Abstract
An increasing number of studies investigated the association between air pollution during pregnancy and the risk of eczema in offspring. However, no meta-analysis has confirmed the existence and size of their association to date. We systematically searched PubMed, Web of Science, Cochrane Library, and Embase databases to select the observational controlled studies published from the inception date to October 16, 2021. Quality evaluation was guided by the Newcastle–Ottawa Scale (NOS). Sensitivity analysis was applied to assess the impact of each included study on the combined effects, and publication bias was examined by Begg’s tests and Egger’s tests. A total of 12 articles involving 69,374 participants met our eligibility criteria. A significant association between the maternal exposure to NO2 (per 10 μg/m3 increased) and childhood eczema was observed, with a pooled risk estimate of 1.13 (95% CI: 1.06–1.19), but no association was observed between exposure to PM10, PM2.5, and SO2 and the risk of eczema in offspring. Besides, the effect of maternal NO2 exposure on childhood eczema was significant in the first and second trimesters, but not in the third trimester. There was notable variability in geographic location (p = 0.037) and air pollutant concentration (p = 0.031) based on meta-regression. Our findings indicated that prenatal exposure to NO2 was a risk factor for elevated risk of eczema in childhood, especially in the first and second trimesters. Further studies with larger sample sizes considering different constituents of air pollution and various exposure windows are needed to validate these associations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eczema, also termed as atopic dermatitis (AD), is a major health issue in childhood affecting a child’s life-quality, often associated with rhinitis, asthma, and other atopic disorders and is characterized by pruritic skin lesions including redness, cracking, scaling, and potential super-infection of the skin (Kanchongkittiphon et al. 2015; Nutten 2015). Eczema occurs most often in early childhood, which generally begins in infancy and can even emerge as early as 1–2 months after birth (Odhiambo et al. 2009). The World Allergy Organization reported that the childhood prevalence of eczema was approximately 10.7% and 15 ~ 20% of children in the USA and Europe, respectively (Drucker et al. 2017). Moreover, it has imposed a significant burden on the life quality of patients and their families. For example, it was conservatively estimated that the total annual cost of treating eczema in the USA was $5.297 billion (in 2015 USD) (Drucker et al. 2017). Risk factors for having eczema are known to include climate, urban versus rural setting, diet, breastfeeding, time of weaning, obesity, physical exercise, tobacco smoke, and ambient air pollution (Nutten 2015).
Data from the Global Burden of Disease Study indicated that ambient air pollution was responsible for 7.5% of deaths and ranked the sixth biggest attributable cause of disability-adjusted life years (DALYs) in 2016 (Bae and Kwon 2019). Numerous previous studies focused on investigating whether postnatal exposure to air pollutants increased the risk of eczema in children or exacerbated eczema symptoms (Brauer et al. 2002; Fuertes et al. 2020; Kim et al. 2016; Liu et al. 2016, 2020; Lu et al. 2021). Although it is well accepted that early life exposure factors are likely to be responsible for the recently rapid rising incidence rate of eczema in childhood, on the other hand, there is growing concern on the correlation between gestational exposure and childhood eczema. Furthermore, emerging evidence suggested that prenatal exposure to ambient air pollution could impact the intrauterine fetal developmental programming through a variety of mechanisms, such as immune dysfunction, oxidative stress, and DNA methylation (Grieger et al. 2016; Kingsley et al. 2016; Lockett et al. 2015).
To date, there have been no consistent conclusions to summarize the previous evidence on the relationship between maternal air pollution exposure and the risk of eczema in the offspring. Abundant researches have demonstrated that prenatal exposure to air pollution was positively associated with the risk of eczema (Deng et al. 2016, 2019; Lee et al. 2018; Liu et al. 2020; Lu et al. 2021), but a few studies have not found the significant association (Aguilera et al. 2013; Granum et al. 2020; Huang et al. 2015). Therefore, we conducted this meta-analysis to evaluate the potential effects of maternal exposure on childhood eczema. The specific goals were (a) to pool the effect estimates of maternal air pollution exposure on childhood eczema; (b) to explore sources of heterogeneity based on gestational windows of exposure, geographic location, sample size, age of disease onset, exposure assessment method, study quality scores, and air pollutant concentration; and (c) to identify the crucial air pollutants and susceptible gestational windows which can provide insights on effective prevention and interventions for childhood eczema.
Method and materials
The protocol of this meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guideline (Moher, et al. 2010). Also, we have registered and published the protocol for this study on the International Prospective Register of Systematic Reviews (PROSPERO, www.crd.york.ac.uk/PROSPERO, ID: CRD42021288028).
Search strategy and study selection
We comprehensively searched articles published before October 16, 2021, in the following electronic bibliographic databases without any limits on the date of first publication: PubMed, EMBASE, Cochrane Library, and Web of Science. Our search strategy is listed as follows.
-
1:
(air pollution) or (particulate matter) or (air pollutants) or (traffic pollution) or (outdoor pollution) or (carbon monoxide) or (sulphur dioxide) or (nitrogen dioxide) or (ozone) or (PM10) or (PM2.5).
-
2:
(eczema) or (ecz) or (atopic eczema) or (atopic dermatitis) or (AD) or (SASSAD) or (atopic).
-
3:
(children) or (childhood) or (child) or (pediatric) or (offspring).
-
4:
(prenatal) or (gestation) or (pregnancy) or (maternal).
#1 AND #2 AND #3 AND #4
Manual searches of the reference lists of included studies were scanned to identify more comprehensive studies. Potentially eligible studies were initially screened by title and abstract after removing duplicates, and further evaluated through the details of full texts by two authors independently (Dengyuan Yue and Jiaqing Mao). All discrepancies were resolved by discussing with the third author (Ting Shen).
