Abstract
Acacia jacquemontii possess has numerous traditional therapeutic uses. The rationale of this study was to investigate the role of Acacia jacquemontii ethyl acetate extract (AJEAE) in the downregulation of hyperglycemia. The current study was performed in two parts, in vitro, through characterization (high-performance liquid chromatography), estimation of total phenolic content, total flavonoid content, antioxidant (2,2-diphenyl-1-picrylhydrazylassay), and α-amylase inhibitory activities of the studied extract, and in vivo using Wistar rats in which animals were divided into five groups NC, DC, GL, AJEAE 250 mg/kg, and AJEAE 500 mg/kg. The effects of AJEAE on fasting plasma glucose, plasma insulin, HOMA-IR, oral glucose tolerance test, glycated hemoglobin (HBA1c), lipid profile, inflammatory cytokines (Interleukin-6, tumor necrosis factor-alpha), and oxidative stress markers (lipid peroxidation, nitic oxide, superoxide dismutase, catalase, glutathione peroxidase) were evaluated. Our findings confirmed the presence of quercetin, kaempferol, gallic acid, vanillic acid, syringic acid, M-coumaric acid, sinapic acid, chlorogenic acid, cinnamic acid, and ferulic acid in AJEAE. Total flavonoid and phenolic contents in AJEAE were 83.83 mg GAE/g and 77.06 mg QE/g, respectively. Significant inhibition of DPPH (69.470%/1 mg/ml) and α-amylase (71.8%/1 mg/ml) activities were exhibited by AJEAE. Alloxan-injected rats showed marked hyperglycemia and hypoinsulinemia, and increased inflammatory marker levels as compared to normal control (p < 0.001). Additionally, raised levels of triglyceride (139.7 ± 2.771), total cholesterol (198.7 ± 1.856), very low-density lipoprotein (33.43 ± 0.2728), low-density lipoprotein (155.5 ± 2.754), lipid peroxidation, and nitric oxide (p < 0.001) and decreased levels of high-density lipoprotein (17.20 ± 0.1732), superoxide dismutase, catalase, and glutathione peroxidase were observed in diabetic rats (p < 0.001). AJEAE significantly (p < 0.05) improved the aforementioned parameters and the protective efficacy was comparable to glibenclamide. Histopathological findings also evidenced the anti-hyperglycemic properties of AJEAE through regeneration of pancreatic β cells. Conclusively, our findings demonstrated the antihyperglycemic, antihyperlipidemic, antioxidant, anti-inflammatory, and pancreatic beta β cell regenerative properties of AJEAE against alloxan-induced diabetes.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a serious and multifaceted metabolic disorder of multiple etiologies, a global public health problem, and is now emerging as an epidemic worldwide, with intense consequences, both acute and chronic (Salehi et al. 2019; Majeed et al. 2021). Data from the International Diabetes Federation (IDF) have expected that 451 million adults are living with diabetes worldwide in 2017 and this number is projected to increase to 693 million by 2045 if no effective prevention methods are adopted (Lin et al. 2020). Diabetes and its associated micro and macrovascular complications have affected approximately 25% of the world population, so management of diabetes is becoming a socioeconomic challenge world wild (Arumugam et al. 2013; Ononamadu et al. 2019). Hyperglycemia, hyperlipidemia, oxidative stress, suppression of antioxidant defense markers, and inflammation are the main consequences of diabetes mellitus (Hammeso et al. 2019). Genetic and environmental factors are also responsible for the development of diabetes in which body cells cannot break down sugar properly due to diminished action of insulin on target tissues resulting in lack of insulin or resistance to insulin (Salehiet al. 2019).
Multiple antidiabetic regimens are used with different mechanisms to counteract the increased level of glucose. However, long-term usage and side effects of available treatment options have increased the demand for novel therapeutically effective agents with minimum side effects for the management of diabetes (Choudhury et al. 2018; Majeed et al. 2018). Medicinal plants have a long history of usage and are valuable sources of new drugs globally (Chen et al. 2016; Calixto 2019). From different regions of the world, different parts of the plants have been investigated for antidiabetic activity as the different plants contain phenols, carotenoids, flavonoids, terpenoids, alkaloids, and glycosides (Moradi et al. 2018). Herbal medicines are most commonly prescribed worldwide due to their easy availability, relatively minimal side effects, reasonable price, and therapeutic efficacy Khan et al. (2018).
The majority of Acacia species are reported to possess pharmacological activities and are effective against a variety of diseases. Acacia jacquemontii Benth locally called Bhu-banwali, Baonli, or Bhunwaliand is native to the “Thar desert” of the Indo-Pak subcontinent. In Pakistan, it is known as “Bable” or “kikri” (Ashfaq et al. 2016; Rasool et al. 2016). It was used in the past by Greek practitioners to treat common ailments, i.e., stomach pain, kidney stones or disorders, toothache, chickenpox, sexual weakness, and controlling inflammation (Choudhary et al. 2009; Rasool et al. 2017). The current study was aimed to characterize the AJEAE using high-performance liquid chromatography (HPLC) to determine the presence of different bioactive constituents as well as hypothesized to investigate the ameliorative impact of AJEAE on glycemia, lipidemia, antioxidant status, inflammatory markers, and pancreatic beta cell apoptosis in alloxan-induced hyperglycemia in rats. To our knowledge, no study has so far been reported to have investigated the anti-hyperglycemic potential of “Acacia jacquemontii.”
