Abstract
Whole-genome sequencing of pathogenic bacteria Stenotrophomonas maltophilia from a less polluted environment of permafrost can help understand the intrinsic resistome of both antibiotics and metals. This study aimed to examine the maximum minimum inhibitory concentration (MIC) of both antibiotics and metals, as well as antibiotic resistance genes and metal resistance genes annotated from whole-genome sequences. The permafrost S. maltophilia was sensitive to ciprofloxacin, tetracycline, streptomycin, and bacitracin, and resistant to chloramphenicol, trimethoprim-sulfamethoxazole, erythromycin, Zn2+, Ni2+, Cu2+, and Cr6+, with a lower maximum MIC, compared with clinical S. maltophilia. The former strain belonged to the lower antibiotic resistance gene (ARG) and metal resistance gene (MRG) clusters compared with the latter ones. The permafrost strain contained no or only one kind of ARG or MRG on a single genomic island, which explained the aforementioned lower maximum MIC and less diversity of ARGs or MRGs. The result indicated that the co-occurrence of antibiotic and metal resistance was due to a certain innate ability of S. maltophilia. The continuous human use of antibiotics or metals induced selective pressure, resulting in higher MIC and more diverse ARGs and MRGs in human-impacted environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The World Health Organization has identified antibiotic resistance genes (ARGs) as one of the most important challenges to human health in the twenty-first century because ARGs are emerging environmental contaminants causing serious public health concerns (Sanderson et al. 2016). The annual number of human deaths due to antimicrobial resistance is expected to reach up to 10 million by 2050 (de Kraker et al. 2016). However, natural antibiotics have existed for billions of years (Barlow and Hall, 2002; Hall and Barlow, 2004; Bhullar et al. 2012; Wright and Poinar 2012). Similar to antibiotics, ARGs are also ancient, as evidenced by the studies identifying various ARGs in ancient permafrost samples (D’Costa et al. 2011; Perron et al. 2015) and isolated cave microbiomes (Bhullar et al. 2012). Then, many studies combined polluted and nonpolluted environments to study the potential sources of ARGs and the influence of human activity on ARGs (Li, et al. 2017; Yuan et al. 2019). The results showed that the nonpolluted environments contained fewer ARG subtypes than the polluted environments. However, a few studies focused on the antibiotic-resistant phenotypes and minimum inhibition concentration (MIC) to verify whether ARGs were expressed in environments with little human activity.
Whole-genome sequencing has become a powerful tool to recover ARGs from the same bacterial species from various sources, such as clinical and environmental settings. Stenotrophomonas maltophilia, a ubiquitous pathogen in hospitals and natural environments (Brooke 2012), has evolved as one of the multidrug-resistant bacteria causing various nosocomial infections, especially in highly debilitated patients (Patil et al. 2018). Some studies were undertaken on ARGs in S. maltophilia from the natural environment and clinical origin. The results showed the absence of smeABC in environmental S. maltophilia (Youenou et al. 2015). Some environmental strains carried more efflux pumps than the clinical ones (Youenou et al. 2015). However, the information on ARGs in S. maltophilia from natural environments with little human activity, such as permafrost, is limited. Comparative genomic analyses are needed to show the diversity of different ARGs among pathogenic S. maltophilia from different sources.
Antibiotic-resistant bacteria can transfer to other bacteria (including potential human pathogens) the ARGs they harbor through mobile genetic elements (MGEs) (Pruden et al. 2006; Zhu et al. 2013). For S. maltophilia, the researches about ARGs and MGEs focused on trimethoprim/sulfamethoxazole and class 1 integrons. The results showed that the most significant contribution of the class 1 integron acquisition to S. maltophilia was the increased resistance to trimethoprim–sulfamethoxazole through the sulI gene (Malekan et al. 2017; Song et al. 2010; Gallo et al. 2016). However, no other MGEs and ARGs were examined, which was important to understand the distribution of ARGs or multidrug resistance mechanism.
ARGs not only correlated with MGEs but also with metal resistance genes (MRGs), due to the potential association of antibiotic resistance and Cu, Zn, Ni, and Hg reported in various environmental settings (Baker-Austin et al. 2006; Berg et al. 2010; Mazhar et al. 2021; Hu et al. 2017; Knapp et al. 2017). MRGs, such as merR, arsR, copG, cadA, and cadC, existed in clinical S. maltophilia (Alonso et al. 2000; Kumar et al. 2020). No related research was undertaken for permafrost S. maltophilia. The comparative genomic analysis of S. maltophilia can reveal the difference in ARGs between human-impacted and less human-impacted environments due to many genomic contents with S. maltophilia, whose whole-genomic sequence data could be downloaded from NCBI. In addition, the widespread occurrence of metals in the environment may facilitate antibiotic resistance via co-selection of ARGs and MRGs. Thus, this study aimed to investigate the relationship between ARGs and MRGs in permafrost S. maltophilia to show whether this co-selection occurred in the pre-antibiotic era.
In this study, an S. maltophilia strain was isolated from the bottom of a ~ 11.7-m deep permafrost core (#B site: 38° 00′ 11.76″ N, 100° 54′ 24.66″ E; altitude 3615 m) of Eboling Mountain, from the Qilian Mountains of the Qinghai-Tibetan Plateau, to test its maximum MIC for both antibiotics and metals. The study then analyzed its whole-genome sequence. Considering the limited knowledge available on the S. maltophilia intrinsic resistome, the aims of this study were to (1) to compare the maximum MIC for antibiotics and metals, (2) the differences in ARGs and MRGs, and (3) genomic islands (GIs) between the ancient and present S. maltophilia.
Materials and methods
Isolation and susceptibility profile characterization
The isolation site of the S. maltophilia strain dates back to 5821 BP (Mu et al. 2014). One of the major features of the clinical isolates of S. maltophilia is their high resistance levels toward most of the currently used antimicrobial agents, including macrolides, fluoroquinolones, aminoglycosides, chloramphenicol, and tetracyclines (Brooke 2012). Thus, the MIC of the strain was determined by broth microdilution, with 40 repeats for each condition tested, in the presence or absence of seven antibiotics [ciprofloxacin, streptomycin, tetracycline, erythromycin, chloramphenicol, bacitracin, and trimethoprim–sulfamethoxazole (TMP-SMZ); Sigma, MO, USA], corresponding to fluoroquinolone, aminoglycosides, tetracycline, macrolide, phenicol, peptide, and sulfonamide, respectively. The strain was grown overnight in Mueller Hinton broth (MHB) using CLSI-recommended incubation conditions. After that, 100 μL of bacterial suspensions, with 40 repeats, and with a final optical density at 550 nm (OD550) of 0.005 were added to the wells containing the 2 × antibiotic dilutions. The clinical breakpoints for the seven antibiotics were established according to the European Society of Clinical Microbiology and Infectious Diseases (ECOFF).
A previous study assessed the levels of 11 different heavy metals Fe, Mn, Zn, Ni, Cr, Cu, As, Co, Mo, Cd, and Hg (Zhang et al. 2021). The MIC of eight metals Zn2+, Mn2+, Ni2+, Sn2+, Cu2+, Cr6+, Hg2+, and Co2+ was determined. The metals were added as ZnCl2, MnCl2.4H2O, NiCl2.6H2O, SnCl2.2H2O, CuCl2.2H2O, K2Cr2O7, HgCl2, and CoCl2, respectively. The tubes containing R2A media were amended with increasing contents of metals (100, 200, 400, 800, and 1600 μg/mL) and incubated at 15℃ for 1 week. The MIC was defined as the lowest concentration of the metal at which the bacterial pellets remained invisible at the bottom of the tubes (Konopka and Zakharova, 1999). The cell concentration was measured using a spectrophotometer (OD600 = 0.2). Escherichia coli K-12, susceptible to many metals, was used as the control (Matyar et al. 2008; Akinbowale et al. 2007; Aleem et al. 2003; Malik and Jaiswal, 2000; Malik and Aleem, 2011). The strains were considered resistant if MIC values exceeded that of the control organism.