Inclusion criteria and exclusion criteria
We identified eligible articles of this meta-analysis utilizing the following inclusion criteria: (1) Study design was observational, including cohort studies, cross-sectional studies, and case–control studies; (2) The outcome of interest was childhood eczema or atopic dermatitis with clear definition; (3) Maternal exposure to at least one pollutant (PM2.5, PM10, NO2, and SO2) was assessed; (4) Risk estimates of the association between pregnant exposure to air pollutants and risk of childhood eczema were reported, including relative risk (RR), odds ratio (OR), hazard ratio (HR), and their 95% confidence intervals (CI), or providing sufficient data to calculate them; (5) If study populations overlapped, the study with larger sample size was included.
The exclusion criteria were as follows: (1) Studies were reviews, conference papers, letters, experimental studies, or other non-epidemiological studies; (2) The exposure level during trimester-specific or entire pregnancy period was not reported, and the effects of specific pollutants were not related to ambient air pollution; (3) Duplicate studies retrieved from different databases.
Data extraction and quality assessment
Two investigators (Dengyuan Yue and Jiaqing Mao) extracted and recorded the following crucial information independently using a predefined data items template: study characteristics (first author, year of publication, country, data collection years, study location, study design, sample size), age of children at evaluation, types and concentrations of air pollutants, exposure window, exposure assessment method, variables adjusted and corresponding risk estimates with 95% CIs. When more than one risk estimates were provided in the same article, we ultimately extracted the risk estimates adjusted for the largest number of covariates. The quality of each study included was assessed by The Newcastle Ottawa Quality Scale (NOS), which was applied because most of the selected studies fall under cohort studies and case–control studies. This tool consists of 8 questions from three domains to evaluate the quality, namely selection of participants, comparability of cases and controls, and ascertainment of outcomes of interest. Scores ranged from 0 to 9 points, and the studies with a quality score of more than 7 points were considered high quality. Two investigators (Dengyuan Yue and Jiaqing Mao) independently gave one score to each item if the criteria were fulfilled. Any disagreement was resolved through joint discussion, and the third investigator (Ting Shen) was available to determine eligibility until a consensus was reached.
Statistical methods
All statistical analyses were conducted utilizing STATA version 14.0. For given data in each study, we sought to obtain the adjusted ORs, RRs, HRs and their 95% CIs for any prenatal exposure to the NO2, SO2, PM10, and PM2.5. Considering that highly correlated types of air pollutants simultaneously included in one model may cause multicollinearity and inverse regression results, we extracted the corresponding results from the single pollutant model (Wang et al. 2018). We regarded RR as OR approximately due to the relatively low incidence of eczema in children (Nelissen et al. 2011), and HR was essentially close to the RR on account of the timing of the development of child outcomes. Based on a previous analysis (Yan et al. 2020), the combination of the three effect values was appropriate to synthesize the effect estimates. Therefore, we directly equated the effect estimates (HRs and RRs) with ORs in our analysis. To compare the effects of air pollutants on childhood eczema across different studies, ORs (and 95% CIs) were scaled to 10 μg/m3 increments by taking the natural logarithm of the risk estimates (and confidence limits) and then standardized to 10 μg/m3 by dividing by the original risk increment and multiplying by 10 (Huangfu and Atkinson 2020), also harmonized the unit of air pollution from ppb to μg/m3 (Klepac et al. 2018). Standardized risks for each study were estimated using the specific formulas were listed as follows (Yang, et al. 2018).
(Conc1 is reported concentration of air pollutant in the original articles [µg/m3], Conc2 stands for the calculated concentration of the air pollutant [ppb], M is molecular weight of an air pollutant.)
We used random-effects models in the DerSimonian and Laird methods to pool the effect estimates and 95% CIs in our meta-analysis whatever the final degree of heterogeneity (DerSimonian and Laird 2015). I2 statistic and Cochran’s Q test were conducted as measures to assess heterogeneity. I2 values of < 25%, 25–50%, and > 50% usually corresponded to small, medium, and high heterogeneity, respectively, and engaged in subgroup analysis and meta-regression to further search potential mechanisms and sources of heterogeneity using study characteristic variables including geographic location (Asia versus Europe), sample size (≤ 2000 versus > 2000), age of disease onset (cut-off point: 3 years old), exposure assessment method, study quality scores (< 7 versus ≥ 7), and air pollutant concentration (low levels versus high levels). In addition, we used visual inspection of funnel plots and Egger’s test as well as Begg’s test in order to detect publication bias, and the p value < 0.05 was assumed as significant publication bias. Since the results of publication bias test and meta-regression analysis were not reliable among limited studies, we did not conduct related analysis when the number of included studies was less than five.
Results
Identification of studies
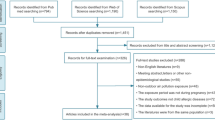
As shown in the PRISMA flow chart (Fig. 1), a total of 1497 studies were yielded through searching the databases. Following the removal of 84 duplicate studies, we excluded 1373 studies described irrelevant air pollutants or other non-eczema allergic diseases according to the titles and abstracts. After further reviewing the full-texts of the 40 records, among which 7 studies were meeting abstract, letters or other non-epidemiological studies, 8 studies used non-pregnant period for air pollution exposure, 8 studies had no access to full-texts or no available data for extraction and 5 studies enrolled the same cohort with other studies. Finally, 12 studies that conformed to the inclusion criteria were eligible for our quantitative synthesis of the current meta-analysis (Aguilera et al. 2013; Deng et al. 2019; Granum et al. 2020; Huang et al. 2015; Jedrychowski et al. 2011a, 2011b; Lee et al. 2018; Liu et al. 2016, 2020; Lu et al. 2021; Yang et al. 2020).