Materials and methods
Plant collection and extraction
The leaves of Acacia jacquemontii were collected from district Bhakkar near Bahawalpur, Pakistan. The plant was authenticated by the Cholistan institute of desert Studies (CIDS) from the Islamia University of Bahawalpur, Pakistan, with voucher number CIDS/ IUB-1901/63. Leaves were cleaned, air-dried under shade, and finally were grounded into a coarse powder using an electric grinder. About 100 gm of powdered material was macerated with ethyl acetate at the ratio of 1:4 (W/V) at room temperature with occasional shaking and stirring for 7 days. After that, whole mixture was filtered through filter paper and then was concentrated by using a rotary evaporator.
Characterization of AJEAE using HPLC
High-performance liquid chromatography analysis was performed for the detection of bioactive compounds. Stationary phase C18 (5.0 µM) (25 cm × 4.6 mm) and SIL-20A autosampler (Shimadzu Scientific Instruments, Kyoto, Japan) were used. A combination of acetic acid and acetonitrile was used as the mobile phase. The flow rate used for analysis was 1 mL/min. UV–visible detector (SPD-10AV) was used for the detection of bioactive compounds at the wavelength of 280 nm. Identification and quantification were done by comparison with a mixture of standards (Imtiaz et al. 2019).
Evaluation of total phenolic and total flavonoid content in AJEAE
The total phenolic content (TPC) was estimated by using Folin-Ciocalteu method. After preparation of the reaction mixture, absorbance was measured at 760 nm wavelength and the results were presented as mg GAE/g (Aryal et al. 2019). The total flavonoid content (TFC) was assessed by the Aluminum chloride colorimetric method. The absorbance was measured at 510 nm wavelength and the results were displayed as mg QE/g of DW (Phuyal et al. 2020).
Evaluation of in vitro antioxidant activity (DPPH assay)
The free radical scavenging activity of AJEAE was measured using DPPH as a free radical model (MogoleL and Mtunzi 2020; Ashraf et al. 2015). Different concentrations of plant extract (0.125–1 µg/mL) were prepared. A total of 1 ml of plant extract was mixed with 3 ml of 5 µg/mL DPPH and then incubated in the dark. Ascorbic acid was used as standard; absorbance was taken at 515 nm using a spectrophotometer, and activity was measured by using the formula below:
% Inhibition = [(A blank − Asample)/Ablank]/100%
In vitro α-amylase inhibition assay
Alpha-amylase inhibitory assay was done to check the in vitro antidiabetic activity of AJEAE (Sangeetha and Vedasree2012; Sathasivampillai et al. 2017). A total of 100 µL of plant extract was allowed to react with 100 µL of 2 mM of phosphate buffer and 200 µL of α-amylase enzyme. The reaction mixture was allowed to incubate for 20 min and then added 100 µL of starch solution (1%). A similar protocol was carried out for the controls where the buffer was used instead of the enzyme. After 5 min of incubation, the dinitro-salicylic acid reagent (500 µL) was mixed in the control and test, and then placed in a boiling water bath for at least 5 min. The absorbance was measured at 540 nm using a spectrophotometer. The inhibitory activity was found by using the equation given below:
% Inhibition = Absorbance of the control − Absorbance of the test sample/Absorbance of the control × 100.
Experimental animals and ethical statements
Seventy-five albino Wistar rats (180–200 g body weight) were caged in the animal house of the Institute of Physiology and Pharmacology, University of Agriculture Faisalabad. Before the start of trail, all rats were acclimatized for 2 weeks. The experimental protocol was planned according to laboratory animal care guidelines permitted by the Graduate studies Research Board, UAF Pakistan. An ethical certificate was issued by the institutional biosafety and bioethics committee with letter no. 1739/ORIC for the conduct of an in vivo experiment. Following the adaptation time, all rats were allocated into five groups, each group having 15 rats.
Induction of experimental diabetes
Alloxan monohydrate (i.p) in 0.9% w/v NaCl was used to induce diabetes (150 mg/kg of BW) in all groups except the normal control group (Majeed et al. 2021). Subsequent to 1st week of the study, glucose levels were measured from all rats according to the tail vein method using On-Call Plus (catalog # G113-214√) glucometer. Rats that showed blood glucose levels higher than 300 mg/dl were confirmed to be diabetic and included in the study (Majeed et al. 2021).
Treatment protocol and sample collection
All rats were divided into the following groups (n = 15/group). Group1: normal control (NC) = daily routine diet + water ad libitum, Group 2: diabetic control (DC), Group 3: treated with (GL) glibenclamide (10 mg/kg) p.o, Group 4: treated with AJEAE (250 mg/kg) p.o, and Group 5: treated with AJEAE (500 mg/kg)p.o (Ashfaq et al. 2016). After completion of the 28th day of the study, all rats were made overnight fast, anesthetized (i.p. 3% sodium pentobarbital), and then sacrificed. Blood samples were obtained, then centrifuged for 10 min at 4000 rpm for serum and for 20 min at 2000 rpm for plasma sample, and obtained samples were stored at − 80 °C for biochemical studies. For histopathological analysis, pancreatic tissues were preserved in a 10% NBF solution. For biochemical investigations, tissue homogenates were prepared by homogenizing the pancreatic and hepatic tissues in a buffer solution containing 50 mMTris-HCl & 1.15% KCl.