Genome sequencing and assembly
The total DNA of the bacterial colony was isolated using the Bacteria Genomic DNA Extraction Kit (TaKaRa Minibeast Ver.3.0, China) and the sample quality was ensured using NanoDrop ND-1000 microspectrophotometer (NanoDrop Technologies, DE, USA). Whole-genome sequencing was performed on an Illumina HiSeq PE150 platform (San Diego, CA, USA). A-tailed ligated paired-end adaptors with polymerase chain reaction (PCR)-amplified 350-bp inserts were used for library construction at Beijing Novogene Bioinformatics Technology Co., Ltd. From the Illumina PCR adapter reads, the low-quality reads were filtered as a quality control step by the sequencing company. All good-quality paired reads were assembled using the SOAP denovo (http://soap.genomics.org.cn/soapdenovo.html) into several scaffolds (Li et al. 2010). The filtered reads were subjected to gap closing. The whole-genome shotgun project was deposited at GenBank (accession PRJNA504495, S. maltophilia).
Genome annotation
To find ARGs, the protein-coding sequences were searched against the comprehensive antibiotic resistance database (McArthur et al. 2013; Jia et al. 2017). A read was considered an ARG-like gene if the BLASTP identity was ≥ 40% (Liu et al. 2020).
MRGs in the metagenomic data were identified as previously described by Gupta et al. (2018). Experimentally confirmed MRGs were downloaded from the BacMet database (Version 2.0; Pal et al. 2014) as a reference source. Then, the clean MRG reads were matched against the reference source using BLASTX with the criteria of e-value < 10−5 and amino acid identity ≥ 90%.
The GIs were identified using Island Viewer 4 (Bertelli et al. 2017) and further analyzed using ICEfinder (Liu et al. 2019). The genes in the GIs were annotated using the Prokaryotic Genome Annotation Pipeline on NCBI3 and RASTtk server (Overbeek et al. 2014; Brettin et al. 2015). The insertion sequence transposases were detected using IS-Finder (Siguier et al. 2006). The integrons (ints) were predicted using the INTEGRALL database (Moura et al. 2009). The sequence alignment was performed with BLAST server2.
Phylogenetic analysis of 16S rRNA gene sequences
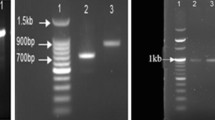
The 16S rRNA gene sequence of the permafrost S. maltophilia was extracted using Prokka. The 16S rRNA gene sequences from S. maltophilia NK-ST, BJ01, NRLFFD179, P4, and EN14ZR5 were downloaded from NCBI and used to construct a phylogenetic tree, with S. tumulicola T5916-2-1b, S. humi R-32729, and S. pictorum JCM 9942 as the members of the same genus. Escherichia coli was selected as an outgroup strain to determine the root of the tree. Multiple sequences were aligned using Clustal W 2.0 (Larkin et al. 2007) and MEGA7 (Kumar et al. 2016). The phylogenetic relationship was determined by phylogeny reconstruction analysis using the neighbor-joining method in MEGA7.
Results
Bacterial taxonomy
The permafrost S. maltophilia formed a cluster with S. maltophilia NK-ST, BJ01, NRLFFD179, P4, and EN14ZR5 (Fig. 1), with sequence similarity of 99.73%, 99.68%, 99.22%, 99.15%, and 99.06, respectively, based on pairwise alignments. This indicated that the permafrost strain was a member of the S. maltophilia group see Table 1.
Antibiotic and metal resistance profiles
The potential co-selections for antibiotic resistance were associated with Cu, Zn, Ni, and Hg in various environmental settings (Baker-Austin et al. 2006; Berg et al. 2010; Mazhar et al. 2021; Hu et al. 2017; Knapp et al. 2017). In addition, the MIC of Zn2+, Ni2+, Cu2+, Cr6+, and Hg2+ for E. coli K-12 was previously reported by Aleem et al. (2003), Malik and Jaiswal (2000), and Malik and Aleem (2011). Thus, the MIC of the aforementioned metals for the permafrost S. maltophilia was compared with that of E. coli K-12 to determine the metal resistance level. The MIC value for Hg2+ in E. coli K-12 was 12.5 μg/mL, while the initial concentration for Hg resistance was 100 μg/mL. Thus, the permafrost S. maltophilia showed resistance to Hg, as well as to other four heavy metals, in the order of Hg2+ > Cr6+ > Zn2+ = Ni2+ > Cu2+ (Table 2). Whether the permafrost S. maltophilia showed resistance to Mn2+, Sn2+, and Co2+ is not clear due to the lack of MIC data for Mn2+, Sn2+, and Co2+ from E. coli K-12.
ARGs
In total, 32 ARGs were identified in the genome of permafrost S. maltophilia, including aminoglycoside resistance genes AAC(6')-Iz and APH(3')-Iic; aminocoumarin resistance genes alaS and mdtC; fluoroquinolone resistance genes emrR and mfd; antibacterial free fatty acid resistance gene farB; β-lactam resistance gene L1 β-lactamase; macrolide resistance genes macA and macB; nitroimidazole resistance gene msbA; triclosan resistance gene gyrA; fosfomycin resistance gene murA; penam resistance gene mecA; peptide resistance gene rosB; elfamycin resistance gene EF-Tu; pleuromutilin resistance gene TaeA; triclosan resistance gene TriC; multidrug resistance genes adeA, adeC, and adeG conferring resistance to tetracycline and glycylcycline; mexJ, mexK, and mexW conferring resistance to tetracycline, macrolide, and triclosan; oprN conferring resistance to phenicol, diaminopyrimidine, and fluoroquinolone; oqxA conferring resistance to tetracycline, nitrofuran, glycylcycline, diaminopyrimidine, and fluoroquinolone; and smeA, smeC, smeD, smeF, smeR, and smeS conferring resistance to ciprofloxacin, tetracycline, chloramphenicol, and erythromycin. These had an amino acid sequence identity of 40.5%–99.2% (Table S1).
MRGs
A total of 36 MRGs were identified in the genome of permafrost S. maltophilia (Table S2), including arsenic resistance genes arsB and arsC; gold resistance genes golS and golT; chromate resistance gene chrR; copper resistance genes copA, copC, pcoB, pcoD, cutA, cutC, cusA, cusB, cueR, and cusS; iron resistance genes fecA and fur; mercury resistance genes merD, merE, and merT; manganese resistance genes mntH and mntR; molybdenum resistance genes modA, modB, modC, moeA, moaE, and mobA; tellurite resistance genes terC; silver resistance genes silA and silB; and cobalt-zinc-cadmium resistance genes czcA, czcB, czcC, and czcD. These had an amino acid sequence identity of 91.1–100.0%.
Genomic islands (GIs)
A total of 12 GIs were identified from 9 scaffolds (Table S3), ranging from 7288 to 40,192 bp (average 15,108 ± 8558 bp). Among these, two GIs contained both resistance genes and MGEs. For instance, GI3 encodes for six Hg resistance genes (merP, merA, merD, merE, merR, and merT), a transposase, and a transposon tnpR. GI4 contained a tetracycline suppressor gene tetR, a transposon tnpA, and two integrases. Three GIs contained only one ARG. For instance, GI2, GI7, and GI11 contained β-lactamase class C gene, acrifervin resistance protein (acr), and aminoglycoside N-acetyltransferase AAC (6′), respectively (Fig. 2). The other 7 GIs contained no resistance genes.
Discussion
This study brings together data on both antibiotic and metal resistant genes, and antibiotic and metal resistant phenotypes, in an environmental organism. This provides mechanistic insights above studies that only consider either genes or phenotypes.
Little is known about antibiotic and metal resistance phenotypes, which is more important than studying ARGs and MRGs only in an environmental context with little human activity. This study showed that metal resistance co-occurred with antibiotic resistance in GIs.
MIC of antibiotics and ARGs
The permafrost S. maltophilia showed resistance to chloramphenicol, erythromycin, and TMP-SMZ, and sensitivity to ciprofloxacin, tetracycline, streptomycin, and bacitracin, which was consistent with the report showing that the culturable bacterial consortiums isolated from Antarctic soils were consistently susceptible to most of the tested antibiotics frequently used in clinical therapies (Yuan et al. 2019). However, Pankuch et al. (1994) showed that S. maltophilia was resistant naturally toward aminoglycosides. They recovered S. maltophilia from the environmental species of captive snakes. Further investigation is still needed to verify whether S. maltophilia from more environments with little human activity was resistant naturally toward aminoglycosides.