Study characteristics
The basic characteristics of these 12 individual studies are presented in Table 1. Among the studies, the effects of PM2.5, PM10, NO2, and SO2 exposure during pregnancy on childhood eczema were five, eight, nine, and four articles, respectively. There were 9 prospective birth cohort studies and 3 cross-sectional studies. Regarding research regions, the majority of articles were conducted in Asia, including 6 in China and 2 in Korea, and 4 articles from Europe. The sample size ranged from 322 to 39,782, and altogether, 69,374 participants were employed occurring during 2010–2018, among which ages of children with eczema ranged from 0 to 11 years old. In terms of the assessment methods of air pollutant concentrations during pregnancy, four studies used land use regression (LUR) model, and three for inverse distance weighted method (IDW), two for personal environmental monitoring sampler (PEMS), two for air-monitoring stations, and one for ordinary kriging (OK) model. Most studies collected information about the outcome based on the structured interviews (N = 6), and the remaining studies used self-reported questionnaires filled out by parents (N = 2) or obtained by clinical diagnosis (N = 4). Based on NOS scores, there were 11 studies scored 7 (Aguilera et al. 2013; Deng et al. 2016, 2019; Granum et al. 2020; Huang et al. 2015; Jedrychowski et al. 2011a, 2011b; Lee et al. 2018; Liu et al. 2020; Lu et al. 2021; Yang et al. 2020), which were considered as high-quality studies in total 12 studies and only one was considered to be moderate-quality study (Table S1).
The association between air pollutants and eczema
Prenatal PM 10 exposure and eczema risk
Eight studies reported the estimates for the relation of PM10 exposure (per 10 μg/m3 increase) to the risk of offspring eczema for the entire pregnancy period. The pooled effect estimates showed that PM10 exposure had a non-significant influence on childhood eczema (OR = 0.98, 95% CI: 0.90–1.07, I2 = 54.5%). Additionally, when the exposure period was taken into account, the pooled effect estimates of PM10 exposure were 1.03 (95% CI: 0.96–1.10), 1.00 (95% CI: 0.95–1.05), 1.00 (95% CI: 0.95–1.04) during the first, second, and third trimesters, respectively (Fig. 2). There was no indication of publication bias present based on the results from Begg’s test (p = 1.000) and Egger’s test (p = 0.589) for the association of PM10 with eczema (Supplementary material, Fig. S1). Meta-regression analysis detected no significant differences in geographic location (p = 0.764), sample size (p = 0.539), age of disease onset (p = 0.579), exposure assessment method (p = 0.733), study quality score (p = 0.207), and air pollutant concentration (p = 0.764). The effect values of the PM10 exposure on childhood eczema in above subgroups yielded the similar negative results (Table S2). One-study-removed analysis was used to adjust the bias, and the results altered after discarding Shuming Deng’s investigation (Deng et al. 2019), with the pooled risk estimate of 0.93 (95% CI: 0.88–0.99) (Supplementary material, Fig. S2), indicating that the results may be unstable.
Prenatal PM 2.5 exposure and eczema risk
Four studies reported the association between prenatal PM2.5 exposure and eczema risk, and results showed a non-significant effect of 1.14 (95% CI = 0.89–1.45, I2 = 46.0%) (Fig. 3). We found that the associations of prenatal PM2.5 exposure in the respective three trimesters and childhood eczema were also not significant when stratified by exposure to gestational windows (Fig. 3). Subgroup analysis by location and exposure assessment method found a statistically significant link between prenatal PM2.5 exposure and childhood eczema among the studies conducted in Asia (OR = 1.62, 95% CI: 1.12–2.34) and using the IDW exposure assessment method (OR = 1.62, 95% CI: 1.12–2.34) (Table S3), with no significant association observed in any of the other subgroups. The one-study-removed analysis indicated that exclusion of each study did not significantly shift the results (Supplementary material, Fig. S3).
Prenatal NO 2 exposure and eczema risk
The pooled effect estimates from nine studies revealed that with per 10 μg/m3 increase of maternal exposure to NO2 during the entire pregnancy, the risk of eczema would rise by 13% (OR = 1.13, 95% CI: 1.06–1.19, I2 = 41.3%) (Fig. 4). In five of the nine publications, risk estimates for each trimester were provided. Of these, we detected that the most hitting positive effect occurring in the first trimester (OR = 1.10, 95% CI: 1.01–1.21), followed by a significant correlation between prenatal NO2 exposure and childhood eczema in the second trimester (OR = 1.09, 95% CI: 1.01–1.18), while there was no significant correlation observed in the third trimester (OR = 1.04, 95% CI: 0.97–1.11) (Fig. 4). The funnel plot exhibited an asymmetric distribution, and the p-values of Begg’s test and Egger’s test were 0.348 and 0.238, respectively (Supplementary material, Fig. S4). The subgroup analysis for the impact of prenatal exposure to NO2 on childhood eczema, heterogeneity might be induced by variances in geographic location (p = 0.037) and air-pollutant concentrations (p = 0.031) according to the results of meta-regression (Table 2). The subgroup analysis of geographic location exhibited a positive significant correlation across Asian studies (OR = 1.15, 95% CI: 1.09–1.21), while non-significant effect in Europe (OR = 1.00, 95% CI: 0.91–1.09). The effect was more considerable in the regions with higher average exposure concentrations (OR = 1.18, 95% CI: 1.09–1.28) in comparison to lower exposure level (OR = 1.11, 95% CI: 1.03–0.19) (Table 2). Furthermore, we also conducted subgroup analysis and meta-regression analysis on the association between prenatal exposure to NO2 and childhood eczema during the first and second trimesters, aiming to find out evidence to explain the high heterogeneity. Heterogeneity might be induced by geographic location (p = 0.046) for the analysis of first trimester in meta-regression (Table S4). However, there was no substantial heterogeneity between strata in the subgroup analysis for second trimester (Table S5). The one-study-removed analysis showed that excluding all studies did not significantly alter the results (Supplementary material, Fig. S5).
