Abstract
Plant species sustaining under a polluted environment for a long time are considered as potentially resistant species. Those plant species can be considered as an eco-sustainable tool used to bio-monitor and mitigate pollution. This study was carried out on a total of ten commonly available plant species to assess their anticipated performance index (API), dust capturing capacity (DCC), and metal accumulation index (MAI) in chromite mine and control areas. According to the anticipated performance index (API), Macaranga peltata (Roxb.) Müll.Arg., Holarrhena pubescens Wall. ex G.Don and Ficus hispida Roxb. ex Wall. are highly tolerant species while Terminalia arjuna (Roxb. ex DC.) Wight & Arn. and Trema orientalis (L.) Blume are intermediate tolerant species. F. hispida was also shown to have the highest dust capturing capacity (5.94 ± 0.43 mg/cm2) whereas that of Woodfordia fruticosa Kurz (1.03 ± 0.11 mg/cm2) was found to be lowest. The metal accumulation index ranged from 17.29 to 4.5 and 6.38 to 1.94 at the mine and control areas, respectively. Two-way ANOVA analysis revealed area-wise significant differences between biochemical and physiological parameters. Also, results showed that the pollution level and heavy metal affected different biochemical and physiological parameters of plant species at the mining area. The plant species with the highest API, DCC, and MAI value could be recommended for greenbelt development in different polluted areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The economic growth and development of a nation is dependent on its mining sector to a great extent which is associated with the exploitation of the mineral resources (Lèbre et al. 2017), whereas mining plays a significant role in economic growth and at the same time its adverse impact on the environment. Worldwide mining activity is one of the serious contributors to the environmental pollution and considered one of the significant sources of air, soil and water pollution (Oluwoye et al. 2017; Golui et al. 2021; Sahu and Basti 2020). From open-cast mining, a massive amount of gaseous pollutants, toxic substances, fine ore particles and dust are released to the environment (Das et al. 2020). These released pollutants mix with the atmosphere and are transported at a long distance through the air from the mine area and lead to pollution in the surrounding undisturbed area (Ni et al. 2018; Khazini et al. 2021).
Green vegetation around the mining area can act as an eco-friendly and cost-effective tool to reduce pollution. In a polluted environment, green plants play a crucial function in improving the environmental quality by accumulating toxic pollutants (Gheorghe and Ion 2011; Kaur and Nagpal 2017). They have great potential to the absorption, adsorption and accumulation of pollutants from both soil and air (Manara 2012; Remon et al. 2013). They transport toxic substances from soil and air to biotic environments. Green plants play a role as pollution sink as they provide a broad surface area for absorption and accumulation of pollutants; hence, plantation of highly tolerant plant species can help in effective green belt development to clean up polluted areas, further improving the environmental quality (Remon et al. 2013; Selmi et al. 2016; Karmakar and Padhy 2019;). Selection of suitable plants for greenbelt development differs from region to region.
The air pollution tolerance index (APTI) and anticipated performance index (API) are considered best and reliable indices to select appropriate tolerant and sensitive plant species. The highly tolerant plant species are chosen to perform against the environmental pollution, and the sensitive species might be used as a bioindicator (Rai 2016; Javanmard et al. 2020; Roy et al. 2020; Molnár et al. 2020). Four biochemical parameters, namely total chlorophyll content (TCC), leaf extract pH (pH), ascorbic acid (AA) and relative water content (RWC), were used to develop the APTI (Singh and Rao, 1991). API is an upgradation of APTI, which is a more appropriate index for the selection of tolerant and sensitive species in a particular region. API is developed with the combination of the APTI value of each individual species along with some morphological (plant habit, canopy structure, types of plant, laminar structure) and socioeconomic characters (Mondal et al. 2011). Dust, particulate matter and heavy metal are the most prevalent environmental pollutants, causing critical problems to all living organisms (Xiu et al. 2020; Kong et al. 2021). Plants provide the most efficient and natural ways to remediate pollution by capturing dust and accumulating particulate matter. In order to combat such pollutants, dust capturing capacity (DCC) and metal accumulation index (MAI) are also considered to select the suitable plant species.
The Sukinda chromite mine area is one of the most populated areas in the world, having a number of open-cast chromite mines. This mining area holds 183 million tons of raw chromite reserves, which is a total of 97% of total reserves of the country and approximately 3.8 million tons of chromite produced per year. Due to various mining activities, a massive amount of ore particles and dust is produced and get blown away through air to surrounding areas, subsequently causing chromium (Cr) contamination by atmospheric deposition (Das et al. 2020). According to a report of Black Smith Institute Report (2007), extensive pollution of the area due to excessive chromite ore mining has made it the fourth most polluted place in the world.