Estimation of serum glycemic markers
For assessment of glucose overloading, an oral glucose tolerance test (OGTT) was performed at the end of the trial. After 16 h of overnight fasting, all groups of rats were treated with their respective treatments. Then 30 min following each treatment, rats were loaded with 4 g/kg glucose solution. Blood samples were collected via tail prick method and serum glucose levels for each rat at 1, 3, and 5 h of the treatment were measured using a glucose assay kit (Sudasinghe and Peiris 2018). The prime hallmark of DM is insulin. Fasting plasma insulin levels were estimated by using an ELISA kit (Thermo Fisher Scientific Catalog# ERINS). Fasting plasma glucose levels of all groups were measured by using a rat glucose assay kit of Crystal Chem, USA # 81,693. According to International Diabetes Federation (IDF), HbA1c is a reliable diagnostic biochemical marker for diabetes. Glycated hemoglobin (HbA1c) was assessed by ELISA kit (Rat HbA1c ELISA kit catalog # MBS2509196). Estimation of insulin resistance was carried out by using the homeostasis model assessment method, HOMA-IR (Chao et al. 2018), and was calculated using the following formula:
Fasting glucose (mmol/L) × fasting insulin (µU/mL)/22.5
Estimation of serum lipid profile
One of the complications observed after diabetic hyperglycemia is dyslipidemia, the serum lipid profile of rats was evaluated in this study. Triglycerides (Rat Triglyceride ELISA Kit, Catalog BS726298), high-density lipoprotein (Rat High-Density Lipoprotein (HDL) ELISA Kit, Catalog MBS26654), low-density lipoprotein (Rat LDL-Cholesterol Assay Kit, Catalog 79,960), total cholesterol (Rat Total cholesterol ELISA Kit, Catalo MBS846775), and very-low-density lipoprotein (Rat very-low-density lipoprotein (VLDL) ELISA Kit, MBS706188) were analyzed according to the manufacturer’s protocol.
Estimation of serum inflammatory markers
Hyperglycemia caused high levels of ROS and pro-inflammatory cytokines, so serum cytokines (TNF-α, IL-6) were measured by commercially available ELISA kits (RayBio® Rat, RayBiotech, Norcross, GA, USA) according to the instruction of the manufacturer.
Estimation of oxidative stress markers
Hyperglycemia induced oxidative stress in terms of an increased generation of reactive oxygen species and suppression of antioxidant defenses system. Oxidative stress was assessed in pancreatic and hepatic tissue homogenates by estimation of lipid peroxidation (malondialdehyde; MDA) using Rat LPO ELISA Kit (Catalog No: MBS2515688) and nitric oxide (NO) level by using nitric oxide Colorimetric Assay Kit (Catalog No: E-BC-K035-M) according to manufacturer’s protocol. Catalase (CAT) activity was measured by using Rat Catalase ELISA kit (Catalog No: MBS726781); CAT ELISA kit applies the quantitative sandwich enzyme immunoassay technique; glutathione peroxidase (GPx) level was accessed by using Rat Glutathione Peroxidase ELISA kit (Catalog Number: MBS744364); the procedure followed the manufacturer’s protocol (Competitive ELISA). Superoxide dismutase (SOD) level was measured by using Rat Superoxide Dismutase ELISA Kit (Catalog No: MBS036924) according to the manufacturer’s protocol; this is quantitative sandwich Elisa kit.
Histopathological analysis
For histopathological analysis, portions of pancreatic tissues were fixed in formalin (10%) for 1 day. Following the fixation, tissues were dehydrated and paraffinized in wax. Serial sections were made via microtomy and stained with H&E dyes for microscopic examination (IRMECO GmbH & Co, no: IM-91) at a magnification power of 40 × , and images were captured using a digital camera inbuilt with the microscope.
Statistics
All data represent at least three autonomous experiments and results were shown as mean ± S.E. Statistically data were analyzed by analysis of variance (ANOVA) followed by Duncan multiple ranges (Graph Pad Prism Software, version 8.0.1, 244). All p values < 0.05 were considered statistically significant.
Results
Characterization of AJEAE by using HPLC
The flavonoids and phenolic fingerprint of the AJEAE are presented in Fig. 1. The HPLC results of AJEAE revealed the occurrence of different flavonoids and phenolics with 30 peaks and retention times ranging from 2.74 to 31.14 min. Based on the retention times and spectral data, AJEAE showed a UV band at 280 nm characteristics for flavonoids and phenolic compounds, possibly quercetin > ferulic acid > sinapic acid > chlorogenic acid > vanillic acid > syringic acid > gallic acid > Kaempferol > M-coumaric acid > cinnamic acid (Table 1, Fig. 1).
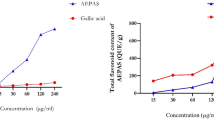
Total phenol and flavonoid contents
Total phenolic contents in AJEAE were determined by using gallic acid as the standard. TPC was 77.06 mg GAE/g. Total flavonoid contents of the AJEAE were determined by using quercetin as standard. TFC was 83.83 mg QE/g.
DPPH activity
The antioxidant activity of AJEAE was assessed on the basis of their capability to scavenge stable free DPPH radicals. The results clearly specified that AJEAE inhibited free radical generation based on the concentration used (Table 2). AJEAE showed percent inhibition of 69.470 at maximum concentrations of 1 mg/ml with IC 50 value of 0.77 mg/ml (+ / − SEM). Reference standard ascorbic acid showed an IC 50 value of 0.54 mg/ml (+ / − SEM).
