Abstract
With the accelerated pace of economic development and modernization, air pollution has become one of the most focused public health problems. However, the impact of particulate matter exposure during pregnancy on childhood asthma and wheezing remains controversial. We performed this meta-analysis to explore the relationship between prenatal exposure to PM2.5 and childhood asthma and wheezing. Candidate papers were searched on PubMed, Web of Science, Embase, and Cochrane Library before July 15, 2019. The main characteristics of the included studies were extracted, and the quality was evaluated by the Newcastle–Ottawa Scale (NOS). A sensitivity analysis was performed to assess the impact of individual studies on the combined effects. The Egger and Begg tests were conducted to examine the publication bias. Nine studies were included in the final analysis. Prenatal exposure to PM2.5 significantly increased the risk of childhood asthma and wheezing (OR = 1.06, 95% CI 1.02–1.11; per 5 μg/m3). Maternal exposure was more strongly related to childhood asthma and wheezing before age 3 (OR = 1.15, 95% CI 1.00–1.31; per 5 μg/m3) than after (OR = 1.04, 95% CI 1.00–1.09; per 5 μg/m3). Children in developed countries showed more severe effects (OR = 1.14, 95% CI 1.02–1.27; per 5 μg/m3). Children who were born to mothers with higher levels of prenatal exposure were at higher risk of asthma and wheezing (OR = 1.07, 95% CI 1.02–1.13; per 5 μg/m3). This meta-analysis indicated that the impact of PM2.5 on childhood asthma and wheezing begins as early as utero, so regulating pollutant emission standards and strengthening prenatal protection are crucial to maternal and child health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the accelerated pace of economic and urban development, air pollution had an increasingly adverse impact on public health all over the world (Kelly and Fussell 2015; Wang et al. 2016; Xie et al. 2016). Among air pollutants, ambient particulate matter (PM) has aroused increased concern, and the primary sources that threaten human health include construction work, road transport, fossil fuels, and tobacco consumption (Yang 2019). It consists of tiny solid or liquid particles suspended in the air with many different chemical components and physical properties (Kelly and Fussell 2012; Ngoc et al. 2017). PM2.5 is also known as fine inhalable particulate matter, which has a small size and large surface area, is light-weight, and has strong reactivity, as well as containing acids, organic chemicals, metals, biological materials, and allergens (de Kok et al. 2006; Guarnieri and Balmes 2014). Compared with coarse particulate matter, inhalable particulate matter easily attaches to poisonous and harmful substances like heavy metals and microorganism, and it often travels for long time and distances in the atmosphere, enters the alveoli via respiration, stimulates the alveolar walls and impairs lung function, and even penetrates the blood vessels and enters the blood circulation (Xing et al. 2016). Previous epidemiological studies have shown that PM exposure have significant impact on the morbidity and mortality of human respiratory diseases (Analitis et al. 2006; Brunekreef and Holgate 2002; Dominici et al. 2006), especially in pregnant women, infants, and adolescents (de Oliveira et al. 2012; Huynh et al. 2006).
Childhood asthma and wheezing are closely related to respiratory tract infections, which are essentially inflammatory hyperresponsiveness of the airway, and frequent wheezing in children can induce asthma (Network 2018). Asthma is characterized by recurrent and variable clinical features including wheezing, chest tightness, persistent cough, shortness of breath, and expiratory airflow limitation (Massoth et al. 2019; Mims 2015). It is estimated that almost 339 million people worldwide suffer from asthma, and about 1000 people die from it every day (Network 2018). Asthma could occur at any stage of life but most commonly develop for the first time in early childhood (Network 2018). Wheezing is a common symptom in patients with bronchospasm and airway obstruction; it is often regarded as a precursor to childhood asthma and has significant prognostic value for the early diagnosis of asthma (Castro-Rodriguez et al. 2000; Martinez 2009; Martinez and Helms 1998). It should be noted that not all wheezing is asthma, although they have similar characteristics (Martinez 2013).
In terms of childhood asthma and wheezing, environmental factors were considered independent but can be combined with genetic factors to affect health. Previous studies have highlighted the impacts of air pollution on asthma and wheezing based on birth region or current residence (Gehring et al. 2015; Molter et al. 2015; Tetreault et al. 2016); however, the view remains controversial. Many studies showed that prenatal exposure to PM2.5 significantly increased the risk of offspring respiratory diseases, including asthma, wheezing, and broncho-pulmonary infections (Chiu et al. 2014; Jedrychowski et al. 2013; Pennington et al. 2018), while some studies have found no similar associations (Lavigne et al. 2018; Sbihi et al. 2016). Furthermore, air quality varies widely across countries with different economic conditions and industrialization levels, which may affect the concentration of pollutants. Multiple prospective birth cohort studies from different developed countries have indicated that exposure to ambient air pollution in early life is more likely to cause asthma in childhood and adolescence (Gehring et al. 2015). Another study based on immigrant populations found that the prevalence of asthma decreased in Japanese descent who had lived in a developing country for at least 6 months, suggesting that living in developing countries may reduce the incidence of asthma (Sakai Bizmark et al. 2016).