Through this study, we provide an integrated approach to the selection of both pollution-tolerant and -sensitive plant species. To know the physiological and biochemical tolerance levels of plants, we studied the air pollution tolerance index (APTI) and anticipated performance index (API). Heavy metal accumulation and dust capturing capacities of a total of ten plants were also examined. This study is confined only on commonly found native plant species in this area. So, our study will contribute convenient knowledge/information to find out the suitability of plant species for phytoremediation of toxic heavy metals in the vicinity of the chromite mine area as well as different other polluted areas to improve the levels of soil and air pollution.
Materials and methods
Study area
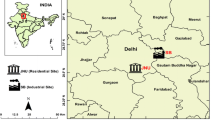
The present study was done at two areas, namely Sukinda chromite mine valley and Tomka Forest, located in Jajpur district, Odisha, India. Sukinda chromite mine valley is the largest chromite producer in India; almost 99% of the country’s chromium is produced from this valley. Stretching between 21° 00′ N to 21° 05′ N latitude and 85° 44′ E to 85° 53′ E longitude occupies an area of 200 km2 which holds an estimated 183 million tons of raw chromium deposits (Nayak and Kale 2020) and consists of an extensive plateau in the interior with a foreground of a wide coastal plain underlain by Precambrian rock. Currently, 20 open-cast and 2 underground mines are functional in the valley producing 160 million tons of overburden. It also releases 11.73 t of hexavalent chromium (Cr(VI)) to the environment every year, making it the fourth-worst polluted place in the world. (Dhal et al. 2011; Das et al. 2020). Tomka Forest is situated 21° 6′12ʺ N to 21° 8′ 11ʺ N latitude and 85° 49′ 12ʺ E to 85°55′ 58ʺ E longitude, considered as a control area due to the pollution-free atmosphere, located about 40 km away from the mining area.
Plant species sampling
In total, leaf samples of ten plant species, namely Ailanthus excelsa Roxb., Bixa Orellana L., Ficus hispida Roxb. Ex Wall., Macaranga peltata (Roxb.) Müll.Arg., Woodfordia fruticosa Kurz, Trema orientalis (L.) Blume, Terminalia arjuna (Roxb. ex DC.) Wight & Arn., Holarrhena pubescens Wall. ex G.Don, Callicarpa tomentosa (L.) Murr. and Combretum roxburghii Spreng. plant species were collected during winter session 2020. These plant species were considered on the basis of dominance, closest to the mining site, visible dust deposition on foliage and ecological importance of the specific plant species. Three replicates of healthy, mature and fully developed plant leaf samples were collected for each species. The replicate plant species were chosen as those individual species having similar height and breast diameter. After being sampled, leaves were placed into zipper-locked bags and kept in a portable ice box, then transferred to the laboratory. Leaves were collected and transferred very carefully form plant to zipper-locked bag, for ensuring least disturbance to their surface dust. For further biochemical analysis, leaves were washed with tap water followed by distilled water, and stored in − 20 °C.
Analysis of the biochemical parameters of the plants
Relative water content
After taking the fresh weight (FW), leaves were submerged in deionized water for 24 h. Then, turgid weight (TW) was recorded by soaking up the surface water of leaves. Overnight dried leaves in the oven at 80 °C were used for taking dry weight (DW) (Liu and Ding 2008).
Total chlorophyll content
The total chlorophyll content of leaves was calculated following the procedure mentioned by Arnon (1949). One gram of fresh leaves was crushed by using 10 ml of 80% acetone and centrifuged at 5000 rpm for 5 min. After collection, the supernatant volume was made up to 30 ml by 80% acetone and absorbance was taken at 645 and 663 nm by using a spectrophotometer (Analytical UV–Vis 3090 V). The calculation of total chlorophyll content was done using the following formula:
where A645 = absorbance at 645 nm, A663 = absorbance at 663 nm, V = total volume of extract (ml), and W = weight of leaf in grams.
Ascorbic acid
Ascorbic acid estimation was done by using the modified colorimetric 2,6-dichlorophenol indophenol technique. Fresh leaves (0.5 g) were extracted in 4% oxalic acid and made up to 30 ml volume and centrifuged at 6000 rpm for 10 min. Ten millilitres of 4% oxalic acid was added with 5 ml of the supernatant and titrated against the dye. Titration was done till a pink colour appeared which was present for a few seconds.
where
- V1:
-
volume of the dye titrated against the working standard
- V2:
-
volume of dye titrated against the sample
Leaf extract pH
The pH of the leaves was determined with the help of a pH meter by crushing 1 g of fresh leaves and homogenizing it in 40 ml of deionized water.
Air pollution tolerance index
APTI was calculated following the equation described by Sing and Rao (1991).
where A = ascorbic acid (mg/g), T = total chlorophyll content (mg/g), P = pH of leaf extract and R = relative water content (%).
Anticipated performance index
Combining some morphological and socio-economic characteristics (plant habit, type of plant, laminar characters, canopy type and economic value) with APTI value, API is estimated. The API score is calculated according to the following formula:
The maximum positive allotted for a plant species is 16. According to API, score plant species were categorized into eight different groups (e.g. not recommended, very poor, poor, moderate, good, very good, excellent and best).