Previous MIC studies for S. maltophilia were performed on four antibiotics, including ciprofloxacin, tetracycline, chloramphenicol, and TMP-SMZ. Thus, the MIC results were compared with the findings on these four antibiotics (Table S4). TMP-SMZ has traditionally been considered the treatment of choice for S. maltophilia (Biagi et al. 2020), with increasing reports of resistance and adverse drug effects causing great concern for S. maltophilia treatment (Hand et al. 2016; Bostanghadiri et al. 2019; Gajdacs and Urban, 2019). Few studies showed the sensitivity of clinical S. maltophilia isolates to TMP-SMZ, with an MIC range of 0.125–2.375 μg/mL (Nakamura et al. 2021; Khan et al. 2021; Krueger et al. 2001). However, the maximum MIC of 304, 608, and 2432 μg/mL was 9.5, 19, and 76 times higher than the MIC of the permafrost strain, respectively, as reported by many researchers (Hejnar et al. 2001; Tatman-Otkun et al. 2005; Nakamura et al. 2021; Zhanel et al. 2008; Weiss et al. 2000; Fung-Tomc et al. 2002; Valdezate et al. 2001). Thus, the MIC of the permafrost S. maltophilia to TMP-SMZ was at the medium level. The maximum MIC of tetracycline (García-León et al. 2015) was > 256 μg/mL for S. maltophilia clinical isolates and 16 μg/mL for the permafrost S. maltophilia. For chloramphenicol, the clinical S. maltophilia isolates could resist up to 96 (Spierer et al. 2018), 152 (Carvalhais et al. 2021), and > 256 μg/mL (García-León et al. 2015), while the permafrost S. maltophilia could resist only 64 μg/mL. The maximum MIC of ciprofloxacin for clinical S. maltophilia isolate was > 32 μg/mL (Grillon et al. 2016; García-León et al. 2015; Spierer et al. 2018), while the permafrost S. maltophilia could resist only 16 μg/mL of ciprofloxacin. Overall, most clinical S. maltophilia isolates had higher resistance (higher MICs) to ciprofloxacin, chloramphenicol, trimethoprim/sulfamethoxazole, and tetracycline compared with the permafrost strain. These results were consistent with the findings of Pankuch et al. (1994), showing that the S. maltophilia isolates from captive snakes were either identically or more susceptible to antibiotics than strains acquired from patients, as well as Balbin et al. (2020), showing that the urban isolates of E. coli showed higher resistance to chloramphenicol, ciprofloxacin, streptomycin, trimethoprim–sulfamethoxazole, and tetracycline than those from the natural area. It was speculated that antibiotic resistance was an innate ability of S. maltophilia. The continuous human use of antibiotics induced selective pressure on antibiotic-resistant bacteria, causing higher MIC compared with less human-impacted environments.
The aforementioned antibiotic resistance phenotypes were related to antibiotic resistance genotypes. For instance, the presence of macA and macB was consistent with the resistance of the permafrost S. maltophilia to erythromycin. The constitutive expression of macABCsm contributed to the intrinsic resistance of S. maltophilia to macrolides (Lin et al. 2014), which verified the result of this study. However, the macABCsm pump also played a physiological role in protecting S. maltophilia from the attack of oxidative and envelope stresses and biofilm formation (Lin et al. 2014), which was also important under permafrost conditions. Therefore, further exploration is still needed with the gene deletion method to show the exact role of macAB in the permafrost S. maltophilia. mexVW usually combined with oprM to form a tripartite multidrug efflux pump (Li et al. 2003). However, mexV and oprM were not recovered from the permafrost S. maltophilia. The smeDEF in the permafrost S. maltophilia was probably related to chloramphenicol resistance (Sánchez and Martínez, 2018). However, smeE and the regulator gene of smeT were not recovered from our permafrost S. maltophilia. In addition, it was reported that smeDEF was an ancient element that evolved over millions of years in S. maltophilia. Quinolone resistance is a recent function of smeDEF and that colonization of plant roots is likely one original function of this efflux pump (García-León et al. 2014). Thus, the mechanism of chloramphenicol resistance is still unexplored and needs further investigation. One way or another, the multidrug efflux systems of macAB, mexW, smeDF could still contribute to antibiotic resistance and their conservation even in environmental strains would cause human risk for therapeutic intervention (Poole 2001).
The aforementioned findings showed the antibiotic resistance phenotypes and the presence of corresponding ARGs. However, the present study showed antibiotic sensitivity and the absence of corresponding ARGs. The smrA conferring resistance to fluoroquinolones and tetracycline (Al-Hamad et al. 2009) was absent, which was consistent with its sensitivity to tetracycline and ciprofloxacin. The qnrB and qnrR conferring resistance to quinolones were absent. Furthermore, oqxAB is a member of the resistance–nodulation–cell division (RND) family of multidrug efflux pumps (Hansen et al. 2004), which can pump out nalidixic acid, flumequine, ciprofloxacin, and norfloxacin, causing an 8- to 64-fold increase in respective MICs (Périchon et al. 2007). The absence of oqxB, as well as the aforementioned qnrB and qnrR, in the permafrost S. maltophilia was consistent with its sensitivity to ciprofloxacin. However, qnrB, qnrR, and smrA were present in clinical isolates (Esposito et al. 2017; Patil et al. 2018; Zhang et al. 2020). oqxB was found in an isolate from the Norwegian University campus pond (Finton et al. 2020) and clinic (Esposito et al. 2017). It was reported that smrA was an acquired and not an intrinsic gene (Al-Hamad et al. 2009), which further demonstrated the natural origin of the permafrost strain with little human influence from antibiotic use. No bcrABC was recovered in the present study, which was consistent with the sensitivity of the strain to bacitracin. strA and strB were absent in the permafrost strain but present in the clinical isolate (Ma et al. 2020; Esposito et al. 2017). The result was consistent with the streptomycin sensitivity of the permafrost strain. Hence, it was speculated that the diversity of ARGs could reflect the risk caused by the human use of antibiotics. This speculation was supported by the promotion and diversification of ARGs under the release of large quantities of anthropogenic antibiotics (Liu et al. 2021, 2018; Tan et al. 2018; Chen et al. 2013, 2016; Ouyang et al. 2015; Sandner-Miranda et al. 2018).
However, a certain discrepancy between phenotype and genotype was also found. Although adeA, adeC, and adeG were recovered, no tetracycline resistance was reported. Also, mfd, gyrA, and emrB were recovered, but no fluoroquinolone resistance of ciprofloxacin was observed. For the recovery of rosB, the permafrost S. maltophilia did not show resistance to bacitracin from peptides. For the recovery of AAC(6′)-Iz, AAC(6′)-31, and APH(3′)-Ic, the permafrost S. maltophilia did not show resistance to streptomycin from aminoglycosides. The difference between the displayed antibiotic resistance phenotypes and the associated ARGs (Smith et al. 2014; Xia et al. 2017; González-Santamarina et al. 2021; Duy et al. 2021) was probably due to the lack of function and expression of ARGs. The observed phenotypic resistance could be a product of additional resistance mechanisms such as multidrug efflux pumps or other unidentified ARGs (Smith et al. 2014).