Prenatal SO 2 exposure and eczema risk
There were four studies investigating the relation between prenatal SO2 exposure and childhood eczema, and the pooled analysis showed a positive non-significant effect of SO2 in the entire trimester (OR = 1.03, 95% CI: 0.98–1.07, I2 = 0.0%) (Fig. 5). In the subgroup analysis of SO2 exposure, there were no statistically significant effect values on the risk of eczema in children in any subgroup and no significant differences in the heterogeneity between strata (Table S6). The exclusion of each study at a time did not significantly alter the results of the analysis, indicating that no individual study significantly affected the pooled results (Supplementary material, Fig. S6).
Discussion
To the best of our knowledge, this is the unprecedented systematic review and meta-analysis to focus on the mother–offspring relationship to inquire into the effects of prenatal air pollution exposure on the incidence of eczema in early-life children. We conducted a synthesis of the evidence from 12 studies and demonstrated that maternal exposure to NO2 was related to a 13% increased risk of eczema. Moreover, we found that exposure to NO2 during the first and second trimesters was slightly more correlated with childhood eczema, rather than the third trimester, whereas the association of other air pollutants (PM10, PM2.5, and SO2) with the risk of childhood eczema was not statistically significant throughout pregnancy or at any stage of pregnancy.
In our current study, we discerned that maternal NO2 exposure was associated with an increased risk of eczema, which was concordant with a majority of preceding parallel individual studies. Furthermore, this result was consistent in the subgroup with a larger sample size (OR = 1.13, 95% CI: 1.06–1.20) and higher study quality (OR = 1.10, 95% CI: 1.01–1.19), which implied the stability and reliability of this result. Our study also found slightly stronger effect of NO2 exposure on eczema in the first (OR = 1.10, 95% CI: 1.01–1.21) and second (OR = 1.09, 95% CI: 1.01–1.18) trimesters of pregnancy in contrast with the third trimester (OR = 1.04, 95% CI: 0.97–1.11). This agreed with the analysis by Lu et al. based on local data from Chongqing city, reporting that prenatal NO2 exposure in the first and second trimesters was associated with lifelong eczema (Lu et al. 2021), whereas Liu et al. discovered that exposure to NO2 in the later trimesters (OR = 1.68, 95% CI: 1.19,2.37) was more strongly associated with eczema than in the early trimesters (OR = 1.59, 95% CI: 1.16, 2.18), which was inconsistent with our findings (Liu et al. 2020). Besides, we yielded the pooled effects of PM2.5 (1.14, 95% CI: 0.89–1.45) and PM10 (0.98, 95% CI: 0.90–1.07) for the entire pregnancy, respectively, which did not provide sufficient evidence to support that exposure to particulate matter was significantly associated with an increased risk of eczema. A study from Shanghai, China, also found no correlation between exposure to environmental PM10 during pregnancy and childhood eczema (Liu et al. 2016). Yet, another study conducted in Wuhan, China, found that PM2.5 and PM10 exposure during pregnancy was significantly associated with a positive increase in the risk of childhood eczema, and this inconsistency may be due to the high levels of particulate matter pollution in this study (Deng et al. 2019). As for SO2, neither the pooled effect estimates nor the risk estimates from the subgroup analysis, non-significant association between SO2 exposure and the risk of eczema in children were found. Prior studies have also put forward little evidence of association between exposure to SO2 and eczema risk (Deng et al. 2016; Huang et al. 2015; Liu et al. 2020; Wang et al. 2018), which may mirror that SO2 is less likely than other pollutants to increase the risk of eczema, but additional studies are warranted. Although our results did not confirm a positive correlation between air pollutants other than NO2 and eczema, this discrepancy may be partially owing to various period-averaged concentrations of the pollutants studied or lagged effects of air pollutants on the incidence and prevalence of eczema.
The exact mechanism whereby in utero exposure to ambient air pollution might increase the risk of postnatal eczema is not known, but it has been hypothesized that the embryo in utero is highly sensitive to the external environment for which it is particularly susceptible to various toxic substances. As a marker of traffic-derived combustion pollutants (Ezratty et al. 2014), exposure to NO2 during pregnancy was found to be positively associated with the risk of eczema in the offspring, consistently with the previous studies (Deng et al. 2016, 2019; Liu et al. 2020; Lu et al. 2021). There were several potential explanations for the process of physiopathology (Fig. 6). First, maternal NO2 exposure significantly increases the intensity of allergic sensitization and the risk of allergic sensitization manifestations in the postnatal offspring, including inflammatory cell infiltration and T helper 2 (Th2) polarization (Muehling et al. 2017; Romagnani 1994). In a pilot mouse toxicology study, it was found that elevated maternal NO2 levels may induce the polarization of naive CD4+ T cells towards Th2 cells (Yue et al. 2017), resulting in substantially higher interleukin 4 (IL-4) and IL-13 levels and lower interferon-γ (IFN-γ) expression in the offspring. Second, epigenetic regulation presents an alternative mechanistic interpretation. As demonstrated in an epigenome-wide meta-analysis (Gruzieva et al. 2017), the differences in epigenomic DNA methylation of several mitochondria-related genes may be attributed to prenatal exposure to NO2. DNA methylation in the promoter regions of the IL-4 and IL-13 genes may activate the allergic phenotype of Th2 (Bégin and Nadeau 2014). Third, oxidative stress caused by NO2 pollution leads to an imbalance between oxidants and antioxidants, and reactive oxygen species and reactive nitrogen species can also reinforce the polarization of Th2 and thus damage the skin barrier (Ahn 2014). It is worth noting that as fetal skin structures develop rapidly during the first trimester (Huang et al. 2015), air pollution is more likely to contribute to fetal immune dysregulation or skin barrier dysfunction in this period, and this fact is consistent with the findings of our study. Future studies are still necessary to elucidate how air pollutants induce epigenetic changes, what level of exposure is required to induce pathophysiological changes, and whether other genes whose expression is altered by methylation (Ahn 2014). In addition, several studies implied that PM-induced oxidative stress could lead to epigenetic changes in DNA repair genes, and thus interfere and affect the development of fetal skin structures (Bowatte et al. 2015; Jedrychowski et al. 2011a; Kannan et al. 2006; Lee et al. 2020; Lu et al. 2021).