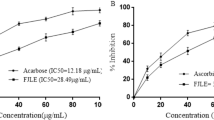
Alpha-amylase inhibition activity
All concentrations of AJEAE were tested on the α-amylase enzyme. The α-amylase activity of the AJEAE exhibited inhibitions of 71.8% at a maximum concentration of 1 mg /ml with an IC 50 value of 0.51 (+ / − SEM). The IC 50 value for standard (acarbose) was 0.29 mg/ml (+ / − SEM) (Table 3).
Effect of AJEAE on glycemic markers
In OGTT, GL and AJEAE induced a significant reduction in serum glucose levels from 1 to 5 h (p < 0.01). Fasting plasma insulin levels were significantly (p < 0.001) elevated in GL- and AJEAE–treated rats in comparison to the diabetic control group. Fasting plasma glucose and HbA1c levels were noticeably (p < 0.001) augmented in all diabetic rats in comparison to the normal control group. However, administration of GL- and AJEAE–graded doses reduced the levels of plasma glucose and HbA1c levels dose-dependently in comparison to the diabetic control group. Insulin resistance leads to a subsequent increase in fasting insulin levels as the disease grows. An increase in fasting insulin is one of the most important signs of insulin resistance in the present study. HOMA-IR is a biomarker of insulin resistance. GL and AJEAE restored HOMA-IR values to near normal (Fig. 2).
Effect of AJEAE on glycemic markers. ### shows p < 0.001, * represents significance at p < 0.05, ** represent significance at p < 0.01, *** represent significance at p < 0.001. Abbreviations: NC: normal control, DC: diabetic control, GL: glibenclamide (10 mg/kg), AJEAE: A. jacquemontii ethyl acetate extract
Effects of AJEAE on serum lipid profile
Substantial (p < 0.05) raise in serum TC, TG, LDL, and VLDL while a decrease in HDL levels was detected in the alloxan-treated group rats compared to the control group. Furthermore, AJEAE treatment especially at high dose (500 mg/kg) showed an anti-lipidemic effect and produced a significant (p < 0.05) reduction in TC, TG, VLDL, and LDL and an increase in HDL levels as compared to the DC group, thus indicating the more pronounced antilipidemic effects of AJEAE high dose (500 mg/kg (Table 4).
Effects of AJEAE on serum inflammatory markers
Figure 3 describes the inflammatory response of hepatic and pancreatic tissues in all groups. Results showed significant (p < 0.001) elevation of TNF-α and IL-6 levels after diabetes induction compared to the control group. Though, GL and AJEAE (250 mg/kg; 500 mg/kg) treatment significantly (p < 0.001) decreased the inflammatory markers compared to diabetic group dose-dependently.
Effects of AJEAE on oxidative stress markers
As shown in Figs. 4 and 5, significant (p < 0.001) decline was observed in hepatic and pancreatic antioxidant enzymes (SOD, GPx, and CAT) in alloxan-treated group compared to the control group. However, GL and AJEAE (250 mg/kg; 500 mg/kg) treatments resulted in a remarkable increase in the antioxidant levels with respect to the diabetic group (p < 0.001). About non-enzymatic oxidative stress markers, a substantial increase of LPO and NO contents in hepatic and pancreatic tissues were observed in the diabetic group in comparison with the control group. However, treatment with GL and AJEAE (250 mg/kg; 500 mg/kg) in diabetic rats decreases LPO and NO levels in both tissues with respect to the diabetic group.
Effect of AJEAE on hepatic oxidative stress markers. ### shows p < 0.001, * represents significance at p < 0.05, ** represent significance at p < 0.01, *** represent significance at p < 0.001. Abbreviations: NC: normal control, DC: diabetic control, GL: glibenclamide (10 mg/kg), AJEAE: A. jacquemontii ethyl acetate extract
Effect of AJEAE on pancreatic oxidative stress markers. ### shows p < 0.001, * represents significance at p < 0.05, ** represent significance at p < 0.01, *** represent significance at p < 0.001. Abbreviations: NC: normal control, DC: diabetic control, GL: glibenclamide (10 mg/kg), AJEAE: A. jacquemontii ethyl acetate extract
Histopathological results
The pancreatic tissues of the NC group showed a normal structure with contact islets of Langerhans (Fig. 6a) while in the DC group the islets of Langerhans exhibited signs of atrophy as well as shrinkage, additional severe damage to the exocrine part. However, GL-treated rats showed improvement in the histopathological changes of islets of Langerhans showing improved β-cell concentration/mass. AJEAE–treated groups preserved the pancreatic tissues, efficiently attenuated the pancreatic lesions, and improved β-cell mass. Damage to the pancreatic β-cells is the main symptom in diabetes and consequently caused impairment of insulin production. Our findings suggest that AJEAE improved the histoarchitecture of pancreatic beta cells and insulin release dose-dependently (Fig. 6a-e).