Dust capturing capacity
Dust capturing capacity was quantified with the help of a Petri dish. A Petri dish was oven dried, and the initial weight (W1) was weighed. The upper and lower surface dust of a leaf was washed with deionized water and transferred to the Petri dish. Then the Petri dish was completely dried and weighed to record the final weight (W2). The surface area (A) of the leaf was measured with the help of graph paper. Dust capturing capacity was calculated by the following formula:
Analysis of heavy metal concentration in soil
The metal concentration in soil was done following the procedure mentioned by Roy et al. (2020). A sieved soil sample (0.2 g) was digested using HCl and H2SO4 in the ratio of 1:3. The digested sample was filtered and made up to 50 ml with 2% HNO3. Then the digested solutions were analysed for six heavy metals by using PerkinElmer ICP-OES (Model: Optima 2100 DV).
Analysis of heavy metal concentration in leaves
The washed leaves were completely dried at 60 °C in a hot air oven. One gram of leaf material was digested using 2 acids, i.e. HCLO4 and HNO3, in the ratio of 1:2 (Samecka-Cymerman and Kempers 1999). Digested solutions were diluted with double-distilled water and the final volume made up to 50 ml. Then, the digested solutions were analysed for Al, Cr, Fe, Ni, Pb and Zn using PerkinElmer ICP-OES (Model: Optima 2100 DV).
Metal accumulation index
The metal accumulation capability of plant species is calculated by the using the metal accumulation index (MAI) formula (Hu et al. 2014).
where N = number of metals and IJ = sub-index of J gained by dividing the metal concentration by its standard deviation. It depends on the collected sample number.
Results and discussions
Ascorbic acid
Ascorbic acid or vitamin C is an important low molecular weight non-enzymatic antioxidant present in plant chloroplasts. This antioxidant plays a crucial function in the light reaction of photosynthesis, regulates various metabolic biosynthesis pathways and protects plants from biotic and abiotic stresses (Singh and Verma 2007). Throughout photosynthetic electron transfer, ascorbic acid reacts and scavenges reactive oxygen species (ROS). Therefore, the biosynthesis and concentration of this antioxidant are directly proportionate to the tolerance level of plants (Smrinoff and Wheeler, 2000; Wang et al. 2018). In the mining area, the highest ascorbic acid content (mg/g in fresh leaves) was found in H. pubescence (26.83 ± 1.01) followed by M. peltata (24.66 ± 0.99), T. orientalis (22.81 ± 0.8) and F. hispida (19.63 ± 2.77), and the lowest content was noticed in C. roxburghii (8.76 ± 0.95), followed by C. tomentosa (9.46 ± 1.93) and W. fruticosa (9.86 ± 1.22). In comparison to the control area, a considerable difference was found in ascorbic acid contents in all plant species (Fig. 1). In the control area, the highest amount was found in M. peltata (14.28 ± 0.93) followed by T. arjuna (12.85 ± 0.57) and H. pubescence (11.36 ± 2.22) and the lowest amount was found in C. tomentosa (4.11 ± 0.7) followed by C. roxburghii (4.72 ± 1.03) and A. excelsa (5.56 ± 0.91).
Ascorbic acid reacts with various ROS (i.e. superoxide radical, singlet oxygen and hydroxyl radical) and protects pigments and nucleic acids. The elevated amount of ascorbic acid increases the tolerance level of plants under pollution environment as well as other stress conditions (like, drought, salt, temperature) (Wang et al. 2018). Hence, plants synthesized with a higher level of ascorbic acid in polluted environments are evaluated as tolerant species. In our study, the ascorbic acid content is higher at the mining area and lower at the control area for all examined plant species. The enhancement of ascorbic acid in all species from the mining area might be due to the elevated production of ROS, through accumulation of toxic metals in the plant body.
Relative water content
The relative water content signifies the balance between plant water uptake and release. The water content maintains turgor pressure and cell wall permeability in the plant body. During pollution conditions, different pollutants enhance cell permeability, which leads to deficiency in water and nutrients, leading to prior senescence of leaves and bark (Achakzai et al. 2017; Safari et al 2019). So, the relative water content of plants is a suitable physiological parameter to measure water condition in the plant body. All the species had a more relative water content in the control area than in the mining area (Fig. 1). The highest relative water content in the mining area was found in M. peltata (93.71 ± 2.69) and the lowest in A. excelsa (64.81 ± 7.76). In the control area, the relative water content of leaves of the plant species differed from a maximum of 98.22 ± 0.33 in F. hispida to a minimum of 69.31 ± 1.37 in A. excelsa (Table 1).