As for heterogeneity, PM10 exposure in the entire pregnancy was the greatest among the studies and that for the exposure to PM2.5 and NO2 displayed moderate heterogeneity. First of all, heterogeneity may be partially explained by the regions with different levels of economy and industrialization, and the correspondingly varying air pollution concentrations across the studies. After stratifying by geographic location, we found that maternal exposure to NO2 increased 15% offspring eczema risk in Asian countries, whereas such significant association was not found in Europe. The urbanization and economic growth in many Asian countries are widely known to have increased industrial activities and vehicle emissions, and has contributed to a surge in air pollution compared to developed countries (Chen et al. 2018), which can explain our findings. For instance, only East Asia had a population-weighted increase of ambient NO2 concentrations compared to North America, Western Europe, and the Asia–Pacific region, which tripled at the 50th percentile between 1996 and 2012 (from 1.0 ppb to 2.9 ppb) (Geddes et al. 2016). WHO reported that less-developed regions such as Asia and Africa suffer 4–5 times greater PM2.5 exposure than more-developed regions (Stahl et al. 2019). We also detected that heterogeneity was declined in both the European (I2 = 8.0%) and Asian (I2 = 0.0%) subgroups of the NO2 group compared to the overall heterogeneity, and similar results were presented in the PM2.5 group, implying that region may be a factor contributing to heterogeneity. But since there was no situation where subgroups of all air pollutants were lower heterogeneity, we cannot determine the source. On the basis, we performed meta-regression and detected that regional (p = 0.046) as well as air pollutant concentration (p = 0.031) factors had an impact on heterogeneity in the NO2 group where a sufficient amount of literature included. It is therefore reasonable to assume that regional factors of different levels of economic and industrial development and corresponding air pollution concentrations are sources of heterogeneity.
In addition, we also looked for other characteristics that may cause heterogeneity by reviewing the literature. The heterogeneity may arise from the differences in the age proportion among the included children. It is reported that age has a strong effect on the incidence rate of eczema, with the highest incidence in infancy and 85% of children are affected before the age of 5 (Ban et al. 2018), while the incidence decreases as children get older. Lower heterogeneity was observed after subgroup analysis based on age at onset than in the overall estimates of heterogeneity in PM10 exposure. Furthermore, different types of air pollution models can be applied to epidemiological studies, resulting in variation across studies. Subgroup analysis by measures of NO2 exposure levels indicated reduced heterogeneity in the air monitoring station group (I2 = 0.0%), the IDW group (I2 = 0.0%), and the LUR group (I2 = 0.1%) compared to the heterogeneity of the overall result (I2 = 41.3%) in our study. Finally, inadequate or inconsistent adjustments for confounding factors across studies may be a source of heterogeneity. Most included studies adjusted the confounders of socioeconomic status and maternal age; however, maternal tobacco exposure was not taken into account in several studies (Aguilera et al. 2013; Granum et al. 2020; Jedrychowski et al. 2011a, 2011b; Lu et al. 2021). Given that maternal tobacco exposure during pregnancy is more likely to carry and pass on allergy risk alleles to their offspring than those who are not exposed (Ducci et al. 2011), the offspring of tobacco-exposed mothers would be at higher risk for eczema. The above potential sources of heterogeneity that might weaken the robustness of the results were not sufficiently evident in the subgroup analyses and meta-regressions, such that I2 in some subgroups still exceeded 50%, so we cannot consider these as exact sources of heterogeneity.
A major strength of our study was the high-quality birth cohort studies accounted for the vast majority of our meta-analysis, which enhanced the reliability of the results in comparison with case–control and cross-sectional studies. According to the NOS risk assessment, eleven studies were at low risk and only one study was at moderate risk of bias. Overall, risk of bias of these studies was low or probably low, was of great significance to support adequate quality. At the same time, it was plausible that the study had several limitations. First, considering the high interaction between prenatal and postnatal exposure, we failed to conclude whether exposure during one or both periods influenced the development of eczema, especially for children who were at an older age of onset. This may lead to false positive effects of prenatal air pollution on eczema risk in early childhood. Second, the included studies were only implemented in Asia and Europe, thus the findings may provide limited information when extrapolated to populations in other regions. Third, focusing on the relationship of single air pollutant and eczema regardless of the interactions between multiple pollutants may bias the risk estimates on account of the inherent limitations of the studies included. Since people in the urban atmosphere are not exposed to a single pollutant but a complex combination of various pollutants at different times and seasons (Han et al. 2018). Multiple pollutants models were expected to provide a more realistic appreciation of benefits and risks of estimates than single pollutant models. Fourth, the reports of eczema onset in children enrolled were derived from parental or physician diagnosis, so the results were inevitably affected by recall bias. In addition, some air pollutants and subgroups included relatively small number of studies and may cause marginal significance of eczema risk, it is still warranted to be considered whether prenatal PM10, PM2.5, and SO2 exposure is associated with the risk of childhood eczema and more epidemiological evidence is needed to confirm the association.