A–E Representative photomicrograph of pancreatic tissue sections (H&E, × 40). A Normal control (NC) rats showing preserved histoarchitecture of pancreatic tissues with normal islets of Langerhans and exocrine element of pancreas. The islets of Langerhans contained normal looking β-cells concentration. B Diabetic control (DC) rats showed destructed/damaged histoarchitecture of pancreatic tissues with abnormal/squeeze islets of Langerhans. The islets of Langerhans showed reduced β-cells concentration/mass compared to NC group. C GL-treated rats showing improvement of the histopathological changes of islets of Langerhans showing improved β-cells concentration/mass compared to DC group as shown in the figure. It is also showing slightly normal acinar cells. D After treatment of rats with AJEAE (250 mg/kg), islet of Langerhans was present in the endocrine element having moderate number of β-cells and acinar cell concentration. E Rats treated with AJEAE (500 mg/kg) showing pancreatic tissue revealed normal looking endocrine and exocrine elements. Endocrine part (islet of Langerhans) was improved and normal looking β-cell concentration is better than rats treated with AJEAE (250 mg/kg). IRMECO GmbH & Co, no: IM-91, scale bar = 40 µm
Discussion
Diabetes mellitus is accompanied by noteworthy changes in lipid and glucose metabolism and the stimulation of oxidative stress which are contributed to the development of DM–related complications (Albasher et al. 2020). Different lifestyle and dietary factors including physical inactivity, weight gain, obesity, and low fiber diet play a substantial role in diabetes development (Idm’hand et al. 2020). Our findings revealed the occurrence of various flavonoids and phenols in AJEAE (Table 1, Fig. 1). Flavonoids are major bioactive compounds and according to previous literature, they have the property to inhibit cell damage as -cell damage is the main factor for the development of diabetes (Manach2004; Wang et al. 2018a, b). A higher intake of total flavonoids has been linked with a lower risk of diabetes in several human studies (Cao et al. 2019). Our results indicated that AJEAE contains a high concentration of flavonoids, i.e., quercetin and kaempferol which have strong anti-oxidant and anti-inflammatory activities (Gavamukulya et al.2014).
Supporting the results of preceding research studies, ferulic acid and sinapic acid have antioxidant, anti-inflammatory, anti-microbial, anticancer, and antidiabetic effects (Zduńska et al. 2018; Chen 2016). Chlorogenic acid is proven to possess antioxidant, anti-inflammatory, antibacterial, and free radical scavenger activities and also has the property to improve glucose homeostasis (Majeed et al.2021; Naveed et al. 2018). Vanillic acid ameliorates hyperglycemia-induced oxidative stress and inflammation (Ji et al.2020) and syringic acid reduces oxidative damages (Sabahi et al. 2020). Gallic acid increases insulin release and has antioxidant and anti-inflammatory properties (Majeed et al. 2021; Kahkeshani et al. 2019). Cinnamic acid is linked with a beneficial influence on the management of diabetes and its complications (Adisakwattana2017). Interestingly, all the phenol and flavonoid compounds detected in AJEAE are responsible to be therapeutically effective against diabetes due to their antioxidant and anti-hyperglycemic activities.
In vitro antioxidant activity of AJEAE was evaluated by DPPH. The result of the present study revealed that AJEAE contains bioactive compounds with a high capability to scavenge free radicals. These phytochemicals could be phenolic and flavonoids which might have potent antioxidant activities. Antidiabetic potential (in vitro) was evaluated by α-amylase inhibition assay; α-amylase is a carbohydrate-digesting enzyme required to hydrolyze complex polysaccharides to simple sugars. We found that AJEAE exhibited 71.8% inhibition of α-amylase enzyme activity (Table 3). This enzyme inhibition has been confirmed to be an effective approach to controlling postprandial sugar levels (Ononamadu et al. 2019). These results indicated that the study plant could demonstrate hypoglycemic activity possibly by inhibition of pancreatic α-amylase.
In the current study, alloxan monohydrate induced diabetes by direct damage to pancreatic β-cells, resulting in loss of insulin secretion and hyperglycemia. We found a remarkable reduction in plasma glucose and improvement in plasma insulin levels in AJEAE– and GL-treated groups (Fig. 2, p < 0.001). To measure insulin sensitivity, HOMA-IR was conducted. The results demonstrated that HOMA-IR in diabetic rats was increased (p < 0.001). However in AJEAE– and GL-treated groups, HOMA-IR significantly decreased (p < 0.001). OGTT is the measure of the body’s ability to utilize glucose that serves as a standard procedure for the diagnosis of borderline diabetic patients in the clinical setup (Alema et al. 2020). Diabetic rats showed statistically elevated blood glucose levels compared to NC rats and AJEAE and treated rats (p < 0.001). The hypoglycemic activity of AJEAE may refer to the inhibition of free radical species–formation induced by alloxan. In agreement with previous research studies, plant extracts containing high flavonoids and polyphenol compounds have the potential to increase insulin secretion, the regenerative potential of β-cells, by inhibiting ATP–sensitive K + channels like glibenclimide (Arunachalam and Parimelazhagan 2014). Flavonoids have the property to inhibit cAMP phosphodiesterase which is responsible for insulin secretion (Albasher et al. 2020). To assess the long-term glycemic control during diabetic treatment, HbA1c is one of the most important markers (Yazdanpanahet al. 2017; Chehregoshaet al. 2019). WHO has also recommended adopting HbA1c as an index to diagnose diabetes in countries and regions where conditions were amenable (Wang et al. 2018a, b). This study observed a significant decrease in HbA1c levels in GL- and AJEAE–treated groups (Fig. 2).