There is a limited number of meta-analyses that assess the relationship between prenatal exposure and childhood respiratory diseases currently. Our study explored the association between prenatal exposure to PM2.5 and asthma and wheezing in offspring by meta-analysis. We also performed subgroup analyses to explore the impact of each trimester of pregnancy, different age groups, and economic levels, as well as potential confounding factors on child health.
Methods
Search strategy
We searched for relevant papers on PubMed, Web of Science, Embase, and Cochrane Library databases, which published before July 15, 2019. The following search terms were used in the databases:
-
#1 (maternal) or (antepartum) or (prenatal) or (pregnancy) or (pregnant)
-
#2 (childhood) or (children) or (infant) or (offspring)
-
#3 (PM2.5) or (particulate matter) or (air pollution) or (air pollutants)
-
#4 (asthma) or (wheeze) or (respiratory allergy) or (respiratory sounds) or (allergic rhinitis) or (hay fever)
-
#5 #1 and #2 and #3 and #4
The terms were entered individually and as combinations in the advanced search fields of each database. Furthermore, we manually searched for relevant papers in reference lists of reviews (Hehua et al. 2017; Yang 2019). All original articles published in English were included, duplicate records and irrelevant topics were excluded, and all candidate literatures were managed by Endnote.
Inclusion and exclusion criteria
The included studies met the following criteria: (1) original research papers were selected; (2) published observational epidemiological studies, including cohort, nested case-control, and cross-sectional studies were included, because they can provide evidence about disease risk factors based on reasonable design; (3) studies were selected that had maternal exposure to PM2.5 during pregnancy as the primary influencing factor, strictly specified childhood “asthma” or “wheezing” diagnosis as outcomes, and explored the relationships among them; (4) the effect sizes that assessed the risk of PM2.5 and corresponding 95% CI were included in research papers.
The exclusion criteria for studies were: (1) reviews, conference abstracts, editorial letters, and comments; (2) explored PM2.5 exposure except during pregnancy; (3) explored other air pollutant exposure except PM2.5 during pregnancy; (4) explored the association between prenatal exposure to PM2.5 and other childhood respiratory diseases or symptoms rather than asthma or wheezing; (5) only childhood asthma or wheezing symptoms or onset was investigated, but no exposure was found; (6) different studies with the same population and overlapping ages.
Data extraction
We screened data from the selected research papers and extracted the main characteristics of each study, including the first author’s last name, published year, study time, study areas, study types, number of subjects, pregnancy phases, exposure assessment methods, PM2.5 exposure distribution, child ages, outcome definitions, effect size, and 95% Cl. Covariates and other important information were also discreetly extracted by reading the full text. If a study applied different data analysis methods, we preferred to select results from common statistical methods to enhance the comparability across original studies. If various pollutant assessment measures were used simultaneously, we tended to extract results from more accurate methods.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was recommended for reporting the quality of observational studies (Stang 2010); NOS consists of 8 items in 3 categories: selection, comparability, and outcome. Each item received a maximum of one star in the selection and outcome categories, and a maximum of two stars in the comparability category (Wells et al. 2011). In our meta-analysis, NOS was used to assess the quality of the included studies.
