of-hemp-cultivation-Alexandra-De-Raeve.pdf
Vlarebo (2008) Besluit van de Vlaamse Regering houdende vaststelling van het Vlaams reglement betreffende de bodemsanering en de bodembescherming, , Bijlage II. Vlaanderen. Retrieved on 6 February 2022 from https://navigator.emis.vito.be/mijn-navigator?woId=23568
Westerhuis W, van Delden SH, van Dam JEG, Pereira Marinho JP, Struik PC, Stomph TJ (2019) Plant weight determines secondary fibre development in fibre hemp (Cannabis sativa L.). Ind. Crops Prod 139:111493. https://doi.org/10.1016/j.indcrop.2019.111493
Xiao Z, Yuan X, Li H, Jiang L, Leng L, Chen X, Zeng G, Li F, Cao L (2015) Chemical speciation, mobility and phyto-accessibility of heavy metals in fly ash and slag from combustion of pelletized municipal sewage sludge. Sci Total Environ 536:774–783. https://doi.org/10.1016/J.SCITOTENV.2015.07.126
Acknowledgements
The authors thank Romain Vion, who provided the soil sites for the field trial and helped with the sowing and harvesting and the management of the plantation on site. The authors are also grateful to Inagro, who provided a drying space for the hemp after harvest, and to HoGent, who provided the USO 31 hemp seeds and gave access to their manual stem breaker for the hemp stem decortication. The authors express their gratitude to the Interreg France-Wallonie-Vlaanderen project New-C-Land Project (with the support of the European Regional Development Fund, grant number:1.2.294), the Public Waste Agency in the region of Flanders (OVAM), the Province West Flanders, and the Walloon Region for their financial support in the accomplished research.
Funding
This research was co-funded by the New-C-Land Project (Interreg France-Wallonie-Vlaanderen, with the support of the European Regional Development Fund, grant number:1.2.294), OVAM, the Province West Flanders, and the Walloon Region.
Author information
Authors and Affiliations
Contributions
Béatrice De Vos: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization. Marcella F. De Souza: conceptualization, methodology, resources, writing—review and editing, supervision, project administration. Evi Michels: writing—review and editing, supervision, project administration, funding acquisition. Erik Meers: conceptualization, methodology, resources, writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously.
Consent to participate
All the authors listed consent to participate.
Consent for publication
All the authors listed have approved the manuscript that is enclosed.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Vos, B., De Souza, M.F., Michels, E. et al. Industrial hemp (Cannabis sativa L.) field cultivation in a phytoattenuation strategy and valorization potential of the fibers for textile production. Environ Sci Pollut Res 30, 41665–41681 (2023). https://doi.org/10.1007/s11356-023-25198-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25198-z