Diabetes mellitus is connected with augmented morbidity and mortality that result from cardiovascular diseases (Lamacchia and Sorrentino 2021). In DM, the metabolism of lipids is also disturbed. Several studies reported that hyperglycemia in STZ-induced diabetic animals caused dyslipidemia (El-Badawy et al. 2019). An altered lipid profile is known to establish danger for atherosclerosis in diabetes. So, control of lipid profile is also vital along with glucose reduction to reduce the risk of diabetes (Albasher et al. 2020). However, treatment with AJEAE stabilized the lipid profile through a reduction in TC, TG, LDL, and VLDL as well as a significant rise in HDL levels in a dose-dependent manner (p < 0.001). The hypolipidemic effect of AJEAE may mention the bioactive compounds, flavonoids, and phenols, which potentiate the release of insulin from pancreatic β-cells and improve glucose oxidation (Table 4).
Oxidative stress is a proposed mechanism for the initiation and development of diabetes, as hyperglycemia is strongly linked with increased superoxide generation via the mitochondrial system (Hassan 2015; Tiwari et al. 2013). According to our results, AJEAE notably amended the pancreatic and hepatic antioxidant markers (SOD, GPx, and CAT). Alloxan significantly increased the concentration of MDA and NO compared to the control group, which indicated powerful oxidative stress due to radical production (Anwar and Meki 2003). AJEAE treatment results in a significant reduction of MDA and NO levels as compared to the diabetic group.
There is a significant link between inflammation and β-cell damage that signifies its association with the pathogenesis of DM. Hyperglycemia results in the formation of advanced glycation products which is related to inflammation. TNF-α and IL-6 are suggested to increase the intensity and occurrence of diabetic complications (Albasher et al. 2020). In this study, we further evaluated the levels of the pro-inflammatory cytokine, TNF-α, and IL-6 as these cytokines are recognized to show a vital role in the development of insulin resistance (Ramadan et al. 2017). In diabetic rats, elevated levels of serum TNF-α and IL-6 were detected, instead AJEAE considerably (p < 0.001) reduced the TNF-α and IL-6 levels in a dose-dependent manner. The projected pathogenic mechanisms of β-cell dysfunction include glucotoxicity, lipotoxicity, endoplasmic reticulum stress, and activation of the renin-angiotensin system. Interestingly, all these pathogenic mechanisms provoke an inflammatory response. Histopathological analysis has revealed that DC rats show damaged histoarchitecture of pancreatic tissues with squeeze islets of Langerhans. The islets of Langerhans showed reduced β-cell concentration. Reduced cell mass or function can both lead to insufficient insulin levels, which can result in hyperglycemia and diabetes. GL-treated rats showed improvement of the histopathological changes of islets of Langerhans with improved β-cell mass. AJEAE considerably normalized the histoarchitecture of pancreatic tissues. Interestingly AJEAE treatment showed significant pancreatic β-cell regenerative potential due to the dramatic rise in pancreatic β-cell population and suppression in uncommon histological changes in comparison to the DC group (Fig. 6a–e). Concerning the mechanism through which AJEAE can improve the histoarchitecture of pancreatic β-cells, earlier research studies have found that flavonoids and phenolics exhibit significant contributions in the regeneration of β-cells (Elshamy et al. 2017). The histopathological findings are in correlation with biochemical results as the bioactive compounds detected in the plant under study play a key role in the improvement of lipid and glucose metabolism and possess significant anti-inflammatory and antioxidant properties. Limitations of our study were we could not perform gene expression analysis and C-peptide tests for further molecular analysis and insulin synthesis information due to lack of facilities. In the future, we have planned to perform the gene expression analysis to understand the molecular mechanisms involved in the antihyperglycemic potential of the plant under study. However, concerning the merits of the present study, we have proposed the hypoglycemic, hypolipidemic, antioxidant, and pancreatic β-cell regenerative properties of Acacia jacquemontii through its protective role in β-cell mass and functioning along with observable improvement in glycemic and lipidemic status and suppression in oxidative status.
Conclusion
Observations of the present study have revealed the ameliorative activity of AJEAE, probably attributed to the presence of bioactive compounds which were previously qualified as key candidates in downregulation of hyperglycemia via modulation of glycemic, lipidemic, anti-inflammatory, and anti-oxidant defense markers. Furthermore, AJEAE also showed strong regenerative pancreatic β-cell potential through improvement in histoarchitechture of pancreatic β-cells. The findings of the present study highlight the therapeutic significance of Acacia jacquemontii in the management of hyperglycemia, providing mainstream for the development of therapeutic alternatives that probably offer cheaper and safe remedies in treating diabetes with minimal side effects.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adisakwattana S (2017) Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications. Nutrients 9(2):163. https://doi.org/10.3390/nu9020163