Statistical analysis
We obtained relative risk (RR), odds ratios (OR), and hazard ratios (HR) as well as 95% confidence intervals (CI) for the PM2.5 exposure during pregnancy. Since the absolute risk of asthma/wheezing events was low, the OR can be approximated as RR (Davies et al. 1998); HR focuses on whether the terminal event occurs, as well as the time and censored data used to reach the terminal, which is essentially a risk ratio that takes time into account. Based on previous meta-analysis experience, the combination of these three effect values is acceptable (Anderson et al. 2013; Khreis et al. 2017). The effect values and 95% CI were extracted in the adjusted models. For some studies where PM2.5 exposure concentration was categorized or the other concentration increment, it is necessary to unify the unit of effect value to 5 μg/m3 through estimation and conversion. The overall effect was estimated by the effect size (ES) and the corresponding standard error (SE). The specific approach was that if included papers only reported the odds ratio and its 95% confidence interval between PM2.5 exposure and asthma and/or wheezing, the effect size can be calculated as ES = Ln OR, and the standard error of effect size may be estimated as SE = OR[Ln (UCI/LCI)/3.92], where UCI and LCI represents the upper and lower confidence limits, respectively (Greenland 1987). Heterogeneity was assessed by the Q and I2 statistics. If the Q statistic corresponds to p < 0.1 or I2 ≥ 50%, it means that there is significant statistical heterogeneity among different studies; otherwise, there is no statistical heterogeneity (Higgins et al. 2003). If there is no significant heterogeneity, the PetoMantel-Haenszel fixed effect model would be performed in the meta-analysis; otherwise, the Dersimonian-Laird random effect model would be used. Subgroup analyses aimed to explain potential heterogeneity across multiple studies (Doi et al. 2015; Mantel and Haenszel 1959). Sensitivity analysis was used to evaluate the impact of individual studies on the pooled effect size. Publication bias was estimated with Egger’s and Begg’s tests. The presentation of the meta-analyses adhered to the meta-analysis of observational studies in epidemiology guidelines. All statistical analyses were performed with Stata 12.0.
Results
Study characteristics
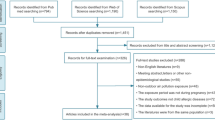
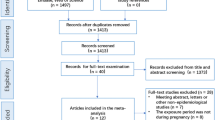
Two thousand two hundred sixty-six records were initially identified from different databases. After applying these criteria, a total of 9 original papers met our inclusion criteria (Hunt et al. 2011; Jedrychowski et al. 2010; Jung et al. 2019; Lavigne et al. 2018; Lee et al. 2018; Norback et al. 2019; Rosa et al. 2017; Sbihi et al. 2016; Soh et al. 2018) (Fig. 1). These papers were published between April 27, 2010, and April 5, 2019. The main characteristics of the included studies were presented in supplementary materials (Table S1). Five studies were conducted in developed countries, and 4 in developing countries. The studies were comprised of 7 cohort studies, 1 nested case-control study, and 1 cross-sectional study. All studies’ exposure assessments and outcome definitions met our inclusion criteria. The longest period of follow-up was 10 years. The participants’ exposure was defined as over entire pregnancy, and parts of reported each trimester exposure. The children’s ages ranged from 0 to 10 years in these studies. Different methods and models were applied to assess maternal air pollution exposure levels. Three studies used aerosol optical depth (AOD) measurements, combined with land use regression (LUR) predictors to obtain the exposure levels of each participant (Jung et al. 2019; Lee et al. 2018; Rosa et al. 2017). Three studies provided the LUR models or the inverse-distance weighted (IDW) averages to estimate pollutants based on high spatial resolution (Lavigne et al. 2018; Norback et al. 2019; Sbihi et al. 2016). Two studies used personal environmental monitoring samplers (PEMS) and 24-h sampling instruments estimated exposure levels (Hunt et al. 2011; Jedrychowski et al. 2010), and one study obtained exposure information from the National Environment Agency (Soh et al. 2018). Children’s health outcomes were determined mainly from hospital diagnosis or reports from the main caregiver.
Quality of included studies
A quality assessment checklist ensured that the included studies met quality requirements. NOS results are shown in (Table S2), indicating that the included studies have high quality (at least six stars), but most of the studies were judged insufficient in the assessment of outcome and adequacy of following up. In terms of outcome assessment, several studies used primary caregivers or questionnaire answers to obtain children’s outcomes (Lee et al. 2018; Norback et al. 2019; Rosa et al. 2017; Soh et al. 2018). Regarding for follow-up adequacy, the loss of follow-up was more than 20% or not mentioned in some studies (Hunt et al. 2011; Lee et al. 2018; Norback et al. 2019; Rosa et al. 2017; Soh et al. 2018).
