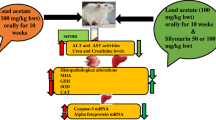
Abstract
Lead, one of the most common heavy metal toxins, seriously affects the health of humans and animals. Sinomenine hydrochloride (SH) shows antioxidative, anti-inflammatory, antiviral, and anticancer properties. Hence, this study investigated the protective effects of SH against Pb-induced liver injury and explored the underlying mechanisms. First, a mouse model of lead acetate (0.5 g/L lead acetate in water, 8 weeks) was established, and SH (100 mg/kg bw in water, 8 weeks) intervention was administered by gavage. Then, the protective effect of SH against lead-induced liver injury was evaluated through serum biochemical analysis, histopathological analysis, and determination of malondialdehyde (MDA) and total antioxidant capacity (T-AOC) levels. The messenger RNA (mRNA) expression levels of the cytokines IL-1β and TNF-α and the apoptosis factors Bax, Bcl-2, and Caspase3 in the liver were detected by quantitative real-time PCR. Then, the expression levels of IL-1β and TNF-α in the liver were detected by ELISA. Immunohistochemical determination of the expression of the apoptosis factors Bax, Bcl-2, and Caspase3 was performed. SH treatment reduced the levels of liver alanine aminotransferase, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and MDA in Pb-treated mice, indicating that SH protected the liver from injury and oxidative stress in Pb-treated mice. SH also increased the liver T-AOC of Pb-treated mice. Quantitative real-time PCR, ELISA, and immunohistochemical analysis showed that SH inhibited apoptosis, as indicated by the regulation of the mRNA expression of Bax and Bcl-2 and the reduced expression of Caspase3 and pro-inflammatory factors (IL-1β and TNF-α) in the livers of Pb-treated mice. These results suggest that SH protects the mouse liver from Pb-induced injury. The underlying mechanism involves antioxidative, anti-inflammatory, and anti-apoptotic processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that there is a global recommendation regarding reducing the use of lead in both industry and agriculture. Pb is a well-known environmental pollutant; the combustion of Pb-containing gasoline and its exhaust emission, the development of Pb ore, metal smelting, and coal combustion have resulted in significant changes in the content of Pb in the ecological environment (Singh et al. 2018). In 2017, 1.06 million people in the world died of health effects related to long-term Pb exposure, with low- and middle-income countries being the most affected (Li et al. 2021a). Due to the irreversible harm of Pb on the environment and living organisms, great attention has been given by countries and organizations worldwide to the study of Pb poisoning. Environmental Pb enters the human or animal body through the food chain including feed, food, and water. Pb accumulates in the kidneys, liver, brain, bones, and other organs. When Pb accumulates in large amounts, it is difficult for organs to decompose it in a short period of time, which leads to chronic Pb poisoning (Li et al. 2021b). Liu et al. (2011) found that the LD50 of lead was approximately 3200 mg/kg bw in 24 h. Many in vitro and animal experiments have shown that oxidative stress is the main contributor to Pb toxicity, and secondary inflammation and apoptosis further aggravate Pb toxicity (Zou et al. 2020). The liver is one of the main organs of Pb toxicity. Approximately 33% of Pb that enters the human body remains in the liver, which is the organ with the largest accumulation of Pb in the body. Some studies have reported on the mechanism of Pb hepatotoxicity. Chronic Pb exposure causes significant elevation of liver-related enzymes (ALP, ALT, AST and GGT), as well as elevated oxidative stress parameters. Pb also has a serious inhibitory effect on the detoxification enzyme system and mitochondrial enzyme system. Pb-induced hepatotoxicity leads to ultrastructural changes in the endoplasmic reticulum and mitochondria and other organelles, affecting the normal physiological function of mitochondria and the normal metabolic process of the liver (Renu et al. 2021). Dimercaptosuccinic acid (DMSA) and disodium edetate calcium (Ca Na2-EDTA) are two complexing agents mainly used for the treatment of Pb poisoning. These two compounds can metabolize the Pb-containing complex and excrete it out of the body through the kidneys, but at the same time, the necessary trace elements (Ca, Mg, Cu, Zn, etc.) in the body are also excreted along with Pb, which causes serious side effects (Bjørklund et al. 2017). Various natural Chinese herbal medicines have been used for the prevention and treatment of various diseases due to their extensive sources, anti-inflammatory and antioxidant physiological characteristics, low toxicity, and few side effects (Xue et al. 2022). Sinomenine is one of the active components in Sinomenium acutum, a Chinese herbal medicine that has been used in the treatment of rheumatoid arthritis in China and Japan for thousands of years (Sharma et al. 2020). In a rat model of temporal lobe epilepsy, sinomenine can regulate the levels of malondialdehyde and SOD and inhibit the signaling pathways of the inflammatory factors TNF-α and NF-κB (Ramazi et al. 2020). Xu et al. (2018) demonstrated that sinomenine can inhibit the NF-κB signaling pathway, downregulate pro-inflammatory factors, and alleviate inflammation in vitro and in vivo.