Albasher G, Alwahaibi M, Abdel-Daim MM, Alkahtani S, Almeer R (2020) Protective effects of Artemisia judaica extract compared to metformin against hepatorenal injury in high-fat diet/streptozotocin-induced diabetic rats. Environ Sci Pollut Res 27(32):40525–40536. https://doi.org/10.1007/s11356-020-09997-2
Alema NM, Periasamy G, Sibhat GG, Tekulu GH, Hiben MG (2020) Antidiabetic activity of extracts of Terminalia brownie Fresen Stem bark in mice. J Exp Pharmacol 12:61
Anwar MM, Meki A-RMA (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A MolIntegr Physiol 135:539–547. https://doi.org/10.1016/S1095-6433(03)00114-4
Arumugam G, Manjula P, Paari N (2013) A review: anti diabetic medicinal plants used for diabetes mellitus. J Acute Dis 2(3):196–200. https://doi.org/10.1016/S2221-6189(13)60126-2
Arunachalam K, Parimelazhagan T (2014) Antidiabetic and enzymatic antioxidant properties from methanol extract of Ficustal boti bark on diabetic rats induced by streptozotocin. Asian Pac J Reprod 3(2):97–105. https://doi.org/10.1016/S2305-0500(14)60011-7
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8(4):96. https://doi.org/10.3390/plants8040096
Ashfaq K, Choudhary BA, Uzair M, Hussain SN, Ghaffari MA, Sarwar W, Manzoor M (2016) Antipyretic, analgesic and anti-inflammatory activities of methanol extract of root bark of Acacia jacquemontii Benth (Fabaceae) in experimental animals. Trop J Pharm Res 15(9):1859–1863. https://doi.org/10.4314/tjpr.v15i9.7
Ashraf A, Sarfraz RA, Rashid MA, Shahid M (2015) Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J Food Drug Anal 23(1):109–115. https://doi.org/10.1016/j.jfda.2014.05.007
Calixto JB (2019) The role of natural products in modern drug discovery. Anais da Academia Brasileira de Ciências 2019:91. https://doi.org/10.1590/0001-3765201920190105
Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, Daglia M, Xiao J (2019) Dietary polyphenols and type 2 diabetes: human study and clinical Trial. Crit Rev Food Sci Nutr 59(20):3371–3379
Chao PC, Li Y, Chang CH, Shieh JP, Cheng JT, Cheng KC (2018) Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed Pharmacother 101:155–61
Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F (2019) A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther 10(3):853–863. https://doi.org/10.1007/s13300-019-0619-1
Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A (2016) Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med J 11(1):1. https://doi.org/10.1186/s13020-016-0108-7
Chen C (2016) Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid Med Cell Longev 2016:1-10. https://doi.org/10.1155/2016/3571614
Choudhary K, Singh M, Shekhawat NS (2009) Ethnobotany of Acacia jacquemontii Benth-an uncharted tree of Thar Desert Rajasthan India. Ethnobot Leaflets 2009(6):1
Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR (2018) An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Trad Complement Med 8(3):361–376. https://doi.org/10.1016/j.jtcme.2017.08.012
El-Badawy RE, Ibrahim KA, Hassan NS, El-Sayed WM (2019) Pterocarpus santalinus ameliorates streptozotocin-induced diabetes mellitus via anti-inflammatory pathways and enhancement of insulin function. Iran J Basic Med Sci 22(8):932
Elshamy AI, El-Shazly M, Yassine YM, El-Bana MA, Farrag AR, Nassar MI, Singab AN, Noji M, Umeyama A (2017). Phenolic constituents, anti-inflammatory and antidiabetic activities of Cyperuslaevigatus L. Pharmacogn J 9(6):828–833. https://doi.org/10.5530/pj.2017.6.129.
Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H (2014) Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med 7:S355–S363. https://doi.org/10.1016/S1995-7645(14)60258-3
Hammeso WW, Emiru YK, Getahun KA, Kahaliw W (2019) Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evid Based Complement Alternat Med 2019:1–9. https://doi.org/10.1155/2019/8263786
Hassan SK (2015) EL-Sammad NM, MousaAM Hypoglycemic and Antioxidant Activities of Caesalpiniaferra Martius Leaf Extract in Streptozotocin-Induced Diabetic Rats Asian. Pac J Trop Med 5:462–473. https://doi.org/10.1016/j.apjtb.2015.03.004
Idm’hand E, Msanda F, Cherifi K (2020) Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin Phytoscience 6:1–32. https://doi.org/10.1186/s40816-020-00166-z
Imtiaz SM, Aleem A, Saqib F, Ormenisan AN, Elena Neculau A, Anastasiu CV (2019) The potential involvement of an ATP-dependent potassium channel-opening mechanism in the smooth muscle relaxant properties of Tamarixdioica Roxb. Biomolecules 9(11):722. https://doi.org/10.3390/biom9110722
Ji G, Sun R, Hu H, Xu F, Yu X, Veeraraghavan VP, Mohan SK, Chi X (2020) Vannilic acid ameliorates hyperglycemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats. J King Saud UnivSci 32(7):2905–11. https://doi.org/10.1016/j.jksus.2020.04.010
Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, Momtaz S, Abbasabadi Z, Rahimi R, Farzaei MH, Bishayee A (2019) Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci 22(3):225. https://doi.org/10.22038/ijbms.2019.32806.7897
Khan MF, Rawat AK, Khatoon S, Hussain MK, Mishra A, Negi DS (2018) In vitro and in vivo antidiabetic effect of extracts of Melia azedarach, Zanthoxylumalatum, and Tanacetumnubigenum. Integr Med Res 7(2):176–183. https://doi.org/10.1016/j.imr.2018.03.00