The study then compared ARGs from the permafrost S. maltophilia with those from the clinical ones (Table S5). mrcA and mrcB were absent in the permafrost S. maltophilia but present in a patient’s isolate (Ma et al. 2020). blaL1 and blaL2 discovered in a clinical isolate (Esposito et al. 2017; Patil et al. 2018; Crossman et al. 2008) were absent in the permafrost strain. All four ARGs were related to β-lactamase expression (Huang et al. 2017). sul1 and sul2 were present in a clinical S. maltophilia isolate (Youenou et al. 2015; Patil et al. 2018) but not in the permafrost strain. This seemed coherent, given the fully synthetic origin of sulfonamide antibiotics (Czekalski et al. 2015). However, sul2 was recovered from an ice core, representing the pre-antibiotic era (Okubo et al. 2019). This was probably because the authors used total DNA and PCR primers targeting sul2, which could better reflect ARG profiles in less human-impacted environments. MacAB, along with a member of the tolC family, formed a tripartite efflux pump. macAB was present in both the permafrost S. maltophilia and the clinical S. maltophilia (Zhang et al. 2020; Esposito et al. 2017; Patil et al. 2018), while tolC was present only in the clinical isolate (Zhang et al. 2020; Esposito et al. 2017; Patil et al. 2018). The absence of tolC in the permafrost S. maltophilia could have affected the function of the macAB-TolC efflux pump (Lu et al. 2018). TolC interacts with a variety of inner membrane transporters, such as acrB, acrD, mdtABC, and mdtEF (Nishino et al. 2003). Among these, only mdtC was present in the permafrost S. maltophilia, while tolC, acrB, acrD, mdtB, and mdtC were found in an isolate from a lung with cystic fibrosis (Esposito et al. 2017). It is known that multiple deletions of acrB, acrD, and mdtABC significantly decrease the export of enterobactin (Horiyama and Nishino, 2014), whether the permafrost S. maltophilia resists enterobactin needs further exploration. The aforementioned results further demonstrated higher ARG diversity in environments with more human activities than those with lesser activities. However, it was reported that no major variation in ARG content was observed from environmental and clinical S. maltophilia. Some environmental S. maltophilia even carried as many multidrug-resistant efflux pumps as the clinical strains or more efflux pumps than the clinical ones (Youenou et al. 2015), which was contrary to the results of this study. That is probably because of different ARGs annonation method. We used CARD, while Youenou et al. (2015) used InterPro database.
MIC of metals and MRGs
Either antibiotics or metals may select both kinds of genes. Thus, much attention has been given to metal resistance, which influences antibiotic resistance in human-impacted environments (Cesare et al. 2016; Che et al. 2019; Ma et al. 2016; Yang et al. 2019; Luo et al. 2017). This study investigated whether metal MIC was lower and MRGs were lesser in the permafrost S. maltophilia than in other environments, just like antibiotic MIC and ARGs.
The permafrost S. maltophilia showed resistance to Hg2+, Cr6+, Zn2+, Ni2+, and Cu2+, which was consistent with the resistance of S. maltophilia to Hg2+, Zn2+, Ni2+, Cu2+, and Cr6+ (Pages et al. 2008; Naguib et al. 2019; Holmes et al. 2009; Baldiris et al. 2018; Nath et al. 2020). A previous study assessed the levels of 11 different heavy metals Fe, Mn, Zn, Ni, Cr, Cu, As, Co, Mo, Cd, and Hg, in which only the As level was higher compared with the upper continental crust (Zhang et al. 2021). The high level of As induced As-resistant bacteria, which could resist not only As but also other metals (Altimira et al. 2012). This explained the resistance of the permafrost S. maltophilia to Hg2+, Cr6+, Zn2+, Ni2+, and Cu2+.
Next, the study compared the MIC of four metals Zn2+, Ni2+, Cu2+, and Cr6+ from the permafrost S. maltophilia with that from other environments because previous MIC studies focused on these four metals (Table S4). The maximum MIC of Zn2+ for the permafrost S. maltophilia was 800 μg/mL, which was much lower than the MIC of those (515,200 μg/mL) recovered from metal-contaminated soil (Chien et al. 2007). The maximum MIC of Ni2+ for the permafrost S. maltophilia was 800 μg/mL, which was lower than that (1000 μg/mL) from the industrial wastewater (Aslam et al. 2018) and much lower than that (495,232 μg/mL) recovered from the metal-contaminated soil (Chien et al. 2007). The maximum MIC of Cu2+ for the permafrost S. maltophilia was 800 μg/mL, which was lower than that (1248.45 μg/mL) from East Fork Poplar Creek (Holmes et al. 2009) and much lower than that (448,000 μg/mL) recovered from the metal-contaminated soil (Chien et al. 2007). The maximum MIC of Cr6+ for the permafrost S. maltophilia was 800 μg/mL, while that recovered from the tannery effluent–contaminated soil, metal-contaminated soil, East Fork Poplar Creek, and industrial wastewater could resist up to 4854 μg/mL (Alam and Ahmad, 2012), 35,280 μg/mL (Chien et al. 2007), 2647.66 μg/mL (Holmes et al. 2009), and 1000 μg/mL of Cr6+ (Aslam et al. 2018), respectively. Overall, the permafrost S. maltophilia showed worse resistance (lower MICs) to the four metals than those from human-impacted environments. The result of this study was consistent with the report showing that the nonpolluted and metal-polluted soils had different responses for metal resistance (Schaeffer et al. 2016). A higher concentration of any metal at a particular site may lead to higher MIC values (Bhardwaj et al. 2018) due to the long-term selective pressure on microbial populations. Importantly, S. maltophilia with metal resistance can be used as an indicator of metal pollution. Since metal pollution exerts both metal and antibiotic resistance (Li et al. 2017; Knapp et al. 2011), special attention must be paid to increasing heavy metal levels in any kind of environment.
For the metal resistance phenotypes and genotypes, Cu2+ resistance was consistent with the recovery of cueR and copA. The cueR switch could activate the S. maltophilia copper transport gene of copA (Baya et al. 2021). The expression of both regulator gene cueR and structure gene copA was related to Cu2+ resistance. In the czcCBA operon, czcC was an outer membrane protein, czcB was a membrane fusion protein, czcA was responsible for Co-Zn-Cd transportation, and czcD was a regulatory protein. Thus, the czcCBA operon combined with its downstream gene of czcD mediated the detoxification of Zn2+ and was consistent with the resistance of the permafrost S. maltophilia to Zn2+ (Sun et al. 2021). Chromate reductase, chrR, is significant because it not only reduces Cr6+ (Ackerley et al. 2004) but also provides protection against Cr6+ toxicity by reducing the concentration of reactive oxygen species (Ahemad 2014). The recovery of chrR was consistent with the resistance of the permafrost S. maltophilia to Cr6+. Hg resistance genes merT, merD, and merE were recovered. The three genes probably controlled Hg resistance in the permafrost S. maltophilia because strains with any mer locus were more likely to be resistant compared with strains without mer (Wireman et al. 1997). This showed consistency between the metal resistance phenotypes and genotypes. Some inconsistency was also noted. For instance, the permafrost S. maltophilia was resistant to Ni2+, but no Ni2+ resistance genes were found. This was probably because some efflux pumps were involved in detoxifying toxic compounds such as heavy metals and solvents, besides antibiotics naturally produced by other microorganisms (Alvarez-Ortega et al. 2013).
Some other MRGs were recovered, but the present study did not analyze the MIC of the corresponding metals, such as resistance genes arsB and arsC, Au resistance genes gold and golT, Fe resistance genes fecA and fur, Mn resistance genes mntH and mntR, Mo resistance genes modA, modB, modC, moeA, moaE, and mobA, Te resistance gene terC, and Ag resistance genes silA and silB. Much more metal MIC standards should be given to E. coli K-12 so that the resistance of the permafrost S. maltophilia to As, Au, Fe Mn, and Mo can be speculated.
Then, MRGs from the permafrost S. maltophilia were compared with those from other environmental isolates (Table S6). Both chrA and chrR were present in the S. maltophilia of wastewater (Naguib et al. 2019), while the permafrost S. maltophilia contained only chrR. Furthermore, copA and copC were present in both the clinical and permafrost S. maltophilia, while copABCD was present in the clinical S. maltophilia D457R (Alonso et al. 2000). The cadmium efflux determinant cadA, together with its transcriptional regulator gene cadC, was identified in the clinical S. maltophilia D457R (Alonso et al. 2000), while these were absent in the permafrost S. maltophilia. merT was found in both the permafrost S. maltophilia and clinical S. maltophilia 279a (Crossman et al. 2008), while merA and merR were present in the clinical S. maltophilia 279a (Crossman et al. 2008) and isolates from seawater, soil (Ge and Ge 2016), and wastewater (Naguib et al. 2019). Hence, it was speculated that S. maltophilia from human-impacted environments contained more MRGs compared with the permafrost S. maltophilia, which was consistent with the report that higher numbers of MGEs existed at the polluted sites compared with their control sites (Jacquiod et al. 2018; Yang et al. 2019). Some other studies reported higher MGE abundance in metal-polluted environments than in nonpolluted ones (Chen et al. 2018; Yang et al. 2019), probably due to a higher number of metal-resistant bacteria (Hemmat-Jouet al. 2021). However, whole-genome sequencing could not reflect the abundance of MRGs. Further exploration with real-time quantitative PCR as well as the standard-curve method of absolute quantification is still needed to show the difference in MRG abundance between the permafrost S. maltophilia and those from other environments.