Sinomenine hydrochloride (SH) is the hydrochloride form of sinomenine and has high stability. Many studies have shown that SH has antioxidative, anti-inflammatory, antiviral, and anticancer properties, among which the anti-inflammatory properties are the most conspicuous (Yin et al. 2020). This finding indicates that SH has pharmacological and therapeutic potential. In a mouse experiment, SH alleviated inflammation and reduced the occurrence of plasma cell mastitis caused by inhibiting overactivated inflammatory signaling (Liu et al. 2020). In vivo and in vitro experiments showed that sinomenine reduced acetaminophen-induced acute liver injury through antioxidative and inflammatory reactions (Chen et al. 2020b). To our knowledge, the investigation of the effect of SH on chronic Pb toxicity injury has not yet been reported. Thus, this study was designed to investigate the protective effects of SH on oxidative stress inflammatory and apoptotic injury in the liver of mice with chronic Pb poisoning, aiming to provide some insights for therapeutic intervention of Pb poisoning.
Materials and methods
Chemicals and reagents
Sinomenine hydrochloride (CAS: 608033-7, 98% purity) was obtained from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Lead acetate ((C2H3O2)2Pb) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Malondialdehyde (Mazumdar and Goswami 2014), total antioxidant capacity (T-AOC), and total protein (TP) detection kits were purchased from Nanjing Jiancheng Institute of Biotechnology (Shanghai, China). Hematoxylin-eosin (HE) dye was supplied by Shenyang Wanlei Biotechnology Co., Ltd. (Shenyang, China). IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from Cusabio Co., Ltd. (Wuhan, China). The SYBR Green Mix kit was procured from Japan. IL-1β, TNF-α, Caspase3, Bax, Bcl-2, and GAPDH primers were obtained from Sangon Biotech Co., Ltd. (Shanghai, China).
Experimental animals
Twenty-four ICR mice (male, 6 weeks, 24–26 g) were procured from Liaoning Changsheng Biotechnology Co., Ltd. Prior to the experiment, the mice were acclimatized for 1 week in a room with a circumambient (temperature of 22 ± 1°C, a 12-h dark/light cycle, relative humidity 40–60%). Then, the mice were randomly divided into 4 groups (n = 6): (1) control group, (2) lead acetate (Pb) group, (3) lead acetate + SH group (Pb+SH), and (4) SH group (Fig. 1). The test period was 8 weeks. The mice in the control group and SH group were given normal drinking water, while those in the Pb group and Pb+SH group were given water supplemented with 0.5 g/L lead acetate to establish chronic Pb poisoning models (Liu et al. 2011). SH (dissolved in normal saline) was administered to mice in the Pb+SH group and SH group by gavage (at doses of 100 mg/kg bw) once a day (Fan et al. 2022). Mice in the control (CON) group and Pb group were intragastrically administered equal volumes of normal saline once a day. During the test, mouse body weight was recorded every week. Two hours after the last dose, the mouse body weight was recorded, mice were euthanized by ether anesthesia, blood was collected by the enucleation method, and blood and liver were collected immediately. Mouse liver weights were measured, and organ indices were calculated using the organ index formula. Liver index (%) = liver weight (mg) / body weight on the last day (g) × 10 (Zhou et al. 2021). Liver tissue was fixed in 4% buffered formalin for histological analysis. One gram of liver tissue was added to 9 mL of normal saline to make a homogenate, and the supernatant was taken after centrifugation for the analysis of malondialdehyde (MDA) and T-AOC concentrations in the liver. The liver (100 mg) was homogenized with PBS (w/v: 1/9) to evaluate pro-inflammatory factor levels. Another piece of liver was digested by 6:1 perchloric acid using a microwave digestion system and was then diluted with deionized water to measure Pb concentrations. The remaining liver tissues were frozen in liquid nitrogen and stored at − 80°C for further analysis.
A Chemical structure of sinomenine hydrochloride. B Experimental design. The mice in the CON and SH groups were given normal drinking water, while those in the Pb group and Pb+SH group were given water supplemented with 0.5 g/L lead acetate to establish chronic Pb poisoning models. SH (dissolved in normal saline) was administered to mice in the Pb+SH group and SH group by gavage (at doses of 100 mg/kg bw) once a day (n = 6 mice per group). (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
The protocols used in this experiment were approved by the Institutional Animal Care and Use Committee, Northeast Agricultural University, and the ethical treatment of animals used in this study was approved by the Animal Welfare Committee protocol (#NEAU-[2013]-9) at Northeast Agricultural University (Harbin, China).
Biochemical analysis
Serum was separated by centrifuging collected blood at 3000 × g for 10 min. The levels of serum AST, ALT, and LDH were determined by an automatic bioanalysis machine (FULLY, Italy) (Fang et al. 2021).
Lead content in liver analysis
The Pb concentrations were determined in the digested hepatic samples using atomic absorption spectrophotometry (Perkin Elmer Analyst 800, USA) with a specific lamp for Pb as defined elsewhere (Almasmoum et al. 2019).
Histological analysis
Liver tissues from each mouse in each group (n = 6 mice per group) were fixed in 4% buffered formaldehyde for 24 h at 4°C, and then the fixed tissues were stained with H&E stain (hematoxylin and eosin stain) followed by dehydration, paraffin embedding, and cutting into 5-μm sections. Sections were examined by a pathologist using an optical microscope (Olympus: BX53, Shanghai, China) to determine the degree of inflammation of the liver (Dou et al. 2022). The pathologist was blinded to the mice’s treatment.
Analysis of liver MDA and T-AOC concentrations
A malondialdehyde assay kit (TBA method) (Cat. No. A003-1; Jiancheng Bioengineering Institute, Nanjing, China) and a T-AOC assay kit (Cat. No. AC10285; Jiancheng Bioengineering Institute, Nanjing, China) were used to detect liver MDA and T-AOC concentrations. The prepared liver homogenate and the reagents in the kit were prepared in a reaction system according to the manufacturer’s instructions. Then, the absorbance value was detected by a microplate reader (Tecan, Australia), and the liver MDA and T-AOC concentrations were calculated according to the standard curve.