The relative water content of plant leaves in the mining area is decreased which might be due to high pollutants and heavy metal contamination; it also specifies the unbalanced physiological condition of the plant. Heavy metal contamination in the plant body reduces the water transport from root to leaves (Barceló and Poschenrieder 1990). Under any stress conditions, a high water content helps to sustain physiological and biochemical balance in a plant body. A higher level of relative water content in plants increases the tolerance strength of plant towards pollution stress (Gupta et al. 2016; Karmakar and Padhy 2019).
Total chlorophyll content
The photosynthetic pigment chlorophyll, found in the chloroplasts of green plants, is an index of productivity and is called a photoreceptor. Chlorophyll plays an important role in plant photosynthesis, so measurement of total chlorophyll content is a significant measure to assess the effect of pollution on the plant. The decreased level of the total chlorophyll content of all species was noticed in the mining area as compared to the control area (Fig. 1). At the mining area, the highest chlorophyll content (mg/g of fresh weight) was found in H. pubescence (1.81 ± 0.01) followed by T. orientalis (1.3 ± 0.11) and M. peltata (1.12 ± 0.05). The lowest content was found in B. orellana (0.44 ± 0.01) followed by W. fruticosa (0.58 ± 0.007) and C. roxburghii (0.61 ± 0.009). At the control area, the highest content was found in H. pubescence (2.33 ± 0.22) followed by M. peltata (1.43 ± 0.24) and T. orientalis (1.39 ± 0.19). The lowest content was found in B. orellana (0.75 ± 0.09) followed by F. hispida (0.9 ± 0.07) and A. excelsa (1.06 ± 0.13). Chlorophyll has a highly organized state that may go through various photochemical reactions like oxidation, reduction and reversible bleaching in different pollutant conditions (Karmakar et al. 2021). The decreased level of chlorophyll at the mining area might be due to dust deposition on the leaf surface and heavy metal accumulation. Protochlorophyllide is the precursor of chlorophyll; during chlorophyll biosynthesis, this precursor is reduced by NADPH to chlorophyllide in the presence of light, but due to the adherence of dust on the leaf surface hindering the pathway of light, thus interfering in the process of chlorophyll formation (Roy et al. 2020). After accumulation of heavy metals, Mg++ ion of chlorophyll is replaced by another metal ion and breakdown into phaeophytin (Karakoti et al 2014). High pollution stress reduces the total chlorophyll content at the polluted site. But some plant species produce tolerance mechanisms for detoxification of metal toxicity. Production of a high level of phytochelatins, metallothioneins, organic acids and thiol-reactive peptides which are bound with toxic metals and compartmentalize into vacuoles is performed. Those species are able to maintain a high level of chlorophyll known as tolerant species.
Leaf extract pH
Leaf extract pH is a biochemical parameter of plants that signifies as an indication of stress. The pH of the leaf extracts ranges from 6.8 ± 0.13 to 5.1 ± 0.06 and 7.17 ± 0.14 to 5.53 ± 0.12, at the polluted and control areas, respectively, with F. hispida and B. orellana having the highest and lowest values. The pH of plants has shown a good relationship with the susceptibility to pollution. Low pH reduced the photosynthetic activity by altering the stomatal activity while a higher level of pH in plants can increase tolerance towards pollution by enhancing the synthesis rate of ascorbic acid from hexose sugar (Escobedo et al. 2008). The presence of acidic pollutants in the environment increases the acidic nature of the leaf by decreasing the pH. Hence, those plant species have a higher leaf extract pH which are considered as pollution-tolerant species. It is evidenced that all the plant species collected from the mining area showed an acidic pH. In comparison with the control area, all plants showed an acidic nature of leaf extract which may be due to the accumulation of acidic nature heavy metals in apoplast. Mostly heavy metals like Cr, Ni and Zn form acidic radicals in the leaf matrix by reacting with cellular water. Karmakar and Padhy (2019) and Nadgórska-Socha et al. (2017) have reported that leaf pH is influenced by the presence of heavy metals like Cd, Cr, Ni and Zn.