Systematic review
Some population-based studies have explored the association between prenatal PM2.5 exposure and wheezing. In growing up in Singapore towards healthy outcomes (GUSTO) study, Soh et al. pointed out that maternal exposure to PM2.5 during entire pregnancy or at each trimester significantly increased the risk of wheeze in children in the first 2 years, and children who were born to overweight or obese women were more likely to suffer from wheezing (Soh et al. 2018). Lee et al. constructed Bayesian distributed lag interaction models to identify susceptible windows and higher risk subgroups in Boston. It is identified that the 19 to 23 weeks of gestation constituted sensitive window, and synergistic effects of prenatal stress and PM2.5 exposure on childhood asthma risk (Lee et al. 2018). With a birth cohort of 76,172 participants in Ontario, Canada, Lavigne et al. found that PM2.5 exposure in the second trimester increased the risk of asthma in offspring (HR 1.07, 95% CI 1.06–1.09). This study has a large sample size, long longitudinal data, and strong causal analysis ability (Lavigne et al. 2018). The study of birth cohort in Krakow found that prenatal exposure was positively correlated with the number of wheezing days in the first 2 years of life, while no longer associated with the frequency of wheezing at age 3 to 4. Wheezing after 3 years old was no longer associated with prenatal exposure, but its occurrence depends on the presence of wheezing in the first 2 years (Jedrychowski et al. 2010). Hunt et al. recruited pregnant women with a history of asthma from inner-city neighborhoods of Syracuse, NY, and found that elevated PM2.5 level was a significant risk factor for childhood wheezing (Hunt et al. 2011). Jung et al. designed the large population-based birth cohort to explore the impact of prenatal and postnatal exposure to PM2.5 on asthma onset, and found that exposure during 6–22 weeks during pregnancy and 9–46 weeks after birth were significantly associated with increased incidence of asthma. The risk ratio increased dramatically when exposure to PM2.5 concentrations was higher than 93 μg/m3 (Jung et al. 2019).
Inconsistent results were found in other longitudinal studies. Rosa et al. found that mothers exposed to higher levels of particulate pollution during pregnancy did not increase the risk of wheezing in their offspring. It is worth noting that pregnant women with high prenatal stress were more frequently exposed to PM2.5 in the first trimester, and that their children have slightly higher risk of wheezing than other children (Rosa et al. 2017). In Greater Vancouver, Sbihi et al. used a nested case-control design compared with cases of preschool children and school-age children with their control group, respectively. After adjusted for economic status and childbirth characteristics, no relationship was found between PM2.5 exposure during pregnancy and general childhood asthma, but the effects of air pollution on children with low birth weight were significant (Sbihi et al. 2016). Norback et al. recruited infants from day care centers in six Chinese cities and found prenatal exposure to PM2.5 was associated with the prevalence of rhinitis; however, no significant association was found in wheezing (Norback et al. 2019). Different studies have obtained controversial results on this subject, but we must recognize that these researchers are constantly trying new methods to accurately assess the association between PM2.5 exposure during pregnancy and asthma and wheezing in children, so as to explore the substantial association.
Meta-analysis
A pooled analysis included 9 studies and are shown in Fig. 2. The reason why there were 10 items in our pooled analysis is that a research paper assigned children into two groups based on ages to explore the effects of prenatal exposure on children. The results indicated that prenatal exposure to PM2.5 significantly increased the risk of childhood asthma and wheezing (OR = 1.06, 95% CI 1.02–1.11; per 5 μg/m3) (Fig. 2).
In order to explore trimester specific effects, we divided the entire pregnancy into three trimesters, and found that PM2.5 exposure had no significant impact on children asthma and wheezing in any trimester (1st trimester OR = 1.03, 95% CI 0.96–1.11; 2nd trimester OR = 1.07, 95% CI 0.98–1.17; 3rd trimester OR = 1.02, 95% CI 0.95–1.09; per 5 μg/m3) (Fig. 3a).
Forest plots of subgroup analyses results. a Maternal exposure to PM2.5 at any single stage of pregnancy was not significantly associated with offspring asthma and wheezing. b Maternal exposure to PM2.5 had strong impact on child asthma and wheezing before 3 years of age. c Prenatal exposure is more strongly associated with child asthma and wheezing in developed countries than in developing countries. d Areas with higher prenatal exposure levels had more stable risk ranges for child asthma and wheezing
Taking the age of 3 as the boundary and dividing the children into two groups, we found that maternal exposure was more strongly associated with infant respiratory symptoms before 3 years old (OR = 1.15, 95% CI 1.00–1.31; per 5 μg/m3) than after (OR = 1.04, 95% CI 1.00–1.09; per 5 μg/m3) (Fig. 3b).
The pooled effect size was also estimated based on the economic development of the research area. The pooled effect indicated that prenatal exposure has a more severe effect on childhood asthma and wheezing in developed countries (OR = 1.14, 95% CI 1.02–1.27; per 5 μg/m3) than in developing countries (OR = 1.05, 95% CI 1.00–1.10; per 5 μg/m3) (Fig. 3c).