Quantitative real-time polymerase chain reaction analysis
Total RNA was extracted with TRIzol reagent (Cat. No. 15596018; Invitrogen, CA, USA). The concentration of RNA was quantified using a NanoDrop 2000c spectrophotometer. RNA was reverse transcribed into complementary DNA (cDNA) using the PrimeScript™ RT kit (Cat. No. 9109; Takara, Otsu, Japan), and the cDNA products were stored at − 80°C. Real-time PCR was performed on the 7500 Real-Time PCR System (ABI, USA). The PCR mixture volume was 20 μL and contained 10 μL of TB Green Premix Ex Taq (Tli RNase H Plus) (2×) (Cat. No. RR420A; Takara, Japan), 0.4 μL of PCR forward primer (10 μM), 0.4 μL of PCR reverse primer (10 μM), 0.4 μL of ROX Reference Dye II (50×) (Cat. No. RR420A; Takara, Otsu, Japan), 0.4 μL of cDNA, and 6.8 μL of sterile water. Amplification was performed with a hot start polymerase activation step for 30 s at 95°C, followed by 40 cycles of 3 s at 95°C and 30 s at 60°C. The range of efficiency primers amplified was between 90 and 110%. The relative expression was calculated using the 2−ΔΔCt method, and the GAPDH gene was regarded as a standard reference. The primers used are shown in Table 1.
Enzyme-linked immunosorbent assay
The TNF-α and IL-1β levels in the liver were analyzed using an ELISA kit (Cat. Nos. E-MSEL-M000 and E-EL-M3063; Cusabio, Wuhan, China) according to the manufacturer’s instructions. Standard working reagents A and B were mixed with the homogenate. Then, the reaction mixture was incubated at 60°C for 30 min, and the absorbance was measured at 562 nm on an ELISA plate reader. The amount of each protein was calculated using the standard curve.
Immunohistochemistry analysis
The distribution of Bax, Bcl-2, and Caspase3 in the liver tissue of mice in each group was detected by immunohistochemistry to understand the changes in liver apoptosis. Liver tissues from each mouse in each group (n = 6 mice per group) were placed on slides and dried. The sections were first dehydrated with xylene and then with different concentrations of ethanol. The endogenous peroxidase was inactivated after incubation with 3% hydrogen peroxide for 15 min. Next, the sections were incubated overnight with the first antibody and then for 60 min with the HRP-labeled second antibody. DAB solution was dropped onto the slides to uniformly cover the tissue sections. The reaction was stopped just after the color became dark, and the sections were stained with hematoxylin and dehydrated. Finally, the staining was observed under a microscope, and the images were taken under × 400 magnification. The quantitative form of IHC is the mean optical density (MOD) parameter detected by Image-Pro Plus.
Statistical analysis
Data represent the mean ± SEM. All statistical analyses were performed using SPSS 26.0 software (IBM, IL, USA). Statistical analysis for the control and experimental groups was performed by one-way analysis of variance (ANOVA) followed by Student’s t test post hoc statistical analysis. P < 0.05 indicated that the differences were statistically significant.
Results
Effects of SH on body weight and organ index in Pb-treated mice
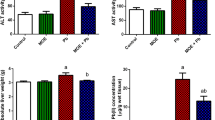
As shown in Fig. 2, compared with the CON group, the body weight of mice in the Pb group was reduced, and the liver index was increased (P < 0.001). Compared with the Pb group, the body weight of the SH group was increased (P < 0.05), and the liver index was decreased (P < 0.01).
Effects of SH on the body weight and organ index of Pb-treated ICR mice. A, B Body weight. C Liver index. D The effect of SH on lead levels in the liver of lead-treated mice. The results are represented as the mean ± SEM (n = 6 mice per group). ***P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01 vs. the Pb-treated group. (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
Effects of SH on serum biochemistry in Pb-treated mice
Changes in serum biochemical levels are considered to be an initial response to body injury. As shown in Table 2, the liver function indices (AST, ALT, and LDH) were higher in the Pb group than in the CON group (P < 0.001). Moreover, compared with the Pb group, the serum ALT, AST, and LDH levels of the Pb+SH group were reduced (P < 0.001).
Effects of SH on liver lead content in Pb-treated mice
Liver Pb levels were used to assess the protective effect of SH against Pb-induced Pb toxicity. As shown in Fig. 2, compared with the CON group, the liver Pb level in the Pb group was significantly increased (P < 0.001). The level of Pb was significantly decreased in the Pb+SH group compared with the Pb group (P < 0.05).
Effects of SH on liver histopathology in Pb-treated mice
The effects of SH on histopathological changes in Pb-treated mice were detected by H&E staining. As shown in Fig. 3, the histopathological results showed that SH had a protective effect on Pb-induced liver injury. In the CON group, the liver tissue structure of the mice was clear, and the hepatocytes were arranged neatly and tightly without degeneration, necrosis, edema, or inflammatory cell infiltration. On the other hand, the hepatocytes in the Pb group were loosely arranged and structurally disordered, with partial cell necrosis and vacuolation. Compared with CON mice, there were no visible histological changes in the liver of the Pb+SH and SH mice.