Conclusions
In summary, our results suggested that exposure to NO2 during pregnancy was a risk factor for childhood eczema. Additionally, we uncovered that the susceptible gestational window may originate in the first and second trimesters. It was beneficial to implement corresponding measures to minimize maternal exposure to traffic air pollutants represented by NO2, such as reducing outdoor activities or wearing masks when the concentration of air pollutants of air pollution is high. Albeit it was not observed a significant association regardless of PM10, PM2.5, and SO2, we cannot rule out the limitations and should consider generating hypothesis rather than conclusive. Future studies are warranted to explore the correlation between different components of air pollutants during pregnancy and childhood eczema, and experiments studies are also encouraged to investigate the underlying mechanisms involved in the association between exposure to air pollution during specific trimester in utero and childhood eczema.
Data availability
The datasets used or analyzed during the current research are available from the corresponding author on request.
References
Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M, Esplugues A, Fernández-Somoano A, Lertxundi A, Tardón A, Sunyer J (2013) Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect 121:387–392
Ahn K (2014) The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 134(993–999):1000
Bae S, Kwon HJ (2019) Current state of research on the risk of morbidity and mortality associated with air pollution in Korea. YONSEI MED J 60:243–256
Ban L, Langan SM, Abuabara K, Thomas KS, Abdul SA, Sach T, McManus E, Santer M, Ratib S (2018) Incidence and sociodemographic characteristics of eczema diagnosis in children: a cohort study. J Allergy Clin Immunol 141:1927–1929
Bégin P, Nadeau KC (2014) Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin Immunol 10:27
Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC (2015) The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 70:245–256
Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, Koopman LP, Neijens HJ, Gerritsen J, Kerkhof M, Heinrich J, Bellander T, Brunekreef B (2002) Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 166:1092–1098
Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, Deng Q, Huang C, Hu Y, Zou Z, Liu W, Wang J, Lu C, Qian H, Yang X, Zhang X, Qu F, Sundell J, Zhang Y, Li B, Sun Y, Zhao Z (2018) The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. ENVIRON POLLUT 232:329–337
Deng Q, Lu C, Li Y, Sundell J, Dan N (2016) Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. ENVIRON RES 150:119–127
Deng S, Huang D, Wang W, Yan H, Li S, Xiang H (2019) Associations of gestational and the first year of life exposure to ambient air pollution with childhood eczema in Hubei, China. Environ Sci Pollut Res Int 26:23842–23849
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. CONTEMP CLIN TRIALS 45:139–145
Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA (2017) The burden of atopic dermatitis: summary of a report for the National Eczema Association. J INVEST DERMATOL 137:26–30
Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, Isohanni M, Charoen P, Coin L, Hoggart C, Ekelund J, Peltonen L, Freimer N, Elliott P, Schumann G, Järvelin MR (2011) TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry 69:650–660
Ezratty V, Guillossou G, Neukirch C, Dehoux M, Koscielny S, Bonay M, Cabanes PA, Samet JM, Mure P, Ropert L, Tokarek S, Lambrozo J, Aubier M (2014) Repeated nitrogen dioxide exposures and eosinophilic airway inflammation in asthmatics: a randomized crossover study. Environ Health Perspect 122:850–855
Fuertes E, Sunyer J, Gehring U, Porta D, Forastiere F, Cesaroni G, Vrijheid M, Guxens M, Annesi-Maesano I, Slama R, Maier D, Kogevinas M, Bousquet J, Chatzi L, Lertxundi A, Basterrechea M, Esplugues A, Ferrero A, Wright J, Mason D, McEachan R, Garcia-Aymerich J, Jacquemin B (2020) Associations between air pollution and pediatric eczema, rhinoconjunctivitis and asthma: a meta-analysis of European birth cohorts. ENVIRON INT 136:105474
Geddes JA, Martin RV, Boys BL, van Donkelaar A (2016) Long-term trends worldwide in ambient NO2 concentrations inferred from satellite observations. Environ Health Perspect 124:281–289
Granum B, Oftedal B, Agier L, Siroux V, Bird P, Casas M, Warembourg C, Wright J, Chatzi L, de Castro M, Donaire D, Grazuleviciene R, Småstuen HL, Maitre L, Robinson O, Tamayo-Uria I, Urquiza J, Nieuwenhuijsen M, Slama R, Thomsen C, Vrijheid M (2020) Multiple environmental exposures in early-life and allergy-related outcomes in childhood. ENVIRON INT 144:106038
Grieger JA, Clifton VL, Tuck AR, Wooldridge AL, Robertson SA, Gatford KL (2016) In utero programming of allergic susceptibility. Int Arch Allergy Immunol 169:80–92