Based on the PM2.5 criterion values (25 μg/m3) set by the World Health Organization (WHO), the studies were categorized into two groups including higher and lower exposure groups, and the effect values were pooled. The subgroup analysis revealed that the effects of maternal prenatal exposure on asthma and wheezing in children were marginal and varied widely in areas with lower average exposure concentrations (OR = 1.10, 95% CI 0.99–1.21; per 5 μg/m3). However, this effect was significant and stable in areas with higher average exposure concentrations (OR = 1.07, 95% CI 1.02–1.13; per 5 μg/m3) (Fig. 3d).
Four studies examined the association between maternal exposure to PM2.5 and childhood asthma, and 5 investigated wheezing. The effect of prenatal exposure on childhood asthma (OR = 1.06, 95% CI 0.98–1.14) and wheezing (OR = 1.08, 95% CI 1.01–1.15) is presented in Table 1. Exposure to PM2.5 during each trimester of pregnancy did not increase the risk of asthma and asthma in children. The pooled effects of PM2.5 on wheezing before the age of 3 was significant (OR = 1.15, 95% CI 1.00–1.31). In the case of wheezing, the pooled effect values for both developed and developing countries showed that exposure to PM2.5 during pregnancy increased the risk of childhood disease (OR = 1.37, 95% CI 1.01–1.86; OR = 1.03, 95% CI 1.02–1.04, respectively).
The pooled effects adjusted for various characteristics of mothers and children are shown in Table 2. Prenatal exposure to PM2.5 was significantly associated with childhood wheezing after adjusting for maternal atopy (OR = 1.08, 95% CI 1.01–1.15), while the association with asthma was not statistically significant under the same conditions (OR = 1.07, 95% CI 0.98–1.17). The pooled effect showed a significant association between prenatal exposure and childhood asthma after adjusting for ETS (OR = 1.06, 95% CI 1.01–1.12). In addition, after adjusting for children’s gender, parity, and breastfeeding, the association between gestational exposure and children’s composite outcomes were statistically significant, but the effects on asthma and wheezing were different respectively. Sensitivity analyses showed the contribution of each study was balanced and our results were robust (Fig. 4a). Egger’s and Begg’s tests showed no significant publication bias (Egger’s test, p = 0.257; Begg’s test, p = 0.210), and the funnel plot of intuitive expression is shown in Fig. 4b. There was no apparent asymmetry in the funnel plot, indicating that the included papers did not have potential publication bias.
Discussion
In this study, we performed systematic review and meta-analysis to assess the association between prenatal PM2.5 and asthma and wheezing in children. A total of 1,030,823 mother-child pairs from 9 studies showed that exposure to PM2.5 during pregnancy had significant effect on asthma and wheezing in offspring. The most association remained significant after adjusting for mother and child characteristics as well as performing the subgroup analysis, suggesting that our results were reliable. Our results also showed that exposure to PM2.5 during each trimester alone did not significantly increase the risk of asthma and wheezing in children, suggesting that exposure throughout pregnancy is harmful (Lee et al. 2018). Therefore, more effective preventive measures to control air pollution and prenatal care are particularly significant during the entire pregnancy (Soh et al. 2018), such as reducing the frequency of outdoor exercise or wearing dust masks in hazy weather. The stronger association was also found between exposure and child asthma and wheezing in the first 3 years. Although there was a weaker significant association after 3 years of age, the frequency and severity of the disease remains dependent on the presence of respiratory symptoms in early life (Jedrychowski et al. 2010). The pooled risk effect in developed countries was higher than in developing countries, which may be due to the large number of studies included in developed countries, and some of them use more accurate exposure measurements (Hunt et al. 2011; Jedrychowski et al. 2010). There were also some studies which indicated that particulate matter in developed countries mainly comes from automobile exhaust emissions (Hime et al. 2018), while from industrial processes, fossil and biomass fuel in developing countries (Li et al. 2014). Both low-exposure and high-exposure areas were associated with respiratory health in children, but the effects of high exposure were more stable.