Effects of SH on the liver histopathology of Pb-treated ICR mice. Paraffin sections of hepatic tissues from the CON group, Pb group, Pb+SH group, and SH group were stained with hematoxylin-eosin (n = 6 mice per group) (H&E staining × 200). The bar is 100 μm. Histopathological results showed that SH had a protective effect on Pb-induced liver injury. In the CON group, the liver tissue structure of the mice was clear, and the hepatocytes were arranged neatly and tightly without degeneration, necrosis, edema, or inflammatory cell infiltration. On the other hand, the hepatocytes in the Pb group were loosely arranged and structurally disordered, with partial cell necrosis and vacuolation. Compared with CON mice, there were no visible histological changes in the liver of the Pb+SH and SH mice. (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
Effects of SH on levels of MDA and T-AOC in Pb-treated mouse liver
MDA and T-AOC levels were used to assess the protective effects of SH on Pb-induced lipid peroxidation. Compared with the CON group, the liver MDA level in the Pb group was significantly increased (P < 0.001) (Fig. 4A), and T-AOC activity was significantly decreased (P < 0.001) (Fig. 4B). The level of MDA was decreased, and the level of T-AOC was elevated in the Pb+SH group compared with the Pb group (P < 0.01).
Effects of SH on the levels of A MDA and B T-AOC in Pb-treated mouse livers. The values are shown as the mean ± SEM (n = 6 mice per group). ***P < 0.001 vs. the control group; ##P < 0.01 vs. the Pb-treated group. (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH), malondialdehyde (MDA), total antioxidant capacity (T-AOC))
Effects of SH on the messenger RNA expression levels of inflammatory and apoptotic cytokines in Pb-treated mouse liver
To determine whether SH can attenuate the inflammatory damage of Pb-treated mice, the expression of inflammatory factors was measured in the liver. Pb treatment increased IL-1β and TNF-α expression compared with the CON treatment (P < 0.05). Treatment with Pb+SH reversed the effects of Pb on inflammatory factors (P < 0.05) (Fig. 5A, B). No difference was observed in the expression of liver inflammatory factors in Pb+SH mice compared with the controls. Compared with the control group, the expression levels of the pro-apoptotic factors Caspase3 and Bax were significantly increased (P < 0.001) (Fig. 5E, F), while Bcl-2 was significantly decreased in the Pb group (Fig. 5G). SH reduced the Pb-induced upregulation of Caspase3 and Bax (P < 0.01) and reversed the Pb-induced downregulation of the expression of Bcl-2 (P < 0.01).
Effects of SH on the mRNA expression levels of inflammatory and apoptotic cytokines in Pb-treated mouse livers (A–G). A, B Pro-inflammatory cytokines (IL-1β and TNF-α) and E Caspase3, F Bax, and G Bcl-2 mRNA expression levels in the liver were detected by qRT-PCR. Effects of SH on inflammatory cytokine production in the liver of Pb-treated mice (C, D). C IL-1β and D TNF-α levels in the liver were detected by ELISA. The values are shown as the mean ± SEM (n = 6 mice per group). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. the Pb-treated group. (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
Effects of SH on the expression levels of inflammatory cytokines in the Pb-treated mouse liver
To further determine whether SH can attenuate the inflammatory damage of Pb-treated mice, the levels of inflammatory factors were measured in the liver. Pb treatment increased IL-1β and TNF-α levels compared with the CON treatment (P < 0.05). Treatment with Pb+SH reversed the effects of Pb on inflammatory factors (P < 0.05). No difference was observed in the level of liver inflammatory factors in Pb+SH mice compared with the controls (Fig. 5C, D).
Effects of SH on the messenger RNA expression levels of NF-κB, NF-κB p65, and IκBα in the Pb-treated mouse liver
To investigate whether SH has a therapeutic effect on Pb-induced inflammation through the NF-κB signaling pathway, the messenger RNA (mRNA) expression levels of NF-κB, NF-κB p65, and IκBα in the NF-κB signaling pathway in the liver were measured. Pb treatment increased NF-κB, NF-κB p65, and IκBα expression compared with the CON treatment (P < 0.001). Treatment with Pb+SH reversed the effects of Pb on NF-κB, NF-κB p65, and IκBα (P < 0.001) (Fig. 6).
Effects of SH on the mRNA expression levels of NF-κB, p65, and IκBα in the NF-κB signaling pathway in the liver of lead-treated mice. A NF-κB, B p65, and C IκBα mRNA expression levels in the liver were detected by qRT-PCR. The values are shown as the mean ± SEM (n = 6 mice per group). ***P < 0.001 vs. the control group; ###P < 0.001 vs. the Pb-treated group. Different letters indicate that the change between each group is statistically significant (P < 0.05). (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
Effects of SH on the protein expression levels of apoptotic cytokines in the Pb-treated mouse liver
To further investigate the anti-apoptotic effect of SH, we detected the distribution of Bax, Bcl-2, and Caspase3 in the liver tissues of mice in each group by immunohistochemistry. Compared with the CON group, the number of Bax- and Caspase3-positive cells in the Pb group was significantly increased (P < 0.001), and the number of Bcl-2-positive cells was decreased (P < 0.01), mainly located around the central portal vein (Fig. 7). However, SH clearly lowered the increased secretion of pro-apoptotic proteins. The results of IHC analysis were quantified as the mean optical density, which was consistent with the trend of qRT-PCR results, indicating that SH effectively inhibited the apoptotic response induced by Pb.