Air pollution tolerance index
The APTI is an established method for the evaluation of plants with regard to their sensitivity to pollutants. Depending on the APTI value, plant species are categorized into four different groups, i.e. ≤ 15 = sensitive, 15–19 = intermediate, 20–24 = moderately tolerant and > 24 = tolerant (Singh et al. 1991). The APTI values of each plant species were calculated using the mean values of T, P, R and A values, so the standard deviation of the APTI values is not mentioned (Table 1). At the mining area, it is shown that the APTI value ranges from 30.24 to 12.48. The highest value was recorded in H. pubescence (30.24) followed by M. peltata (25.96), T. orientalis (25.62) and F. hispida (22.52). According to the APTI range, H. pubescence, M. peltata and T. orientalis are tolerant species as their APTI value ≥ 24. F. hispida (22.52) is an only moderately tolerant species. C. roxburghii (18.71) and T. arjuna (16.51) are intermediate species, whereas A. excelsa, B. orellana and C. tomentosa are sensitive species (APTI ≤ 15). At the control area, the APTI showed a declining (Fig. 1) value ranging from 21.51 to 11.41, with M. peltata and C. roxburghii having the highest and lowest values, respectively. All species are grouped into three categories, i.e. sensitive, intermediate and moderately tolerant; no species could qualify as tolerant at the control areas. A strong correlation between ascorbic acid and APTI at both mining (R2 = 0.86) and control area (R2 = 0.83) was noticed, whereas the remaining three parameters (pH, total chlorophyll and relative water content) have an insignificant low correlation with APTI. This indicates that, as the pollution level increases ascorbic acid in plants also increases to combat the pollution stress. Some previous studies have also found a similar kind of correlation between ascorbic acid and APTI value (Rai and Panda 2014; Bharti et al. 2018; Roy et al. 2020), since the concentration of heavy metal is higher in the mining area (Table 4) and the MAI of the plants is also higher, which leads to an increase in the antioxidant level to combat such high stress. In the control area, such stressors are below the permissible level, plant species not increasing their antioxidant level. Depending on the stressor level, same species showed different APTI indices in both areas. Plantation of tolerant plant species, to mitigate environmental pollution, is a sustainable prospective to meet industrial and commercial growth.
Anticipated performance index
Different species act differently to different pollution stresses. Therefore, grouping of species on the basis of the APTI value is only useful to evaluate the tolerance status of the plant. Hence, only the APTI value is not enough for the selection of appropriate tolerant species to develop greenbelts in polluted areas. By incorporating the APTI value with economic value and morphological characters like plant habit, canopy structure, laminar character and plant types, the API grade of different species is calculated. In the mining area, M. peltata scored the highest API value followed by H. pubescens and F. hispida which were qualified as excellent, very good and good species, respectively, for greenbelt development (Table 2). Among the rest of the species, two (T. arjuna and T. orientalis) were qualified as moderate. C. tomentosa, C. roxburghii, A. excelsa, Bixa orellana and W. fruticosa are poor, very poor and not recommended for greenbelt development, because of their low API value (50- 25). In control areas, a similar result was also found. M. peltata scored the highest API value followed by H. pubescens and F. hispida. But all three species were qualified as good species for greenbelt development. Among the rest of the plant species, T. arjuna is moderate, C. tomentosa is poor, three species (A. excelsa, B. orellana and C. roxburghii) are very poor and W. fruticosa is not recommended for greenbelt development. Screening all the species by using only the APTI value, H. pubescence, M. peltata and T. orientalis have been assessed as tolerant species and F. hispida was a moderately tolerant species. But after combining the APTI value with the morphological and economic value, then F. hispida qualified as a tolerant species along with H. pubescence and M. peltata and T. orientalis qualified as moderate along with T. arjuna.
Dust capturing capacity
The variation of dust capturing capacity of collected species at the mining and control areas are depicted in Table 3. F. hispida was found to have the highest dust capturing capacity (5.94 mg/cm2) in the mining area followed by C. tomentosa (4.26 mg/cm2), H. pubescence (3.6 mg/cm2), M. peltata (3.51 mg/cm2) and T. orientalis (3.5 mg/cm2). The remaining species (A. excelsa, B. orellana, C. roxburghii, T. arjuna, W. fruticosa) showed the lower dust capturing capacities in the mining area. In control areas, M. peltata (0.39 mg/cm2) showed the highest dust capturing capacities followed by C. tomentosa (0.37 mg/cm2) and F. hispida (0.36 mg/cm2) and the lowest dust capturing capacities shown by W. fruticosa (0.14 mg/cm2) followed by C. roxburghii (0.16 mg/cm2) and B. orellana (0.17 mg/cm2). The dust capturing capacity of plants significantly depended on species to species. Several micro-characters, macro-characters and the surrounding environment affect the plant’s dust capturing capacity. Micro-characters such as stomatal size and density, presence of trichome, pubescence, wax layer and macro-characters like height of plant, canopy structure, leaf arrangement on stem and petiole area significantly influence the dust capturing capacity of plant species (Mo et al. 2015; Leonard et al. 2016). F. hispida, C. tomentosa, H. pubescence, M. peltata and T. orientalis contribute a high level of dust capturing capacities due to the presence of trichome in both sides of the leaf surface. Similar findings were supported by some recent studies by Roy et al. (2020), and Chaudhary and Rathore (2019) found higher dust capturing capacities in genus Ficus. Roy et al. (2020) also found that A. excelsa had the lowest value due to its small and glabrous leaf petiole. Single-factor ANOVA showed significant differences in dust capturing capacities in both areas (F = 24.11, Fcritical = 4.41), at the 0.05 significance level. Area-wise differences may be due to high atmospheric pollution and anthropogenic activity.