Our results are consistent with most of the included studies, but there are still several inconsistencies. Sbihi et al. used a nested case-control design to explore the relationship between air pollution exposure during pregnancy and asthma in offspring. The incidence of asthma in children aged 0–5 and 6–10 was 11.92% and 2.69%, respectively. Furthermore, the air quality in the study area was quite good, with the average concentration of PM2.5 being 4.10 ± 1.6 μg/m3 and per IQR 1.45 μg/m3, which may be the reason why no significant correlation was found (Sbihi et al. 2016). Rosa et al. did not find the effect of prenatal PM2.5 exposure on children’s wheezing, but showed that psychological stress could regulate the association between particulate exposure and children’s wheezing. The study involved 552 mother and child pairs, using a hybrid satellite-based method and residential addresses to estimate the exposure of pregnant women during pregnancy. Asthma was assessed using a questionnaire: “ Has your child ever had wheezing or whistling in the chest at any time in the past?”; such assessments are highly subjective, and it is difficult for most caregivers to tell whether the child has asthma (Rosa et al. 2017). Norback et al. conducted a cross-sectional questionnaire survey on the guardians of children aged 3–6 in day care centers in 6 cities of China. The assessment of pollutant exposure was based on the date of birth and the location of day care centers, and retrospective modeling was performed using official air pollution data to estimate prenatal PM2.5 exposure levels. The research team suggested that most children live close to day care centers in China, where air pollution levels can be approximated as household exposure levels, but this is different from the reality in Chinese urban areas. Then, the retrospective modeling may be biased, and the calculation of PM2.5 levels based on PM10 may make the bias even greater (Norback et al. 2019). It also should be noted that Hunt et al. focused on pregnant women with a history of asthma, which may be an important potential confounding factor for offspring asthma, making the risk value higher than in other studies (Hunt et al. 2011).
From the point of methodology, although randomized controlled studies provide stronger evidence than observational studies, observational studies are considered more appropriate for meta-analyses based on the characteristics of air pollution and its impact on health. Subgroup analyses and sensitivity analysis were carried out for the included studies, and random effect models were adopted. After applying the random effect model, the interval of effect value was small, indicating that the result was stable. However, considering that subgroup analysis cannot eliminate the heterogeneity among studies, the results still need to be viewed with caution and objectivity. There were some possible explanations for large heterogeneity in the meta-analysis. First, there were significant regional differences in the concentration of ambient particulate, from an average exposure of 4.10 μg/m3 for pregnant women in the greater Vancouver metropolitan region to 69 μg/m3 in six provincial capitals in China (Norback et al. 2019; Sbihi et al. 2016). Second, different methods were measured and estimated exposure levels across the studies. PM2.5 evaluation models included IDW, LUR, and AOD, as well as PEMS. IDW is less accurate than LUR in estimating individual PM2.5 exposure levels; AOD use satellite remote sensing inversion technology to establish a high spatial and temporal resolution exposure prediction model with better accuracy than LUR; PEMS are considered the most accurate method currently used to monitor individual exposures (Hehua et al. 2017). Some studies have compared the evaluation effectiveness of different models and concluded that different models were acceptable (Yu et al. 2018). Furthermore, the estimated spaces in different studies varied from indoor to outdoor, and the locations included ZIP code, residential address, and day care center address. Some measurements provided daily exposure, while others provided average exposure levels over time. Third, the number of subjects in different studies varied widely from 103 to 761,172 (Hunt et al. 2011; Lavigne et al. 2018); most of the studies focused on symptoms before the age of 6 and only one investigated child who aged 6–10 years. Four studies used hospital diagnoses to determine the disease outcomes, while others were obtained through questionnaires by primary caregivers. Postnatal exposure is related to the outcome of childhood diseases, but some studies failed to adjust the PM2.5 pollution level after birth when focusing on the health effects of exposure during pregnancy, and the underlying bias cannot be ignored, which is also the fourth reason for the large heterogeneity. Atmospheric particulate matter is an important risk factor in childhood allergic diseases. Prenatal exposure to PM may affect children’s respiratory health through direct placental exposure or compromising placental function (Yang 2019). Certainly, more evidence is needed on the potential impacts of prenatal exposure to PM2.5 on children’s health to reveal the underlying mechanisms via which PM2.5 may cause asthma and wheezing in offspring.
This is a comprehensive analysis about the effect of prenatal exposure to PM2.5 on childhood wheezing and asthma. After identifying relevant studies through a strict publication screening and quality assessment, we conducted a pooled analysis to comprehensively assess the risk impact of prenatal exposure on offspring’s asthma and wheezing. It not only provides a timely contribution to antenatal care, but illustrates the importance of preventing air pollution. However, there are some limitations. First, we only examined the impact of a single pollutant on children’s respiratory health. In fact, there are various air pollutants that have synergistic effects, and there is a high correlation between the different pollutants, which may lead to a false positive association between exposure and outcomes. Second, most of our included studies used residential exposure instead of individual exposure, and we only extracted exposure concentration and effect size, so we could provide more concise and visual risk estimates. Third, our results showed a high heterogeneity level across the included studies, this may be due to the study design, exposure assessment, and childhood age groups were different across studies, but the good sign is the results were robust. Air pollution and childhood respiratory health are significant problems in the current field of public health, so further research with more scientific epidemiologic designs, more accurate exposure assessment models, and larger sample sizes (Dong et al. 2018) are urgently needed to clarify the causal association between maternal exposure to PM2.5 and childhood asthma as well as wheezing.