Effects of SH on the expression levels of apoptosis-related proteins in Pb-treated mouse livers. The black arrow indicates the increase of positive cells. The optical density is shown as the mean ± SEM (n = 6 mice per group). **P < 0.01, and ***P < 0.001 vs. the control group; #P < 0.05, and ###P < 0.001 vs. the Pb-treated group. (Control group (CON), lead acetate group (Pb), lead acetate + SH group (Pb+SH), sinomenine hydrochloride group (SH))
Discussion
Pb is one of the most common and toxic heavy metal elements. Long-term Pb exposure can lead to contamination of animal feed, drinking water, and animal products, which, in turn, may lead to chronic Pb poisoning in humans (Zhang et al. 2019). Pb can disrupt cellular biological functions by modulating molecular interactions and cell signaling, causing liver inflammation and oxidative damage (Almasmoum et al. 2019). In some previous studies, sinomenine was found to have antioxidant and anti-inflammatory effects (Fan et al. 2022); thus, this experiment planned to explore the protective effect of sinomenine on Pb-induced hepatotoxicity.
The results of this experiment show that SH can inhibit weight loss and increase the organ index in Pb-poisoned mice and that the addition of SH can reduce the accumulation of Pb in the liver tissue of mice. Histopathology is the most substantial detection index of liver injury. The results of H&E staining showed that the hepatocytes of the mice in the control group were closely arranged, the structure was complete, and there were no swelling or obvious pathological changes in the tissue. In the pathological sections of the Pb group, clear abnormal arrangement of liver cells and infiltration of inflammatory cells were observed, and cell vacuoles appeared, indicating serious liver damage. Compared with the Pb group, the liver tissue of the Pb+SH group had a clear structure, the hepatocytes were clearly outlined and neatly arranged, and the pathological changes in the liver tissue of the mice were significantly improved, indicating that only slight liver damage was caused. Liu et al. (2011) found that chronic Pb poisoning can cause histological changes in the livers of mice, and the mice developed hepatocyte damage and cell infiltration, which is consistent with the results of this study. ALT, AST, and LDH in serum are often used as indirect indicators of hepatocyte injury and are important markers of hepatocyte injury (Pandurangan and Kim 2015). When the content of reactive oxygen species (ROS) increases in the liver due to Pb poisoning, the permeability of the cell membrane and mitochondria of hepatocytes increases; thus, ALT, AST, and LDH are released into the blood (Wang et al. 2011; Liu and Song 2022). In this study, the activities of AST, ALT, and LDH in the serum of mice in the Pb group were significantly increased, indicating that Pb poisoning caused damage to the liver. Compared with the Pb group, the serum levels of AST, ALT, and LDH in the Pb+SH group were significantly lower, indicating that SH has a therapeutic effect on Pb-induced chronic Pb poisoning in the liver.
The increase in ROS levels caused by Pb in animal cells has been a hot research topic. Oxidative stress is the result of an imbalance between the generation and scavenging of ROS. In a healthy body, the concentration of various ROS and the action of antioxidant molecules and antioxidant enzymes maintain a safe level of ROS (Bedard and Krause 2007). Pb does not cause oxidative stress by directly participating in ROS generation but by disrupting the antioxidant system in the liver. MDA is the product of lipid peroxidation in living organisms; it has active chemical properties and is the final product of lipid peroxidation under the action of free radicals in living organisms. In a healthy state, the body produces more MDA, but mammals have a complete MDA degradation pathway to maintain a dynamic balance between the production and decomposition of MDA in the body. MDA can cause the cross-linking and polymerization of biological macromolecules such as proteins and nucleic acids, thereby changing the functions of DNA and proteins, hindering the normal transcription and replication of DNA, and in severe cases, causing chromosome breakage or deletion and even cell death. Excessive MDA in the liver of mice has varying degrees of effects on the mitochondrial respiratory chain, α-ketoglutarate dehydrogenase, pyruvate dehydrogenase, and malate dehydrogenase, causing damage to liver cells (Duvvuri et al. 2015). Therefore, MDA is often used as an important physiological indicator of oxidative stress and lipid peroxidation in the body. There are many antioxidants in the body, including antioxidant molecules and enzymes, which can remove various ROS generated in the body and prevent ROS from inducing oxidative stress. The total level of these antioxidant molecules and enzymes is the total antioxidant capacity. The level of T-AOC is negatively correlated with the level of ROS in the body. Elevated MDA and decreased GSH, GPx, and CAT in the liver can be detected in a mouse model of hepatic Pb intoxication. This result indicated that Pb could damage the liver by affecting the level of MDA and T-AOC (Almasmoum et al. 2019). In another study on alcohol-induced acute liver injury, SH enhanced antioxidant capacity in mice by downregulating MDA levels and upregulating CAT, SOD, and GSH-Px levels (Fan et al. 2022). Compared with the Pb group, the MDA level in the Pb+SH group decreased and the T-AOC level increased, indicating that SH may ameliorate the injury of mouse liver caused by chronic Pb poisoning by inhibiting lipid peroxidation and enhancing antioxidant defense capacity.