Assessment of heavy metal in soil
Mining area soils showed a relatively very high amount of different heavy metals in order Fe > Cr > Al > Ni > Zn > Pb. The concentration of Fe is higher than those of other metals which is 154,766.67 ± 10,150.92 mg/kg. The high Fe content in the mining soil is due to the presence of hematite and goethite. The concentrations of the other five heavy metals namely Cr, Al, Ni, Zn and Pb are depicted in Table 4. All heavy metal concentrations in the mining area soils surpass the ecotoxicological level as per WHO standard. On the other hand, all the heavy metal concentrations in the control forest area are within the limit of the WHO standard.
Assessment of heavy metal within the leaf
Heavy metal accumulation in plants can be from both adsorption through stomata from areal dust deposition and root uptake from soil. So, it is impossible to distinguish whether the accumulated heavy metal came from the soil or from the air (Serbula et al. 2012; Norouzi et al. 2015). In mining areas, the analysed plant leaves revealed that they surpassed the permissible limit of the WHO (1996) standards. The Al, Cr and Fe contents were extremely higher within all investigated plant leaves. The average metal accumulation ranges of all investigated plants at both the areas are represented in Table 5. The Fe concentration was found to be higher in all plant leaves of the mining area ranging from 1028.87 ± 55 69 to 6793.93 ± 797.15 mg/kg and in the control area ranging between 245.89 ± 36.76 and 57.04 ± 15.18. The highest Fe accumulation was found in F. hispida leaves in the mining area (6793.93 ± 797.15 mg/kg) and in C. tomentosa leaves in the control area (245.89 ± 36.76 mg/kg). Al accumulation ranged between 159.43 ± and 1358.70 ± mg/kg at the mining area. The highest Al accumulation was detected in F. hispida followed by M. peltata (833.07 ± mg/kg), and the lowest was detected in W. fruticosa followed by T. orientalis (183.20 ± mg/kg). Compared with the control area, the highest accumulation was found in F. hispida (5.23 ± mg/kg) and the lowest was found in T. orientalis (0.62 ± mg/kg). The highest content of Cr accumulation was found in F. hispida (432.27 ± mg/kg) followed by M. peltata (328.03 ± mg/kg), and the lowest accumulation was found in C. tomentosa (48.97 ± mg/kg) followed by B. orellana (52.83 ± mg/kg). The maximum accumulation of Ni was found in C. roxburghii (64.57 ± mg/kg) followed by M. peltata (62.57 ± mg/kg).
Metal accumulation index
MAI is used to evaluate the heavy metal accumulation capacities in plants using a standard formula (Hu et al. 2014; Karmakar and Padhy 2019). The MAI values of the analysed plant species are shown in Table 5. At the mining area, MAI ranged from 4.5 (W. fruticosa) to 17.29 (C. tomentosa). The trend followed was C. tomentosa (17.29) > F. hispida (10.31) > M. peltata (10.16) > H. pubescens (9.74) > A. excelsa (7.46) > T. arjuna (7.05) > T. orientalis (6.89) > B. orellana (5.17) > W. fruticosa (4.5). At the control area, the trend followed was F. hispida (6.38) > C. roxburghii (3.4) > T. arjuna (3.17) > M. peltata (3.15) > C. tomentosa (2.88) > A. excelsa (2.82) > W. fruticosa (2.57) > H. pubescens (2.34) > T. orientalis (2.26) > B. orellana (1.94). In both areas, F. hispida had a higher MAI value (mining area = 10.31, control area = 6.38), indicating that it has higher metal accumulation capacity. However, the MAI of A. excelsa (7.46) in the Sukinda chromite mine area is higher than that previously reported by Roy et al. (2020) in an industrial area (2.13) and a commercial area (2.03). In another study in China, a similar kind of result was found by Hu et al. (2014). The MAI value of Sophora japonica in Beijing (9.0) was higher than that reported in Yan’an city (2.56). Nadgórska-Socha et al. (2017) and Karmakar and Padhy (2019) also found a positive correlation between heavy metal uptakes in plant leaves and their concentration in soil. So, this fluctuation of MAI value could be due to a higher metal concentration in mining areas compared previously with the industrial and commercial areas. Plant species with higher MAI values have a better metal accumulation capacity and considered metal-tolerant species; those species can use an impediment between contaminated and uncontaminated areas. Those plant species that have a lower MAI value are considered sensitive to metal and can be used as bioindicators of metal pollution.
Conclusion
Based on dust capturing capacities and metal accumulation index as well as APTI and API, F. hispida, M. peltata and H. pubescens can be used as tolerant species and could be recommended for greenbelt development to mitigate pollution. Those plant species (A. excelsa, B. orellana, C. roxburghii, W. fruticosa, T. arjuna, T. orientalis) have lower APTI and API values which could be used as sensitive species for biomonitoring of environmental pollution. Our study also finds that C. tomentosa is the highest metal accumulation capacities, but according to the anticipated performance index it is a poor performer towards pollution. So, these species could be used as hyper-accumulator to mitigate heavy metal contamination from air and soil. The physiological and biochemical responses of plants vary from species to species and also from area to area. But more biochemical and physiological research is needed to confirm the effect and response of plant species in a particular pollution stress condition.