Genomic islands
GIs are frequently associated with a particular microbial adaptation, such as antibiotic resistance or metal resistance (Hsiao et al. 2005). They also harbor genes coding for an integrase or transposons, contributing to the mobilization of gene clusters (AL-Jabri et al. 2018). This study aimed to investigate whether GIs in the permafrost S. maltophilia contained both ARGs and MRGs, and to demonstrate whether the combination of ARGs and MRGs occurred during the pre-antibiotic era.
Only one kind of resistance gene cluster was located on a single GI in the permafrost S. maltophilia. On the contrary, S. maltophilia from other environments exhibited a minimum of two kinds of antibiotics or MRG clusters on a single GI. For instance, ARGs of aadA2, qacE, sul1, strA, strB, tetA, and tetR, as well as two ints, were all located on a single GI of S. maltophilia GZP-Sm1 from porcine (He et al. 2015). In addition, cop, cus operons, and czc genes were all located on the GI K25 of the clinical S. maltophilia strain isolated from the blood of a cancer patient K279a (Rocco et al. 2009). Thus, it was speculated that S. maltophilia from human-impacted environments showed more multi-resistance to antibiotics or metals than those from less human-impacted environments. The result of this study also explained less diversity of ARGs and MRGs in the former than in the latter due to the possibility of horizontal gene transfer (Youenou et al. 2015). However, ARGs and MRGs present in GIs still pose a threat to human health and can not be ignored (Martinez 2009).
Conclusion
To conclude, the permafrost S. maltophilia exhibited lower ARG or MRG cluster components and only one kind of ARG or MRG in GIs compared with the strains from human-impacted areas, which confirmed the lower maximum MIC of antibiotics and metals. The present study suggested that the clinical S. maltophilia developed higher antibiotic and metal resistance due to the horizontal gene transfer of GIs. However, only one permafrost S. maltophilia strain was recovered and sequenced from the study site. In addition, complete genome sequences could not be generated due to the constraints inherent in using short-read Illumina sequencing data. Further analyses supplemented with long-read sequencing technology, such as PacBio sequencing, along with more S. maltophilia strains from more permafrosts and a broad range of antibiotics and metals, are required to precisely determine the role and mechanism ARGs and MRGs in permafrost S. maltophilia.
Data availability
The data are available at NCBI: PRJNA504495.
References
Ackerley DF, Gonzalez CF, Park CH et al (2004) Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl Environ Microbiol 70:873–882. https://doi.org/10.1128/AEM.70.2.873-882.2004
Ahemad M (2014) Bacterial mechanisms for Cr(VI) resistance and reduction: an overview and recent advances. Folia Microbiol 59:321–332. https://doi.org/10.1007/s12223-014-0304-8
Akinbowale OL, Peng H, Grant P et al (2007) Antibiotic and metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int J Antimicrob Ag 30:177–182. https://doi.org/10.1016/j.ijantimicag.2007.03.012
Alam MZ, Ahmad S (2012) Toxic chromate reduction by resistant and sensitive bacteria isolated from tannery effluent contaminated soil. Ann Microbiol 62:113–121. https://doi.org/10.1007/s13213-011-0235-4
Aleem A, Isar J, Malik A (2003) Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizospheric soil. Bioresour Technol 86:7–13. https://doi.org/10.1016/S0960-8524(02)00134-7
Al-Hamad A, Upton M, Burnie J (2009) Molecular cloning and characterization of SmrA, a novel ABC multidrug efflux pump from Stenotrophomonas maltophilia. J Antimicrob Chemoth 64:731–734. https://doi.org/10.1093/jac/dkp271
Al-Jabri Z, Zamudio R, Horvath-Papp E et al (2018) Integrase-controlled excision of metal-resistance genomic islands in Acinetobacter baumannii. Genes 9:366. https://doi.org/10.3390/genes9070366
Alonso A, Sanchez P, Martínez JL (2000) Stenotrophomonas maltophilia D457R contains a cluster of genes from gram-positive bacteria involved in antibiotic and heavy metal resistance. Antimicrob Agents Chemother 44:1778–1782. https://doi.org/10.1128/AAC.44.7.1778-1782.2000
Altimira F, Yáñez C, Bravo G (2012) Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of central Chile. BMC Microbiol 12:193. https://doi.org/10.1186/1471-2180-12-193
Alvarez-Ortega C, Olivares J, Martinez JL (2013) RND multidrug efflux pumps: what are they good for? Front Microbiol 5:4–7. https://doi.org/10.3389/fmicb.2013.00007
Aslam F, Yasmin A, Thomas T (2018) Essential gene clusters identified in Stenotrophomonas MB339 for multiple metal/antibiotic resistance and xenobiotic degradation. Curr Microbiol 75:1484–1492. https://doi.org/10.1007/s00284-018-1549-2
Baker-Austin C, Wright MS, Stepanauskas R et al (2006) Co-selection of antibiotic and metal resistance. Trends Microbiol 14:176–182. https://doi.org/10.1016/j.tim.2006.02.006
Balbin MM, Hull D, Guest C et al (2020) Antimicrobial resistance and virulence factors profile of Salmonella spp. and Escherichia coli isolated from different environments exposed to anthropogenic activity. J Glob Antimicrob Re 22:578–583. https://doi.org/10.1016/j.jgar.2020.05.016
Baldiris R, Acosta-Tapia N, Montes A et al (2018) Reduction of hexavalent chromium and detection of chromate reductase (ChrR) in Stenotrophomonas maltophilia. Molecules 23:406. https://doi.org/10.3390/molecules23020406. https://doi.org/10.3390/molecules23020406.
Barlow M, Hall BG (2002) Phylogenetic analysis shows that the OXA b-lactamase genes have been on plasmids for millions of years. J Mol Evol 55:314–321. https://doi.org/10.1007/s00239-002-2328-y
Baya G, Muhindi S, Ngendahimana V et al (2021) Potential whole-cell biosensors for detection of metal using merR family proteins from Enterobacter sp. YSU and Stenotrophomonas maltophilia OR02. Micromachines 12:142. https://doi.org/10.3390/mi12020142.