Studies have shown that excessive ROS produced by the body can activate the NF-κB signaling pathway and regulate the expression of multiple cytokines (IL-1β and TNF-α) during inflammation (Yang et al. 2020). The NF-κB signaling pathway is an important signaling pathway that regulates inflammation (Bian et al. 2022). Once activated, it induces large amounts of pro-inflammatory cytokines. There are five proteins in the mammalian NF-κB family, Rel A (p65), Rel B, c-Rel, p50/p105, and p52/p100. The IκB family is regulated by (IκBα, IκBβ, IκBε, BCL-3, p100, and p105) NF-κB activity. The IκB protein masks the nuclear localization signal (NLS) of the NF-κB protein and keeps it in an inactive state in the nucleus. IκB kinase (IKK) promotes protein degradation by phosphorylating IκB; thus, NF-κB dimers from the nucleus regulate target gene expression (Lv et al. 2020; Miao et al. 2021). The effect of SH on NF-κB has been found, and SH treatment can inhibit the NF-κB signaling pathway in a rat model of temporal lobe epilepsy (Chen et al. 2020a). In this study, SH inhibited Pb-induced mRNA expression of NF-κB, p65, and IκB-α. Therefore, the abovementioned data suggest that SH can inhibit the NF-κB pathway to regulate Pb-induced expression of inflammatory factors in the liver. It has been reported that SH can inhibit the NF-κB signaling pathway by activating the Nrf2/HO-1 signaling pathway to relieve chondrocyte arthritis in mice (Wu et al. 2019). NF-κB plays a central role in SH on chronic Pb intoxication–induced liver inflammation in this study.
After NF-κB translocates into the nucleus, it induces the release of pro-inflammatory factors such as IL-1β and TNF-α (Annoscia et al. 2020). The inflammatory response plays an important role in Pb-induced chronic liver injury. TNF-α and IL-1β, two main pro-inflammatory factors, can aggravate liver diseases by participating in the production of secondary inflammatory mediators and inducing apoptosis (Manoj et al. 2017). Studies have shown a significant increase in liver IL-1β and TNF-α, which is associated with inflammatory cell infiltration and hepatocellular degeneration (Chen et al. 2019; Kebieche et al. 2009). In this study, the content of IL-1β and TNF-α in liver tissue and their mRNA expression levels were determined. We found that SH could inhibit chronic Pb poisoning–induced liver inflammation by regulating the levels of IL-1β and TNF-α in hepatocytes and their mRNA expression.
In addition, signals such as IL-1β, TNF-α, ROS, and damaged DNA can induce apoptosis, activate Bax protein, and thereby induce the activation of Caspase3, which ultimately leads to apoptosis. The bcl-2 family is divided into two categories: anti-apoptotic and pro-apoptotic proteins. Bcl-2 and bax are the most important apoptosis-inhibiting and pro-apoptotic proteins in the Bcl-2 family, respectively. Bax inactivates bax when it interacts with Bcl-2 (Dong et al. 2020; Li et al. 2015). Therefore, the ratio of Bax/Bcl-2 can also reflect the degree of apoptosis of cells. As an executor of apoptosis in cells, Caspase3 cleaves the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) after being activated, making it unable to perform its normal function, resulting in cell cleavage of DNA between nucleosomes and apoptosis death (Chi et al. 2017). A previous study showed that SH can regulate Caspase3 and the ratio of Bax/Bcl-2, attenuate liver apoptosis in mice, and protect against alcohol-induced acute liver injury (Fan et al. 2022). In this study, it was determined by immunohistochemistry that SH can treat hepatocyte apoptosis caused by chronic Pb poisoning by regulating the protein levels of Bax, Bcl-2, and Caspase3 in hepatocytes.
We found that SH (100 mg/kg bw) could exert antioxidant, anti-inflammatory, and anti-apoptotic effects in chronic Pb poisoning–induced liver injury, and SH could inhibit the liver damage induced by chronic Pb poisoning in mice by inhibiting the NF-κB signaling pathway, inducing liver injury progression. However, this experiment is only a preliminary study of the NF-κB signaling pathway mechanism of SH in the treatment of chronic Pb poisoning. Whether other inflammatory pathways are also involved in the treatment of SH is still unclear. This study provides theoretical support for SH in the treatment of liver injury caused by chronic Pb poisoning, and further experiments are needed to study the mechanism of SH action.