Gruzieva O, Xu CJ, Breton CV, Annesi-Maesano I, Antó JM, Auffray C, Ballereau S, Bellander T, Bousquet J, Bustamante M, Charles MA, de Kluizenaar Y, den Dekker HT, Duijts L, Felix JF, Gehring U, Guxens M, Jaddoe VV, Jankipersadsing SA, Merid SK, Kere J, Kumar A, Lemonnier N, Lepeule J, Nystad W, Page CM, Panasevich S, Postma D, Slama R, Sunyer J, Söderhäll C, Yao J, London SJ, Pershagen G, Koppelman GH, Melén E (2017) Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect 125:104–110
Han L, Zhou W, Pickett ST, Li W, Qian Y (2018) Multicontaminant air pollution in Chinese cities. Bull World Health Organ 96:233–242
Huang CC, Wen HJ, Chen PC, Chiang TL, Lin SJ, Guo YL (2015) Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol 173:981–988
Huangfu P, Atkinson R (2020) Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. ENVIRON INT 144:105998
Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Miller RL, Flak E, Mroz E, Jacek R, Spengler JD (2011) Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol 155:275–281
Jedrychowski W, Spengler JD, Maugeri U, Miller RL, Budzyn-Mrozek D, Perzanowski M, Flak E, Mroz E, Majewska R, Kaim I, Perera F (2011) Effect of prenatal exposure to fine particulate matter and intake of paracetamol (acetaminophen) in pregnancy on eczema occurrence in early childhood. SCI TOTAL ENVIRON 409:5205–5209
Kanchongkittiphon W, Gaffin JM, Phipatanakul W (2015) Child with atopic dermatitis. Ann Allergy Asthma Immunol 114:6–11
Kannan S, Misra DP, Dvonch JT, Krishnakumar A (2006) Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 114:1636–1642
Kim HH, Lee CS, Yu SD, Lee JS, Chang JY, Jeon JM, Son HR, Park CJ, Shin DC, Lim YW (2016) Near-road exposure and impact of air pollution on allergic diseases in elementary school children: a cross-sectional study. YONSEI MED J 57:698–713
Kingsley SL, Eliot MN, Whitsel EA, Huang YT, Kelsey KT, Marsit CJ, Wellenius GA (2016) Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. ENVIRON INT 92–93:43–49
Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A (2018) Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. ENVIRON RES 167:144–159
Lee E, Lee SY, Kim HC, Choi KY, Kim HB, Park MJ, Rhee ES, Yoon JS, Cho HJ, Jung S, Ahn K, Kim KW, Sheen YH, Suh DI, Hong SJ (2020) Prenatal particulate matter exposure with skin barrier dysfunction affects offspring’s atopic dermatitis: COCOA study. J Allergy Clin Immunol Pract 8:2062–2065
Lee JY, Lamichhane DK, Lee M, Ye S, Kwon JH, Park MS, Kim HC, Leem JH, Hong YC, Kim Y, Ha M, Ha E (2018) Preventive effect of residential green space on infantile atopic dermatitis associated with prenatal air pollution exposure. Int J Environ Res Public Health 15(1):102
Liu W, Cai J, Huang C, Hu Y, Fu Q, Zou Z, Sun C, Shen L, Wang X, Pan J, Huang Y, Chang J, Zhao Z, Sun Y, Sundell J (2016) Associations of gestational and early life exposures to ambient air pollution with childhood atopic eczema in Shanghai, China. SCI TOTAL ENVIRON 572:34–42
Liu W, Huang C, Cai J, Fu Q, Zou Z, Sun C, Zhang J (2020) Prenatal and postnatal exposures to ambient air pollutants associated with allergies and airway diseases in childhood: a retrospective observational study. ENVIRON INT 142:105853
Lockett GA, Huoman J, Holloway JW (2015) Does allergy begin in utero? Pediatr Allergy Immunol 26:394–402
Lu C, Norbäck D, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Qian H, Sun Y, Sundell J, Wang J, Liu W, Deng Q (2021) Onset and remission of eczema at pre-school age in relation to prenatal and postnatal air pollution and home environment across China. SCI TOTAL ENVIRON 755:142467
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. INT J SURG 8:336–341
Muehling LM, Lawrence MG, Woodfolk JA (2017) Pathogenic CD4+ T cells in patients with asthma. J Allergy Clin Immunol 140:1523–1540
Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL (2011) Epigenetics and the placenta. HUM REPROD UPDATE 17:397–417
Nutten S (2015) Atopic dermatitis: global epidemiology and risk factors. ANN NUTR METAB 66(Suppl 1):8–16
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI (2009) Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 124:1251–1258
Romagnani S (1994) Regulation of the development of type 2 T-helper cells in allergy. CURR OPIN IMMUNOL 6:838–846
Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, Mattheisen M, Wang Y, Coleman J, Gaspar HA, de Leeuw CA, Steinberg S, Pavlides J, Trzaskowski M, Byrne EM, Pers TH, Holmans PA, Richards AL, Abbott L, Agerbo E, Akil H, Albani D, Alliey-Rodriguez N, Als TD, Anjorin A, Antilla V, Awasthi S, Badner JA, Bækvad-Hansen M, Barchas JD, Bass N, Bauer M, Belliveau R, Bergen SE, Pedersen CB, Bøen E, Boks MP, Boocock J, Budde M, Bunney W, Burmeister M, Bybjerg-Grauholm J, Byerley W, Casas M, Cerrato F, Cervantes P, Chambert K, Charney AW, Chen D, Churchhouse C, Clarke TK, Coryell W, Craig DW, Cruceanu C, Curtis D, Czerski PM, Dale AM, de Jong S, Degenhardt F, Del-Favero J, DePaulo JR, Djurovic S, Dobbyn AL, Dumont A, Elvsåshagen T, Escott-Price V, Fan CC, Fischer SB, Flickinger M, Foroud TM, Forty L, Frank J, Fraser C, Freimer NB, Frisén L, Gade K, Gage D, Garnham J, Giambartolomei C, Pedersen MG, Goldstein J, Gordon SD, Gordon-Smith K, Green EK, Green MJ, Greenwood TA, Grove J, Guan W, Guzman-Parra J, Hamshere ML, Hautzinger M, Heilbronner U, Herms S, Hipolito M, Hoffmann P, Holland D, Huckins L, Jamain S, Johnson JS, Juréus A, Kandaswamy R, Karlsson R, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koller AC, Kupka R, Lavebratt C, Lawrence J, Lawson WB, Leber M, Lee PH, Levy SE, Li JZ, Liu C, Lucae S, Maaser A, MacIntyre DJ, Mahon PB, Maier W, Martinsson L, McCarroll S, McGuffin P, McInnis MG, McKay JD, Medeiros H, Medland SE, Meng F, Milani L, Montgomery GW, Morris DW, Mühleisen TW, Mullins N, Nguyen H, Nievergelt CM, Adolfsson AN, Nwulia EA, O’Donovan C, Loohuis L, Ori A, Oruc L, Ösby U, Perlis RH, Perry A, Pfennig A, Potash JB, Purcell SM, Regeer EJ, Reif A, Reinbold CS, Rice JP, Rivas F, Rivera M, Roussos P, Ruderfer DM, Ryu E, Sánchez-Mora C, Schatzberg AF, Scheftner WA, Schork NJ, Shannon WC, Shehktman T, Shilling PD, Sigurdsson E, Slaney C, Smeland OB, Sobell JL, Søholm HC, Spijker AT, St CD, Steffens M, Strauss JS, Streit F, Strohmaier J, Szelinger S, Thompson RC, Thorgeirsson TE, Treutlein J, Vedder H, Wang W, Watson SJ, Weickert TW, Witt SH, Xi S, Xu W, Young AH, Zandi P, Zhang P, Zöllner S, Adolfsson R, Agartz I, Alda M, Backlund L, Baune BT, Bellivier F, Berrettini WH, Biernacka JM, Blackwood D, Boehnke M, Børglum AD, Corvin A, Craddock N, Daly MJ, Dannlowski U, Esko T, Etain B, Frye M, Fullerton JM, Gershon ES, Gill M, Goes F, Grigoroiu-Serbanescu M, Hauser J, Hougaard DM, Hultman CM, Jones I, Jones LA, Kahn RS, Kirov G, Landén M, Leboyer M, Lewis CM, Li QS, Lissowska J, Martin NG, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Metspalu A, Mitchell PB, Morken G, Mors O, Mortensen PB, Müller-Myhsok B, Myers RM, Neale BM, Nimgaonkar V, Nordentoft M, Nöthen MM, O’Donovan MC, Oedegaard KJ, Owen MJ, Paciga SA, Pato C, Pato MT, Posthuma D, Ramos-Quiroga JA, Ribasés M, Rietschel M, Rouleau GA, Schalling M, Schofield PR, Schulze TG, Serretti A, Smoller JW, Stefansson H, Stefansson K, Stordal E, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Werge T, Nurnberger JI, Wray NR, Di Florio A, Edenberg HJ, Cichon S, Ophoff RA, Scott LJ, Andreassen OA, Kelsoe J, Sklar P (2019) Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet 51:793–803
Wang Q, Zhang H, Liang Q, Knibbs LD, Ren M, Li C, Bao J, Wang S, He Y, Zhu L, Wang X, Zhao Q, Huang C (2018) Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environ Pollut 237:18–27
Yan W, Wang X, Dong T, Sun M, Zhang M, Fang K, Chen Y, Chen R, Sun Z, Xia Y (2020) The impact of prenatal exposure to PM2.5 on childhood asthma and wheezing: a meta-analysis of observational studies. Environ Sci Pollut Res Int 27:29280–29290
Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, Dong GH (2018) Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. ENVIRON POLLUT 235:576–588
Yang SI, Lee SH, Lee SY, Kim HC, Kim HB, Kim JH, Lim H, Park MJ, Cho HJ, Yoon J, Jung S, Yang HJ, Ahn K, Kim KW, Shin YH, Suh DI, Won HS, Lee MY, Kim SH, Choi SJ, Kwon JY, Jun JK, Hong SJ (2020) Prenatal PM2.5 exposure and vitamin D-associated early persistent atopic dermatitis via placental methylation. Ann Allergy Asthma Immunol 125:665–673
Yue H, Yan W, Ji X, Gao R, Ma J, Rao Z, Li G, Sang N (2017) Maternal exposure of BALB/c mice to indoor NO2 and allergic asthma syndrome in offspring at adulthood with evaluation of DNA methylation associated Th2 polarization. Environ Health Perspect 125:97011
Acknowledgements
The authors of this study would like to thank all authors of the studies included in this paper for their efforts to provide information on the relevant results.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81973663, 22106143); the Natural Science Foundation of Zhejiang Province (LQ20H260008, LQ20B07005).
Author information
Authors and Affiliations
Contributions
Dengyuan Yue conceived the study. Dengyuan Yue and Jiaqing Mao searched the databases and checked them according to the eligible criteria and exclusion criteria. Ting Shen helped develop search strategies. Dengyuan Yue, Ting Shen, and Qing Su analyzed the data and wrote the draft of the paper. Ding Ye contributed to reviewing or revising the paper. Ding Ye, Xiaoqing Ye and Yingying Mao are the guarantors of this work. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Supplementary information.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yue, D., Shen, T., Mao, J. et al. Prenatal exposure to air pollution and the risk of eczema in childhood: a systematic review and meta-analysis. Environ Sci Pollut Res 29, 48233–48249 (2022). https://doi.org/10.1007/s11356-022-20844-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20844-4