Data availability
All data generated or analysed during this study are included in this published article.
References
Achakzai K, Khalid S, Adrees M, Bibi A, Ali S, Nawaz R, Rizwan M (2017) Air pollution tolerance index of plants around brick kilns in Rawalpindi, Pakistan. J Environ Manag 190:252–258
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol 24(1):1
Barceló JUAN, Poschenrieder C (1990) Plant water relations as affected by heavy metal stress: a review. J Plant Nutr 13(1):1–37
Bharti SK, Trivedi A, Kumar N (2018) Air pollution tolerance index of plants growing near an industrial site. Urban Climate 24:820–829
Black Smith Institute Report (2007) The world’s worst polluted places. A project of Blacksmith Institute, pp 16–17
Chaudhary IJ, Rathore D (2019) Dust pollution: its removal and effect on foliage physiology of urban trees. Sustain Cities Soc 51:101696
Das PK, Das BP, Dash P (2021) Chromite mining pollution, environmental impact, toxicity and phytoremediation: a review. Environmental Chemistry Letters 19(2):1369–1381
Dhal B, Das NN, Pandey BD, Thatoi HN (2011) Environmental quality of the Boula-Nuasahi chromite mine area in India. Mine Water Environ 30(3):191–196
Escobedo FJ, Wagner JE, Nowak DJ, De la Maza CL, Rodriguez M, Crane DE (2008) Analyzing the cost effectiveness of Santiago, Chile’s policy of using urban forests to improve air quality. J Environ Manag 86(1):148–157
Gheorghe IF, Ion B (2011) The effects of air pollutants on vegetation and the role of vegetation in reducing atmospheric pollution. Impact Air Pollut Health Econ Environ Agric Sour 29:241–80
Golui D, Datta SP, Dwivedi BS, Meena MC, Trivedi VK, Jaggi S, Bandyopadhyay KK (2021) Assessing geoavailability of zinc, copper, nickel, lead and cadmium in polluted soils using short sequential extraction scheme. Soil Sediment Contam Int J 30(1):74–91
Gupta GP, Kumar B, Kulshrestha UC (2016) Impact and pollution indices of urban dust on selected plant species for green belt development: mitigation of the air pollution in NCR Delhi. India Arab J Geosci 9(2):136
Hu Y, Wang D, Wei L, Zhang X, Song B (2014) Bioaccumulation of heavy metals in plant leaves from Yan׳ an city of the Loess Plateau, China. Ecotoxicol Environ Saf 110:82–88
Javanmard Z, Kouchaksaraei MT, Hosseini SM, Pandey AK (2020) Assessment of anticipated performance index of some deciduous plant species under dust air pollution. Environ Sci Pollut Res 27(31):38987–38994
Karakoti N, Bajpai R, Upreti DK, Mishra GK, Srivastava A, Nayaka S (2014) Effect of metal content on chlorophyll fluorescence and chlorophyll degradation in lichen Pyxine cocoes (Sw.) Nyl.: a case study from Uttar Pradesh, India. Environ Earth Sci 71(5):2177–2183
Karmakar D, Padhy PK (2019) Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 237:124522
Karmakar D, Deb K, Padhy PK (2021) Ecophysiological responses of tree species due to air pollution for biomonitoring of environmental health in urban area. Urban Climate 35:100741
Kaur M, Nagpal AK (2017) Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ Sci Pollut Res 24(23):18881–18895
Khazini L, Dehkharghanian ME, Vaezihir A (2022) Dispersion and modeling discussion of aerosol air pollution caused during mining and processing of open-cast mines. Int J Environ Sci Technol 19(2):913–924
Kong SSK, Fu JS, Dong X, Chuang MT, Ooi MCG, Huang WS, Lin NH (2021) Sensitivity analysis of the dust emission treatment in CMAQv5. 2.1 and its application to long-range transport over East Asia. Atmos Environ 257:118441
Lèbre É, Corder G, Golev A (2017) The role of the mining industry in a circular economy: a framework for resource management at the mine site level. J Ind Ecol 21(3):662–672
Leonard RJ, McArthur C, Hochuli DF (2016) Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For Urban Green 20:249–253
Liu YJ, Ding HUI (2008) Variation in air pollution tolerance index of plants near a steel factory: implication for landscape-plant species selection for industrial areas. WSEAS Trans Environ Dev 4(1):24–32
Manara A (2012) Plant responses to heavy metal toxicity. In Plants and heavy metals (pp. 27–53). Springer, Dordrecht
Mo L, Ma Z, Xu Y, Sun F, Lun X, Liu X, Chen J, Yu X (2015) Assessing the capacity of plant species to accumulate particulate matter in Beijing, China. Plos One 10(10):140664