Conclusion
In conclusion, SH is a potential therapeutic substance for hepatic Pb poisoning which alleviates the liver damage caused by chronic Pb poisoning. According to the experimental results, it is speculated that the protective mechanism of SH toxicity may be to alleviate chronic Pb poisoning by reducing oxidative stress, inhibiting the NF-κB signaling pathway to enhance anti-inflammatory ability, and regulating the effects of cytokines Bax, Bcl-2, and Caspase3 to inhibit apoptosis-induced liver damage. Our study may provide some evidence for the protective mechanism of SH in Pb-induced hepatotoxicity injury.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
References
Almasmoum H, Refaat B, Ghaith MM, Almaimani RA, Idris S, Ahmad J, Abdelghany AH, Ba Salamah MA, El-Boshy M (2019) Protective effect of vitamin D3 against lead induced hepatotoxicity, oxidative stress, immunosuppressive and calcium homeostasis disorders in rat. Environ Toxicol Pharmacol 72(undefined):103246. https://doi.org/10.1016/j.etap.2019.103246
Annoscia D, Di Prisco G, Becchimanzi A, Caprio E, Frizzera D, Linguadoca A, Nazzi F, Pennacchio F (2020) Neonicotinoid clothianidin reduces honey bee immune response and contributes to Varroa mite proliferation. Nat Commun 11(1):5887. https://doi.org/10.1038/s41467-020-19715-8
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. https://doi.org/10.1152/physrev.00044.2005
Bian Y, Lei J, Zhong J, Wang B, Wan Y, Li J, Liao C, He Y, Liu Z, Ito K, Zhang B (2022) Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J Nutr Biochem 99:108840. https://doi.org/10.1016/j.jnutbio.2021.108840
Bjørklund G, Mutter J, Aaseth J (2017) Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol 91(12):3787–3797. https://doi.org/10.1007/s00204-017-2100-0
Chen C, Lin B, Qi S, He J, Zheng H (2019) Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley rats. Biol Trace Elem Res 191(2):426–434. https://doi.org/10.1007/s12011-019-1635-8
Chen D, Ning F, Zhang J, Tang Y, Teng X (2020a) NF-κB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic lymphocytes. Sci Total Environ 729(undefined):139017. https://doi.org/10.1016/j.scitotenv.2020.139017
Chen H, Wang Y, Jiao FZ, Yang F, Li X, Wang LW (2020b) Sinomenine attenuates acetaminophen-induced acute liver injury by decreasing oxidative stress and inflammatory response via regulating TGF-β/Smad pathway in vitro and in vivo. Drug Des Devel Ther 14(undefined):2393–2403. https://doi.org/10.2147/DDDT.S248823
Chi Q, Liu T, Sun Z, Tan S, Li S, Li S (2017) Involvement of mitochondrial pathway in environmental metal pollutant lead-induced apoptosis of chicken liver: perspectives from oxidative stress and energy metabolism. Environ Sci Pollut Res Int 24(36):28121–28131. https://doi.org/10.1007/s11356-017-0411-6
Dong N, Li X, Xue C, Zhang L, Wang C, Xu X, Shan A (2020) Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol 235(null):5525–5540. https://doi.org/10.1002/jcp.29452
Dou X, Ma Z, Yan D, Gao N, Li Z, Li Y, Feng X, Meng L, Shan A (2022) Sodium butyrate alleviates intestinal injury and microbial flora disturbance induced by lipopolysaccharides in rats. Food Funct 13(3):1360–1369. https://doi.org/10.1039/d1fo03183j
Duvvuri LS, Katiyar S, Kumar A, Khan W (2015) Delivery aspects of antioxidants in diabetes management. Expert Opin Drug Deliv 12(5):827–844. https://doi.org/10.1517/17425247.2015.992413
Fan H, Tu T, Zhang X, Yang Q, Liu G, Zhang T, Bao Y, Lu Y, Dong Z, Dong J, Zhao P (2022) Sinomenine attenuates alcohol-induced acute liver injury via inhibiting oxidative stress, inflammation and apoptosis in mice. Food Chem Toxicol 159(undefined):112759. https://doi.org/10.1016/j.fct.2021.112759
Fang Y, Zhu Y, Li L, Lai Z, Dong N, Shan A (2021) Biomaterial-interrelated bacterial sweeper: simplified self-assembled octapeptides with double-layered Trp zipper induces membrane destabilization and bacterial apoptosis-like death. Small Methods 5(12):e2101304. https://doi.org/10.1002/smtd.202101304
Kebieche M, Lakroun Z, Lahouel M, Bouayed J, Meraihi Z, Soulimani R (2009) Evaluation of epirubicin-induced acute oxidative stress toxicity in rat liver cells and mitochondria, and the prevention of toxicity through quercetin administration. Exp Toxicol Pathol 61(2):161–167. https://doi.org/10.1016/j.etp.2008.06.002
Li N, Liu X, Zhang P, Qiao M, Li H, Li X, Zhang H, Yu Z (2015) The effects of early life lead exposure on the expression of interleukin (IL) 1β, IL-6, and glial fibrillary acidic protein in the hippocampus of mouse pups. Hum Exp Toxicol 34(4):357–363. https://doi.org/10.1177/0960327114529451
Li D, Liang H, Li Y, Zhang J, Qiao L, Luo H (2021a) Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis via SIRT1/FOXO1 pathway in mice. Biol Trace Elem Res 199(1):237–243. https://doi.org/10.1007/s12011-020-02136-5
Li Y, Lv H, Xue C, Dong N, Bi C, Shan A (2021b) Plant polyphenols: potential antidotes for lead exposure. Biol Trace Elem Res 199(10):3960–3976. https://doi.org/10.1007/s12011-020-02498-w
Liu X, Song L (2022) Quercetin protects human liver cells from o,p'-DDT-induced toxicity by suppressing Nrf2 and NADPH oxidase-regulated ROS production. Food Chem Toxicol 161(undefined):112849. https://doi.org/10.1016/j.fct.2022.112849
Liu CM, Ma JQ, Sun YZ (2011) Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol 49(12):3119–3127. https://doi.org/10.1016/j.fct.2011.09.007