Berg J, Thorsen MK, Holm PE et al (2010) Cu exposure under field conditions coselects for antibiotic resistance as determined by a novel cultivation-independent bacterial community tolerance assay. Environ Sci Technol 44:8724–8728. https://doi.org/10.1021/es101798r
Bertelli C, Laird MR, Williams KP et al (2017) IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res 45:W30–W35. https://doi.org/10.1093/nar/gkx343
Bhardwaj R, Gupta A, Garg JK (2018) Impact of metals on inhibitory concentration of Escherichia coli—a case study of river Yamuna system, Delhi. India Environ Monit Assess 190:674. https://doi.org/10.1007/s10661-018-7061-0
Bhullar K, Waglechner N, Pawlowski A et al (2012) Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS ONE 7:e34953. https://doi.org/10.1371/journal.pone.0034953
Biagi M, Tan X, Wu T et al (2020) Activity of potential alternative treatment agents for Stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or TMP-SMZ. J Clin Microbiol 58:e01603-e1619. https://doi.org/10.1128/JCM.01603-19
Bostanghadiri N, Ghalavand Z, Fallah F et al (2019) Characterization of phenotypic and genotypic diversity of Stenotrophomonas maltophilia strains isolated from selected hospitals in Iran. Front Microbiol 10:1191. https://doi.org/10.3389/fmicb.2019.01191
Brettin T, Davis JJ, Disz T et al (2015) RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. https://doi.org/10.1038/srep08365
Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. https://doi.org/10.1128/CMR.00019-11
Carvalhais BES, Silva CS, Santos KV (2021) Effect of antimicrobials on Stenotrophomonas maltophilia biofilm. Future Microbiol 16:83–93. https://doi.org/10.2217/fmb-2020-0115
Cesare AD, Eckert EM, D’Urso S et al (2016) Co-occurrence of integrase 1, antibiotic and metal resistance genes in municipal wastewater treatment plants. Water Res 94:208–214. https://doi.org/10.1016/j.watres.2016.02.049
Che Y, Xia Y, Liu L et al (2019) (2019) Mobile antibiotic resistome in wastewater treatment plants revealed by nanopore metagenomic sequencing. Microbiome 7:1–13. https://doi.org/10.1186/s40168-019-0663-0
Chen Y, Jiang Y, Huang H et al (2018) Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ 637:1400–1412. https://doi.org/10.1016/j.scitotenv.2018.05.109
Chen B, Yang Y, Liang X et al (2013) Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ Sci Technol 47:12753–12760. https://doi.org/10.1021/es403818e
Chen B, Yuan K, Chen X et al (2016) Metagenomic analysis revealing antibiotic resistance genes (ARGs) and their genetic compartments in the Tibetan environment. Environ Sci Te 50:6670–6679. https://doi.org/10.1021/acs.est.6b00619
Chien CC, Hung CW, Han CT (2007) Removal of cadmium ions during stationary growth phase by an extremely cadmium-resistant strain of Stenotrophomonas sp. Environ Toxicol Chem 26:664–668. https://doi.org/10.1897/06-280R.1
Crossman LC, Gould VC, Dow JM et al (2008) The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9:R74. https://doi.org/10.1186/gb-2008-9-4-r74
Czekalski N, Sigdel R, Birtel J et al (2015) Does human activity impact the natural antibiotic resistance background? Abundance of antibiotic resistance genes in 21 Swiss lakes. Environ Int 81:45–55. https://doi.org/10.1016/j.envint.2015.04.005
D’Costa VM, King CE, Kalan L et al (2011) Antibiotic resistance is ancient. Nature 477:457–461. https://doi.org/10.1038/nature10388
de Kraker ME, Stewardson AJ, Harbarth S (2016) Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med 13:e1002184. https://doi.org/10.1371/journal.pmed.1002184
Duy DT, Anh NLL, Thoa NTK et al (2021) Identification of genotype and phenotype of antimicrobial resistance of Escherichia coli isolates from pigs in southern Vietnam. Thai J Vet Med 51:125–132. https://doi.org/10.14456/tjvm.2021.17
Esposito A, Pompilio A, Bettua C et al (2017) Evolution of Stenotrophomonas maltophilia in cystic fibrosis lung over chronic infection: a genomic and phenotypic population study. Front Microbiol 8:1590. https://doi.org/10.3389/fmicb.2017.01590
Finton MD, Meisal R, Porcellato D et al (2020) Whole genome sequencing and characterization of multidrug-resistant (MDR) bacterial strains isolated from a Norwegian University Campus Pond. Front Microbiol 11:1273. https://doi.org/10.3389/fmicb.2020.01273
Fung-Tomc JC, Gradelski E, Valera L et al (2002) Synergistic activity of the novel des-fluoro(6) quinolone, garenoxacin (bms-284756), in combination with other antimicrobial agents against Pseudomonas aeruginosa and related species. Int J Antimicrob Ag 20:57–60. https://doi.org/10.1016/S0924-8579(02)00109-7
Gajdacs M, Urban E (2019) Prevalence and antibiotic resistance of Stenotrophomonas maltophilia in respiratory tract samples: a 10-year epidemiological snapshot. Health Serv Res Manag Epidemiol 6:2333392819870774. https://doi.org/10.1177/2333392819870774
Gallo SW, Figueiredo TP, Bessa MC et al (2016) Isolation and characterization of Stenotrophomonas maltophilia isolates from a Brazilian hospital. Microb Drug Resist 22:688–695. https://doi.org/10.1089/mdr.2015.0306
García-León G, de Alegría Puig CR, De La Fuente CG et al (2015) High-level quinolone resistance is associated with the overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin Microbiol Infec 21:464–467. https://doi.org/10.1016/j.cmi.2015.01.007
García-León G, Hernández A, Hernando-Amado S et al (2014) A function of SmeDEF, the major quinolone resistance determinant of Stenotrophomonas maltophilia, is the colonization of plant roots. Appl Environ Microb 80:4559–4565. https://doi.org/10.1128/AEM.01058-14
Ge S, Ge SC (2016) Simultaneous Cr(VI) reduction and Zn(II) biosorption by Stenotrophomonas sp. and constitutive expression of related genes. Biotechnol Lett 38:877–884. https://doi.org/10.1007/s10529-016-2057-8
González-Santamarina B, García-Soto S, Dang-Xuan S et al (2021) Genomic characterization of multidrug-resistant Salmonella serovars derby and rissen from the pig value chain in Vietnam. Front Vet Sci 8:705044. https://doi.org/10.3389/fvets.2021.705044
Grillon A, Schramm F, Kleinberg M et al (2016) Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS ONE 11:e0156690. https://doi.org/10.1371/journal.pone.0156690
Gupta SK, Shin H, Han D et al (2018) Metagenomic analysis reveals the prevalence and persistence of antibiotic- and metal-resistance genes in wastewater treatment plant. J Microbiol 56:408–415. https://doi.org/10.1007/s12275-018-8195-z
Hall BG, Barlow M (2004) Evolution of the serine β-lactamases: past, present and future. Drug Resist Update 7:111–123. https://doi.org/10.1016/j.drup.2004.02.003
Hand E, Davis H, Kim T et al (2016) Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 71:1071–1075. https://doi.org/10.1093/jac/dkv456
Hansen LH, Johannesen E, Burmølle M et al (2004) Plasmid encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob Agents Ch 48:3332–3337. https://doi.org/10.1128/aac.48.9.3332-3337.2004
He T, Shen J, Schwarz S et al (2015) Characterization of a genomic island in Stenotrophomonas maltophilia that carries a novel floR gene variant. J Antimicrob Chemoth 70:1031–1036. https://doi.org/10.1093/jac/dku491
Hejnar P, Kolář M, Hájek V et al (2001) Occurrence of variants with temperature-dependent susceptibility (TDS) to antibiotics among Stenotrophomonas maltophilia clinical strains. Folia Microbiol 46:151–155. https://doi.org/10.1007/BF02873595
Hemmat-Jou MH, Safari-Sinegani AA, Che R et al (2021) Toxic trace element resistance genes and systems identified using the shotgun metagenomics approach in an Iranian mine soil. Environ Sci Pollut Res 28:4845–4856. https://doi.org/10.1007/s11356-020-10824-x
Holmes A, Vinayak A, Benton C et al (2009) Comparison of two multimetal resistant bacterial strains: Enterobacter sp. YSU and Stenotrophomonas maltophilia ORO2. Curr Microbiol 59:526–531. https://doi.org/10.1007/s00284-009-9471-2
Horiyama T, Nishino K (2014) AcrB, AcrD, and MdtABC multidrug efflux systems are involved in Enterobactin export in Escherichia coli. PLoS ONE 9:e108642. https://doi.org/10.1371/journal.pone.0108642
Hsiao WWL, Ung K, Aeschliman D et al (2005) Evidence of a large novel gene pool associated with prokaryotic genomic islands. PLoS Genet 1:e62. https://doi.org/10.1371/journal.pgen.0010062
Hu HW, Wang JT, Li J et al (2017) Long-term nickel contamination increases the occurrence of antibiotic resistance genes in agricultural soils. Environ Sci Technol 51:790–800. https://doi.org/10.1021/acs.est.6b03383
Huang Y-W, Wang Y, Lin Y et al (2017) Impacts of penicillin binding protein 2 inactivation on, β-lactamase expression and muropeptide, profile in Stenotrophomonas maltophilia. mSystems 2:e00077–17. https://doi.org/10.1128/mSystems.00077-17.