Molnár VÉ, Simon E, Tóthmérész B, Ninsawat S, Szabó S (2020) Air pollution induced vegetation stress–the air pollution tolerance index as a quick tool for city health evaluation. Ecol Indic 113:106234
Mondal D, Gupta S, Datta JK (2011) Anticipated performance index of some tree species considered for green belt development in an urban area. Int Res J Plant Sci 2(4):99–106
Nadgórska-Socha A, Kandziora-Ciupa M, Trzęsicki M, Barczyk G (2017) Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere 183:471–482
Nayak S, Kale P (2020) A review of chromite mining in Sukinda Valley of India: impact and potential remediation measures. Int J Phytorem 22(8):804–818
Ni ZZ, Luo K, Zhang JX, Feng R, Zheng HX, Zhu HR, Wang JF, Fan JR, Gao X, Cen KF (2018) Assessment of winter air pollution episodes using long-range transport modeling in Hangzhou, China, during World Internet Conference, 2015. Environ Pollut 236:550–561
Norouzi S, Khademi H, Cano AF, Acosta JA (2015) Using plane tree leaves for biomonitoring of dust borne heavy metals: a case study from Isfahan, Central Iran. Ecol Ind 57:64–73
Oluwoye I, Dlugogorski BZ, Gore J, Oskierski HC, Altarawneh M (2017) Atmospheric emission of NOx from mining explosives: a critical review. Atmos Environ 167:81–96
Rai PK, Panda LL (2014) Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Qual Atmos Health 7(1):93–101
Rai PK (2016) Impacts of particulate matter pollution on plants: implications for environmental biomonitoring. Ecotoxicol Environ Saf 129:120–136
Remon E, Bouchardon JL, Le Guédard M, Bessoule JJ, Conord C, Faure O (2013) Are plants useful as accumulation indicators of metal bioavailability? Environ Pollut 175:1–7
Roy A, Bhattacharya T, Kumari M (2020) Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci Total Environ 722:137622
Safari F, Akramian M, Salehi-Arjmand H, Khadivi A (2019) Physiological and molecular mechanisms underlying salicylic acid-mitigated mercury toxicity in lemon balm (Melissa officinalis L.). Ecotoxicol Environ Saf 183:109542
Sahu C, Basti S, Sahu SK (2021) Particulate Collection Potential of Trees as a Means to Improve the Air Quality in Urban Areas in India. Environ Process 8(1):377–395
Samecka-Cymerman A, Kempers AJ (1999) Bioindication of heavy metals in the town Wrocław (Poland) with evergreen plants. Atmos Environ 33(3):419–430
Selmi W, Weber C, Rivière E, Blond N, Mehdi L, Nowak D (2016) Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For Urban Green 17:192–201
Serbula SM, Kalinovic TS, Ilic AA, Kalinovic JV, Steharnik MM (2012) Assessment of airborne heavy metal pollution using Pinus spp. and Tilia spp. Aerosol Air Qual Res 13(2):563–573
Singh SK, Rao DN, Agrawal M, Pandey J, Naryan D (1991) Air pollution tolerance index of plants. J Environ Manage 32(1):45–55
Singh SN, Verma A (2007) Phytoremediation of air pollutants: a review. Environ Bioremed Technol 293–314
Wang Y, Zhao H, Qin H, Li Z, Liu H, Wang J, Zhang H, Quan R, Huang R, Zhang Z (2018) The synthesis of ascorbic acid in rice roots plays an important role in the salt tolerance of rice by scavenging ROS. Int J Mol Sci 19(11):3347
WHO (1996) Denneman and Robberse 1990. Ministry of Housing, Netherlands, p 1994
Xiu Z, Nie W, Yan J, Chen D, Cai P, Liu Q, Du T, Yang B (2020) Numerical simulation study on dust pollution characteristics and optimal dust control air flow rates during coal mine production. J Clean Prod 482:119197
Acknowledgements
The authors are thankful to Director of CSIR-IMMT, Bhubaneswar, Odisha, India, for providing laboratory facility for the work. Mr. K Mandal is also thankful to the University Grand Commission (UGC), India, for providing fellowship as a Ph.D. scholar.
Author information
Authors and Affiliations
Contributions
1. Nabin Kumar Dhal: (corresponding author)
Contribution: conceptualization of the work, supervision, preparing the manuscript.
2. Kalicharan Mandal: (first author)
Contribution: methodology, sample collection, data analysis and interpretation, preparation of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mandal, K., Dhal, N.K. Pollution resistance assessment of plants around chromite mine based on anticipated performance index, dust capturing capacity and metal accumulation index. Environ Sci Pollut Res 29, 63357–63368 (2022). https://doi.org/10.1007/s11356-022-20246-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20246-6