Liu Y, Sun Y, Zhou Y, Tang X, Wang K, Ren Y, He J (2020) Sinomenine hydrochloride inhibits the progression of plasma cell mastitis by regulating IL-6/JAK2/STAT3 pathway. Int Immunopharmacol 81(undefined):106025. https://doi.org/10.1016/j.intimp.2019.106025
Lv Y, Bing Q, Lv Z, Xue J, Li S, Han B, Yang Q, Wang X, Zhang Z (2020) Imidacloprid-induced liver fibrosis in quails via activation of the TGF-β1/Smad pathway. Sci Total Environ 705(undefined):135915. https://doi.org/10.1016/j.scitotenv.2019.135915
Manoj KV, Henley AK, Nelson CJ, Indumati O, Prabhakara RY, Rajanna S, Rajanna B (2017) Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ Sci Pollut Res Int 24(2):1544–1552. https://doi.org/10.1007/s11356-016-7923-3
Mazumdar I, Goswami K (2014) Chronic exposure to lead: a cause of oxidative stress and altered liver function in plastic industry workers in Kolkata, India. Indian J Clin Biochem 29(1):89–92. https://doi.org/10.1007/s12291-013-0337-9
Miao Z, Zhang K, Bao R, Li J, Tang Y, Teng X (2021) Th1/Th2 imbalance and heat shock protein mediated inflammatory damage triggered by manganese via activating NF-κB pathway in chicken nervous system in vivo and in vitro. Environ Sci Pollut Res Int 28(32):44361–44373. https://doi.org/10.1007/s11356-021-13782-0
Pandurangan M, Kim DH (2015) ZnO nanoparticles augment ALT, AST, ALP and LDH expressions in C2C12 cells. Saudi J Biol Sci 22(6):679–684. https://doi.org/10.1016/j.sjbs.2015.03.013
Ramazi S, Fahanik-Babaei J, Mohamadi-Zarch SM, Tashakori-Miyanroudi M, Nourabadi D, Nazari-Serenjeh M, Roghani M, Baluchnejadmojarad T (2020) Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: involvement of oxidative stress, inflammation and pyroptosis. J Chem Neuroanat 108(undefined):101800. https://doi.org/10.1016/j.jchemneu.2020.101800
Renu K, Chakraborty R, Myakala H, Koti R, Famurewa AC, Madhyastha H, Vellingiri B, George A, Gopalakrishnan AV (2021) Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium) - induced hepatotoxicity - a review. Chemosphere 271(undefined):129735. https://doi.org/10.1016/j.chemosphere.2021.129735
Singh N, Kumar A, Gupta VK, Sharma B (2018) Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem Res Toxicol 31(10):1009–1021. https://doi.org/10.1021/acs.chemrestox.8b00193
Sharma R, Kambhampati SP, Zhang Z et al (2020) Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury.[J] .J Control Release 323:361–375. https://doi.org/10.1016/j.jconrel.2020.04.036
Wang JB, Zhao HP, Zhao YL, Jin C, Liu DJ, Kong WJ, Fang F, Zhang L, Wang HJ, Xiao XH (2011) Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS One 6(9):e24498. https://doi.org/10.1371/journal.pone.0024498
Wu Y, Lin Z, Yan Z, Wang Z, Fu X, Yu K (2019) Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-κB signaling pathways. Int Immunopharmacol 75(undefined):105715. https://doi.org/10.1016/j.intimp.2019.105715
Xu M, Liu S, Wan R, Chen Y (2018) Combined treatment with sinomenine and acupuncture on collagen-induced arthritis through the NF-κB and MAPK signaling pathway. Oncol Lett 15(6):8770–8776. https://doi.org/10.3892/ol.2018.8394
Xue C, Lv H, Li Y, Dong N, Wang Y, Zhou J, Shi B, Shan A (2022) Oleanolic acid reshapes the gut microbiota and alters immune-related gene expression of intestinal epithelial cells. J Sci Food Agric 102(2):764–773. https://doi.org/10.1002/jsfa.11410
Yang H, Huang J, Gao Y, Wen Z, Peng L, Ci X (2020) Oridonin attenuates carrageenan-induced pleurisy via activation of the KEAP-1/Nrf2 pathway and inhibition of the TXNIP/NLRP3 and NF-κB pathway in mice. Inflammopharmacology 28(2):513–523. https://doi.org/10.1007/s10787-019-00644-y
Yin N, Xiong Y, Tao W, Chen J, Wang Z (2020) Sinomenine alleviates lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. Immunopharmacol Immunotoxicol 42(2):147–155. https://doi.org/10.1080/08923973.2020.1732407
Zhang Y, Jiang Q, Xie S, Wu X, Zhou J, Sun H (2019) Lead induced ototoxicity and neurotoxicity in adult guinea pig. Biomed Res Int 2019(undefined):3626032. https://doi.org/10.1155/2019/3626032
Zhou Y, Chen S, Gu W, Sun X, Wang L, Tang L (2021) Sinomenine hydrochloride ameliorates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota composition whilst suppressing the activation of the NLRP3 inflammasome. Exp Ther Med 22(5):1287. https://doi.org/10.3892/etm.2021.10722
Zou H, Sun J, Wu B, Yuan Y, Gu J, Bian J, Liu X, Liu Z (2020) Effects of cadmium and/or lead on autophagy and liver injury in rats. Biol Trace Elem Res 198(1):206–215. https://doi.org/10.1007/s12011-020-02045-7
Funding
This work was financially supported by the China Agriculture Research System of MOF and MARA.
Author information
Authors and Affiliations
Contributions
Y.L.: conceptualization, methodology, and writing of the original draft; W.C.: methodology and investigation; C.X.: methodology and investigation; R.C.: data curation and visualization; Z.A.: resources; N.D.: supervision, writing (including review and editing), and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. The care and management of animals were in compliance with the standards of the NEAU Animal Welfare Committee protocol (#NEAU-[2013]-9).
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Cai, W., Ai, Z. et al. Protective effects of sinomenine hydrochloride on lead-induced oxidative stress, inflammation, and apoptosis in mouse liver. Environ Sci Pollut Res 30, 7510–7521 (2023). https://doi.org/10.1007/s11356-022-22386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22386-1