Jacquiod S, Cyriaque V, Riber L et al (2018) Long-term industrial metal contamination unexpectedly shaped diversity and activity response of sediment microbiome. J Hazard Mater 344:299–307. https://doi.org/10.1016/j.jhazmat.2017.09.046
Jia B, Raphenya AR, Alcock B et al (2017) CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. https://doi.org/10.1093/nar/gkw1004
Khan A, Pettaway CH, Dien Bard J et al (2021) Evaluation of the performance of manual antimicrobial susceptibility testing methods and disk breakpoints for Stenotrophomonas maltophilia. Antimicrob Agents Chemother 65:e02631-e2720. https://doi.org/10.1128/AAC.02631-20
Knapp CW, McCluskey SM, Singh BK et al (2011) Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils. PLoS ONE 6(11):e27300. https://doi.org/10.1371/journal.pone.0027300
Knapp CW, Callan AC, Aitken B et al (2017) Relationship between antibiotic resistance genes and metals in residential soil samples from Western Australia. Environ Sci Pollut Res Int 24:2484–2494. https://doi.org/10.1007/s11356-016-7997-y
Konopka A, Zakharova T (1999) Quantification of bacterial lead resistance via activity assays. J Microbiol Meth 37:17–22. https://doi.org/10.1016/S0167-7012(99)00032-9
Krueger TS, Clark EA, Nix DE (2001) In vitro susceptibility of Stenotrophomonas maltophilia to various antimicrobial combinations. Diagn Micr Infec Dis 41:71–78. https://doi.org/10.1016/S0732-8893(01)00281-4
Kumar S, Bansal K, Patil PP et al (2020) Genomic insights into evolution of extensive drug resistance in Stenotrophomonas maltophilia complex. Genomics 112:4171–4178. https://doi.org/10.1016/j.ygeno.2020.06.049
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and clustal X version 2.0. Bioinformatics 21:2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Li LG, Xia Y, Zhang T (2017) Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection. ISME J 11:651–662. https://doi.org/10.1038/ismej.2016.155
Li R, Zhu H, Ruan J et al (2010) De novo assembly of human genomes with massively parallel short read sequencing. Genome Res 20:265–272. https://doi.org/10.1101/gr.097261.109
Li Y, Mima T, Komori Y et al (2003) A new member of the tripartite multidrug efflux pumps, MexVW–OprM, in Pseudomonas aeruginosa. J Antimicrob Chemoth 52:572–575. https://doi.org/10.1093/jac/dkg390
Lin YT, Huang YW, Liou RS et al (2014) MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J Antimicrob Chemother 69:3221–3226. https://doi.org/10.1093/jac/dku317
Liu L, Shen P, Zheng B et al (2020) Comparative genomic analysis of 19 clinical isolates of tigecycline-resistant Acinetobacter baumannii. Front Microbiol 11:1321. https://doi.org/10.3389/fmicb.2020.01321
Liu L, Su JQ, Guo Y et al (2018) Large-scale biogeographical patterns of bacterial antibiotic resistome in the waterbodies of China. Environ Int 117:292–299. https://doi.org/10.1016/j.envint.2018.05.023
Liu M, Li X, Xie Y et al (2019) ICEberg 2.0: an updated database of bacterial integrative and conjugative elements. Nucleic Acids Res 47:D660–D665. https://doi.org/10.1093/nar/gky1123
Liu S, Wang P, Wang X et al (2021) Ecological insights into the elevational biogeography of antibiotic resistance genes in a pristine river: metagenomic analysis along the Yarlung Tsangpo River on the Tibetan Plateau. Environ Pollut 286:117101. https://doi.org/10.1016/j.envpol.2021.117101
Lu WJ, Lin HJ, Janganan TK et al (2018) ATP-binding cassette transporter VcaM from Vibrio cholerae is dependent on the outer membrane factor family for its function. Int J Mol Sci 19:1000. https://doi.org/10.3390/ijms19041000
Luo G, Li B, Li LG et al (2017) Antibiotic resistance genes and correlations with microbial community and metal resistance genes in full-scale biogas reactors as revealed by metagenomic analysis. Environ Sci Technol 51:4069–4080. https://doi.org/10.1021/acs.est.6b05100
Ma J, Feng J, Shan Y et al (2020) Characteristic antimicrobial resistance of clinically isolated Stenotrophomonas maltophilia CYZ via complete genome sequence. J Glob Antimicrob Re 23:186–193. https://doi.org/10.1016/j.jgar.2020.09.008
Ma L, Xia Y, Li B et al (2016) Metagenomic assembly reveals hosts of antibiotic resistance genes and the shared resistome in pig, chicken, and human feces. Environ Sci Technol 50:420–427. https://doi.org/10.1021/acs.est.5b03522
Malekan M, Tabaraie B, Akhoundtabar L et al (2017) Distribution of class I integron and smqnr resistance gene among Stenotrophomonas maltophilia isolated from clinical samples in Iran. Avicenna J Med Biotechnol 9:138–141
Malik A, Aleem A (2011) Incidence of metal and antibiotic resistance in Pseudomonas spp. from the river water, agricultural soil irrigated with wastewater and groundwater. Environ Monit Assess 178:293–308. https://doi.org/10.1007/s10661-010-1690-2
Malik A, Jaiswal R (2000) Metal resistance in Pseudomonas strains isolated from soil treated with industrial wastewater. World J Microb Biot 16:177–182. https://doi.org/10.1023/A:1008905902282
Martinez JL (2009) The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc R Soc B 276:2521–2530. https://doi.org/10.1098/rspb.2009.0320
Matyar F, Kaya A, Dinçer S (2008) Antibacterial agents and metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Sci Total Environ 407:279–285. https://doi.org/10.1016/j.scitotenv.2008.08.014
Mazhar SH, Li X, Rashid A et al (2021) Co-selection of antibiotic resistance genes, and mobile genetic elements in the presence of heavy metals in poultry farm environments. Sci Total Environ 755:142702. https://doi.org/10.1016/j.scitotenv.2020.142702
McArthur AG, Waglechner N, Nizam F et al (2013) The comprehensive antibiotic resistance database. Antimicrob Agents Ch 57:3348–3357. https://doi.org/10.1128/AAC.00419-13
Moura A, Soares M, Pereira C et al (2009) INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics 25:1096–1098. https://doi.org/10.1093/bioinformatics/btp105
Mu C, Zhang T, Wu Q et al (2014) Stable carbon isotopes as indicators for permafrost carbon vulnerability in upper reach of Heihe River basin, northwestern China. Quatern Int 321:71–77. https://doi.org/10.1016/j.quaint.2013.12.001
Naguib MM, Khairalla AS, El-Gendy A et al (2019) Isolation and characterization of mercury-resistant bacteria from wastewater sources in Egypt. Can J Microbiol 65:308–321. https://doi.org/10.1139/cjm-2018-0379
Nakamura R, Oota M, Matsumoto S et al (2021) In vitro activity and in vivo efficacy of cefiderocol against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 65:e01436-e1520. https://doi.org/10.1128/AAC.01436-20
Nath S, Sinha A, Singha YS et al (2020) Prevalence of antibiotic-resistant, toxic metal-tolerant and biofilm-forming bacteria in hospital surroundings. Environ Anal Health Toxicol 35:e2020018. https://doi.org/10.5620/eaht.2020018
Nishino K, Yamada J, Hirakawa H et al (2003) Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to β-lactams. Antimicrob Agents Ch 47:3030–3033. https://doi.org/10.1128/aac.47.9.3030-3033.2003
Okubo T, Ae R, Noda J et al (2019) Detection of the sul2–strA–strB gene cluster in an ice core from Dome Fuji Station, East Antarctica. J Glob Antimicrob Re 17:72–78. https://doi.org/10.1016/j.jgar.2018.11.005
Ouyang W, Huang F, Zhao Y et al (2015) Increased levels of antibiotic resistance in urban stream of Jiulongjiang River, China. Appl Microbiol Biot 99:5697–5707. https://doi.org/10.1007/s00253-015-6416-5
Overbeek R, Olson R, Pusch GD et al (2014) The SEED and the rapid annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. https://doi.org/10.1093/nar/gkt1226
Pages D, Rose J, Conrod S et al (2008) Heavy metal tolerance in Stenotrophomonas maltophilia. PLoS ONE 3:e1539. https://doi.org/10.1371/journal.pone.0001539
Pal C, Bengtsson-Palme J, Rensing C et al (2014) BacMet: antibacterial biocide and metal resistance genes database. Nucleic Acids Res 42:D737–D743. https://doi.org/10.1093/nar/gkt1252
Pankuch GA, Jacobs MR, Rittenhouse SF et al (1994) Susceptibilities of 123 strains of Xanthomonas maltophilia to eight beta-lactams (including beta-lactam-beta-lactamase inhibitor combinations) and ciprofloxacin tested by five methods. Antimicrob Agents Ch 38:2317–2322. https://doi.org/10.1128/AAC.38.10.2317