Conclusions
The results from the meta-analysis suggested that maternal exposure to PM2.5 was significantly associated with childhood asthma and wheezing. Gestation is a crucial period of fetal respiratory development, and exposure may increase the risk of subsequent asthma and wheezing in children; however, our conclusions need to be treated with caution because of heterogeneity. We suggest that regulating pollutant emission standards and enhancing prenatal care measures should be carried out to promote maternal and child health.
References
Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, Touloumi G, Schwartz J, Anderson HR, Cambra K, Forastiere F, Zmirou D, Vonk JM, Clancy L, Kriz B, Bobvos J, Pekkanen J (2006) Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology 17:230–233
Anderson HR, Favarato G, Atkinson RW (2013) Erratum to: Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health 6:541–542
Brunekreef B, Holgate ST (2002) Air pollution and health. Lancet 360:1233–1242
Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD (2000) A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 162:1403–1406
Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, Wright RJ (2014) Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol 133:713–22 e4
Davies HT, Crombie IK, Tavakoli M (1998) When can odds ratios mislead? BMJ 316:989–991
de Kok TM, Driece HA, Hogervorst JG, Briede JJ (2006) Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat Res 613:103–122
de Oliveira BF, Ignotti E, Artaxo P, Saldiva PH, Junger WL, Hacon S (2012) Risk assessment of PM(2.5) to child residents in Brazilian Amazon region with biofuel production. Environ Health 11:64
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM (2015) Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 45:130–138
Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM (2006) Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295:1127–1134
Dong T, Hu W, Zhou X, Lin H, Lan L, Hang B, Lv W, Geng Q, Xia Y (2018) Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Reprod Toxicol 76:63–70
Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, Fuertes E, Gruzieva O, Heinrich J, Hoffmann B, de Jongste JC, Klümper C, Koppelman GH, Korek M, Krämer U, Maier D, Melén E, Pershagen G, Postma DS, Standl M, von Berg A, Anto JM, Bousquet J, Keil T, Smit HA, Brunekreef B (2015) Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 3:933–942
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Guarnieri M, Balmes JR (2014) Outdoor air pollution and asthma. Lancet 383:1581–1592
Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z (2017) The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ Res 159:519–530
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Hime NJ, Marks GB, Cowie CT (2018) A comparison of the health effects of ambient particulate matter air pollution from five emission sources. Int J Environ Res Public Health 15
Hunt A, Crawford JA, Rosenbaum PF, Abraham JL (2011) Levels of household particulate matter and environmental tobacco smoke exposure in the first year of life for a cohort at risk for asthma in urban Syracuse, NY. Environ Int 37:1196–1205
Huynh M, Woodruff TJ, Parker JD, Schoendorf KC (2006) Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol 20:454–461
Jedrychowski WA, Perera FP, Maugeri U, Mrozek-Budzyn D, Mroz E, Klimaszewska-Rembiasz M, Flak E, Edwards S, Spengler J, Jacek R, Sowa A (2010) Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr. Allerg Immunol 21:e723–e732
Jedrychowski WA, Perera FP, Spengler JD, Mroz E, Stigter L, Flak E, Majewska R, Klimaszewska-Rembiasz M, Jacek R (2013) Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int J Hyg Environ Health 216:395–401
Jung CR, Chen WT, Tang YH, Hwang BF (2019) Fine particulate matter exposure during pregnancy and infancy and incident asthma. J Allergy Clin Immunol 143:2254–2262.e5
Kelly FJ, Fussell JC (2012) Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 60:504–526
Kelly FJ, Fussell JC (2015) Air pollution and public health: emerging hazards and improved understanding of risk. Environ Geochem Health 37:631–649
Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M (2017) Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int 100:1–31
Lavigne E, Bélair MA, Rodriguez Duque D, Do MT, Stieb DM, Hystad P, van Donkelaar A, Martin RV, Crouse DL, Crighton E, Chen H, Burnett RT, Weichenthal S, Villeneuve PJ, To T, Brook JR, Johnson M, Cakmak S, Yasseen AS III, Walker M (2018) Effect modification of perinatal exposure to air pollution and childhood asthma incidence. Eur Respir J 51:1701884
Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, Wilson A, Schwartz J, Cohen S, Coull BA, Wright RO, Wright RJ (2018) Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol 141:1880–1886