Patil PP, Kumar S, Midha S et al (2018) Taxonogenomics reveal multiple novel genomospecies associated with clinical isolates of Stenotrophomonas maltophilia. Microbial Genomics 4:e000207. https://doi.org/10.1099/mgen.0.000207
Périchon B, Courvalin P, Galimand M (2007) Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob Agents Ch 51:2464–2469. https://doi.org/10.1128/aac.00143-07
Perron GG, Whyte L, Turnbaugh PJ et al (2015) Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. PLoS ONE 10:e0069533. https://doi.org/10.1371/journal.pone.0069533
Poole K (2001) Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol 3:255
Pruden A, Pei RT, Storteboom H et al (2006) Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environ Sci Technol 40:7445–7450. https://doi.org/10.1021/es060413l
Rocco F, Gregorioa ED, Colonna B et al (2009) Stenotrophomonas maltophilia genomes: a start-up comparison. Int J Med Microbiol 299:535–546. https://doi.org/10.1016/j.ijmm.2009.05.004
Sánchez MB, Martínez JL (2018) Overexpression of the efflux pumps SmeVWX and SmeDEF is a major cause of resistance to co-trimoxazole in Stenotrophomonas maltophilia. Antimicrob Agents Ch 62:e00301-e318. https://doi.org/10.1128/AAC.00301-18
Sanderson H, Fricker C, Brown RS et al (2016) Antibiotic resistance genes as an emerging environmental contaminant. Environ Rev 24:205–218. https://doi.org/10.1139/er-2015-0069
Sandner-Miranda L, Vinuesa P, Cravioto A et al (2018) The genomic basis of intrinsic and acquired antibiotic resistance in the genus Serratia. Front Microbiol 9:828. https://doi.org/10.3389/fmicb.2018.00828
Schaeffer A, Amelung W, Hollert H et al (2016) The impact of chemical pollution on the resilience of soils under multiple stresses: a conceptual framework for future research. Sci Total Environ 568:1076–1085. https://doi.org/10.1016/j.scitotenv.2016.06.161
Siguier P, Perochon J, Lestrade L et al (2006) ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Re 34:D32–D36. https://doi.org/10.1093/nar/gkj014
Smith M, Do TN, Gibson JS et al (2014) Comparison of antimicrobial resistance phenotypes and genotypes in enterotoxigenic Escherichia coli isolated from Australian and Vietnamese pigs. J Glob Antimicrob Re 2:162–167. https://doi.org/10.1016/j.jgar.2014.03.008
Song JH, Sung JY, Kwon KC et al (2010) Analysis of acquired resistance genes in Stenotrophomonas maltophilia. Korean J Lab Med 30: 295–300. https://doi.org/10.3343/kjlm.2010.30.3.295
Spierer O, Miller D, O’Brien TP (2018) Comparative activity of antimicrobials against Pseudomonas aeruginosa, Achromobacter xylosoxidans and Stenotrophomonas maltophilia keratitis isolates. Brit J Ophthalmol 102:708–712. https://doi.org/10.1136/bjophthalmol-2017-311751
Sun SC, Wang CJX, YG, et al (2021) Molecular mechanisms of heavy metals resistance of Stenotrophomonas rhizophila JC1 by whole genome sequencing. Arch Microbiol 203:2699–2709. https://doi.org/10.1007/s00203-021-02271-0
Tatman-Otkun M, Gürcan Ş, Özer B et al (2005) The antimicrobial susceptibility of Stenotrophomonas maltophilia isolates using three different methods and their genetic relatedness. BMC Microbiol 5:1–6. https://doi.org/10.1186/1471-2180-5-24
Tan L, Li L, Ashbolt N et al (2018) Arctic antibiotic resistance gene contamination, a result of anthropogenic activities and natural origin. Sci Total Environ 621:1176–1184. https://doi.org/10.1016/j.scitotenv.2017.10.110
Valdezate S, Vindel A, Loza E et al (2001) Antimicrobial susceptibilities of unique Stenotrophomonas maltophilia clinical strains. Antimicrob Agent Ch 45:1581–1584. https://doi.org/10.1128/aac.45.5.1581-1584.2001
Weiss K, Restieri C, De Carolis E et al (2000) Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemoth 45:363–365. https://doi.org/10.1093/jac/45.3.363
Wireman J, Liebert CA, Smith T et al (1997) Association of mercury resistance with antibiotic resistance in the gram-negative fecal bacteria of primates. Appl Environ Microb 63:4494–4503. https://doi.org/10.1128/aem.63.11.4494-4503.1997
Wright GD, Poinar H (2012) Antibiotic resistance is ancient: implications for drug discovery. Trends microbiol 20:157–159. https://doi.org/10.1016/j.tim.2012.01.002
Xia Y, Li AD, Deng Y et al (2017) MinION nanopore sequencing enables correlation between resistome phenotype and genotype of coliform bacteria in municipal sewage. Front Microbiol 8:2105. https://doi.org/10.3389/fmicb.2017.02105
Yang Y, Li Z, Song W et al (2019) Metagenomic insights into the abundance and composition of resistance genes in aquatic environments: influence of stratification and geography. Environ Int 127:371–380. https://doi.org/10.1016/j.envint.2019.03.062
Youenou B, Favre-Bonté S, Bodilis J et al (2015) Comparative genomics of environmental and clinical Stenotrophomonas maltophilia strains with different antibiotic resistance profiles. Genome Biol Evol 7:2484–2505. https://doi.org/10.1093/gbe/evv161
Yuan K, Yu K, Yang R et al (2019) Metagenomic characterization of antibiotic resistance genes in Antarctic soils. Ecotox Environ Safe 176:300–308. https://doi.org/10.1016/j.ecoenv.2019.03.099
Zhanel GG, DeCorby M, Nichol KA et al (2008) Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect 62:67–80. https://doi.org/10.1016/j.diagmicrobio.2008.04.012
Zhang S, Yang G, Hou S et al (2021) Analysis of heavy metal-related indices in the Eboling permafrost on the Tibetan Plateau. CATENA 196:104907. https://doi.org/10.1016/j.catena.2020.104907
Zhang X, Shang B, Li X et al (2020) Complete genome sequence data of multidrug-resistant Stenotrophomonas sp. strain SXG-1. J Glob Antimicrob Re 222:206–209. https://doi.org/10.1016/j.jgar.2020.03.005
Zhu YG, Johnson TA, Su JQ et al (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. P Natl Acad Sci USA 110:3435–3440. https://doi.org/10.1073/pnas.1222743110
Funding
We thank the Science and Technology Research Project of Henan Province (No. 222102320010) for financial support, the Program for Science & Technology Innovative Research Team in University of Henan Province (21IRTSTHN025), and the Program of Ecological Conservation and High-quality Development of the Old Course of Yellow River (2021KYFZ06) for instrument supply.
Author information
Authors and Affiliations
Contributions
SHZ, GLY, YLJ were equally contributed to all stages of preparing, drafting, writing this manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work during different preparation stages. All authors read, revised, and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11356_2022_22888_MOESM1_ESM.xlsx
Supplementary file1 Table S1 ARGs recovered from permafrost Stenotrophomonas maltophilia in our study and their identities. (XLSX 10 KB)
11356_2022_22888_MOESM2_ESM.xlsx
Supplementary file2 Table S2 MRGs recovered from permafrost Stenotrophomonas maltophilia in our study and their identities. (XLSX 11 KB)
11356_2022_22888_MOESM3_ESM.xlsx
Supplementary file3 Table S3 Annotated resistance genes in genomic islands from Stenotrophomonas maltophilia (XLSX 949 KB)
11356_2022_22888_MOESM4_ESM.docx
Supplementary file4 Table S4 The maximum MIC for antibiotics and metals of Stenotrophomonas maltophilia from different environments. (DOCX 18 KB)
11356_2022_22888_MOESM5_ESM.xlsx
Supplementary file5 Table S5 Comparison of ARGs from our permafrost Stenotrophomonas maltophilia and clinical Stenotrophomonas maltophilia. (XLSX 17 KB)
11356_2022_22888_MOESM6_ESM.xlsx
Supplementary file6 Table S6 Comparison of MRGs from our permafrost Stenotrophomonas maltophilia and clinical Stenotrophomonas maltophilia. (XLSX 11 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Yang, G. & Jiang, Y. Antibiotic and metal resistance of Stenotrophomonas maltophilia isolates from Eboling permafrost of the Tibetan Plateau. Environ Sci Pollut Res 30, 11798–11810 (2023). https://doi.org/10.1007/s11356-022-22888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22888-y