Li W, Wang C, Wang H, Chen J, Yuan C, Li T, Wang W, Shen H, Huang Y, Wang R, Wang B, Zhang Y, Chen H, Chen Y, Tang J, Wang X, Liu J, Coveney RM Jr, Tao S (2014) Distribution of atmospheric particulate matter (PM) in rural field, rural village and urban areas of northern China. Environ Pollut 185:134–140
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Martinez FD (2009) The connection between early life wheezing and subsequent asthma: the viral march. Allergol Immunopathol (Madr) 37:249–251
Martinez JA (2013) Not all that wheezes is asthma! J Bras Pneumol 39:518–520
Martinez FD, Helms PJ (1998) Types of asthma and wheezing. Eur Respir J Suppl 27:3s–8s
Massoth L, Anderson C, McKinney KA (2019) Asthma and chronic rhinosinusitis: diagnosis and medical management. Med Sci 7
Mims JW (2015) Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol 5(Suppl 1):S2–S6
Molter A et al (2015) A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J 45:610–624
Network NZGA (2018) The Global Asthma Report 2018
Ngoc LTN, Park D, Lee Y, Lee YC (2017) Systematic review and meta-analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health 14
Norback D, Lu C, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Qian H, Sun Y, Sundell J, Juan W, Liu W, Deng Q (2019) Onset and remission of childhood wheeze and rhinitis across China - associations with early life indoor and outdoor air pollution. Environ Int 123:61–69
Pennington AF, Strickland MJ, Klein M, Zhai X, Bates JT, Drews-Botsch C, Hansen C, Russell AG, Tolbert PE, Darrow LA (2018) Exposure to mobile source air pollution in early-life and childhood asthma incidence: the Kaiser air pollution and pediatric asthma study. Epidemiology 29:22–30
Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, Bose S, Chiu YM, Hsu HL, Coull B, Schwartz J, Cohen S, Tellez Rojo MM, Wright RO, Wright RJ (2017) Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol 119(232–237):e1
Sakai Bizmark R, Kumamaru H, Nagata S (2016) Reduced asthma susceptibility from early childhood exposure to residing in developing country. Pediatr Allergy Immunol 27:876–880
Sbihi H, Tamburic L, Koehoorn M, Brauer M (2016) Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J 47:1062–1071
Soh SE, Goh A, Teoh OH, Godfrey KM, Gluckman PD, Shek LP, Chong YS (2018) Pregnancy trimester-specific exposure to ambient air pollution and child respiratory health outcomes in the first 2 years of life: effect modification by maternal pre-pregnancy BMI. Int J Environ Res Public Health 15
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Tetreault LF, Doucet M, Gamache P, Fournier M, Brand A, Kosatsky T, Smargiassi A (2016) Childhood exposure to ambient air pollutants and the onset of asthma: an administrative cohort study in Quebec. Environ Health Perspect 124:1276–1282
Wang L, Zhong B, Vardoulakis S, Zhang F, Pilot E, Li Y, Yang L, Wang W, Krafft T (2016) Air quality strategies on public health and health equity in Europe-a systematic review. Int J Environ Res Public Health 13
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis
Xie R, Sabel CE, Lu X, Zhu W, Kan H, Nielsen CP, Wang H (2016) Long-term trend and spatial pattern of PM2.5 induced premature mortality in China. Environ Int 97:180–186
Xing YF, Xu YH, Shi MH, Lian YX (2016) The impact of PM2.5 on the human respiratory system. J Thorac Dis 8:E69–E74
Yang SI (2019) Particulate matter and childhood allergic diseases. Korean J Pediatr 62:22–29
Yu H, Russell A, Mullholland J, Odman T, Hu Y, Chang HH, Kumar N (2018) Cross-comparison and evaluation of air pollution field estimation methods[J]. Atmospheric environment, 179:49–60
Acknowledgments
This work was supported by National Key Research and Development Program of China (2017YFC0211605); Qing Lan Project of Jiangsu Province, Six Talent Peaks Project of Jiangsu Province (JY-052); Second Level of Training Object of Jiangsu Province “333” Project; the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Yankai Xia received support from the funding. Yankai Xia conceived and designed the study. Wu Yan, Mengqi Sun, and Xu Wang performed eligibility screening and data extraction. Tianyu Dong and Mingzhi Zhang assessed the quality of the included studies. Wu Yan analyzed the data and performed the statistical analysis, as well as wrote the initial manuscript. Yi Chen, Rui Chen, and Zhiwei Sun critically revised the manuscript. Kacey Fang polished the English language.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Yan, W., Wang, X., Dong, T. et al. The impact of prenatal exposure to PM2.5 on childhood asthma and wheezing: a meta-analysis of observational studies. Environ Sci Pollut Res 27, 29280–29290 (2020). https://doi.org/10.1007/s11356-020-09014-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09014-6