Lamacchia O, Sorrentino MR (2021) Diabetes mellitus, arterial stiffness and cardiovascular disease: clinical implications and the influence of SGLT2i. CurrVascPharmacol 19(2):233–240. https://doi.org/10.2174/1570161118666200317150359
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF (2020) Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific reports 10(1):1–1
Majeed W, Aslam B, Iftikhar A, Awan AM, Javed F, Daud M, Shahab N, Syed M, Iqbal H (2021) Acacia nilotica polyphenol extract restores glucose homeostasis by upregulating the insulin secretion and lowering the oxidative stress through down regulation of c-Jun N-terminal kinase (JNK) signaling cascade. J King Saud UnivSci 33(5):101474. https://doi.org/10.1016/j.jksus.2021.101474
Majeed W, Khaliq T, Aslam B, Khan JA (2018) Polyherbal formulation prevents hyperglycemia by modulating the biochemical parameters and upregulating the insulin signaling cascade in alloxan induced hyperglycemic rats. Pak Vet J 38(2):121–126. https://doi.org/10.29261/pakvetj/2018.035
Manach C (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747. https://doi.org/10.1093/ajcn/79.5.727
MogoleL OW, Mtunzi F (2020) Phytochemical screening, antioxidant activity and α-amylase inhibition study using different extracts of loquat (Eriobotrya japonica) leaves. Heliyon 6(8):04736. https://doi.org/10.1016/j.heliyon.2020.e04736
Moradi B, Abbaszadeh S, Shahsavari S, Alizadeh M, Beyranvand F (2018) The most useful medicinal herbs to treat diabetes. Biomed Res Ther 5(8):2538–2551. https://doi.org/10.15419/bmrat.v5i8.463
Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, Ahmad F, Babazadeh D, Fang Fang X, Modarresi-Ghazani F, WenHua L (2018) Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother 97:67–74. https://doi.org/10.1016/j.biopha.2017.10.064
Ononamadu CJ, Alhassan AJ, Imam AA, Ibrahim A, Ihegboro GO, Owolarafe AT, Sule MS (2019) In vitro and in vivo anti-diabetic and anti-oxidant activities of methanolic leaf extracts of Ocimum canum. Casp J Intern Med 10(2):162. https://doi.org/10.22088/cjim.10.2.162
Phuyal N, Jha PK, Raturi PP, Rajbhandary S (2020) Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Scientific World J 2020:1-7. https://doi.org/10.1155/2020/8780704
Ramadan BK, Schaalan MF, Tolba AM (2017) Hypoglycemic and pancreatic protective effects of Portulacaoleracea extract in alloxan induced diabetic rats. BMC Complement Altern Med 17(1):1–10. https://doi.org/10.1186/s12906-016-1530-1
Rasool F, Murtaza G, Zeshan M, Habib R, Yousaf MM, Ayub MM, Ayub MA, Sardar K, Irshad HA (2016) Comprehensive review on ecology and ethnobotany of Acacias and Acacia jacquemontii Benth in dry environment. IJAR 2(12):103–109
Rasool F, Ishaque M, Yaqoob S, Tanveer A (2017) Chemical composition and ethnobotanical uses of Acacia jacquemontii Benth. in the Thal desert in Pakistan. Bois & Forets Des Tropiques 331:67–76. https://doi.org/10.19182/bft2017.331.a31327
Sabahi Z, Khoshnoud MJ, Khalvati B, Hashemi SS, Farsani ZG, Gerashi HM, Rashedinia M (2020) Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 10(3):111. https://doi.org/10.4103/2221-1691.276317
Salehi B, Ata AV, Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, AbdulmajidAyatollahi S, ValereTsouhFokou P, Kobarfard F, AmiruddinZakaria Z, Iriti M (2019) Antidiabetic potential of medicinal plants and their active components. Biomolecules 9(10):551. https://doi.org/10.3390/biom9100551
Sangeetha R, Vedasree N (2012) In vitro α-amylase inhibitory activity of the leaves of Thespesia populnea. ISRN Pharmacol 2012:1–4. https://doi.org/10.5402/2012/515634
Sathasivampillai SV, Rajamanoharan PR, Munday M, Heinrich M (2017) Plants used to treat diabetes in Sri Lankan Siddha Medicine–an ethnopharmacological review of historical and modern sources. J Ethnopharmacol 198:531–599. https://doi.org/10.1016/j.jep.2016.07.053
Sudasinghe HP, Peiris DC (2018) Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflorasuberosa L. Peer J 6:e4389
Takahara S, Hamilton HB, Neel JV, Kobara TY, Ogura Y, Nishimura ET (1960) Hypocatalasemia a new genetic carrier state. J ClinInvestig 39(4):610–9. https://doi.org/10.1172/JCI104075
Tiwari BK, Pandey KB, Abidi AB, Rizvi SI (2013) Markers of oxidative stress during diabetes mellitus. J Biomark 2013:1–8. https://doi.org/10.1155/2013/378790
Wang TY, Li Q, Bi KS (2018a) Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci 13(1):12–23. https://doi.org/10.1016/j.ajps.2017.08.004
Wang Q, Zhang X, Fang L, Guan Q, Guan L, Li Q (2018) Prevalence, awareness, treatment and control of diabetes mellitus among middle-aged and elderly people in a rural Chinese population: a cross-sectional study. PloS one 13(6):e0198343
Yazdanpanah S, Rabiee M, Tahriri M, Abdolrahim M, Rajab A, Jazayeri HE, Tayebi L (2017) Evaluation of glycated albumin GA and GA/HbA1c ratio for diagnosis of diabetes and glycemic control a comprehensive review. Crit Rev Clin Lab Sci 54(4):219–32. https://doi.org/10.1080/10408363.2017.1299684
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin PharmacolPhysiol 31(6):332–6. https://doi.org/10.1159/000491755
Acknowledgements
Authors acknowledge the respected lab staff of the Institute of Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan, for collaboration in the lab and research facilities. The authors also acknowledge Daniel Villafruela (https://en.wikipedia.org/wiki/Vachellia_jacquemontii) for a picture of Acacia jacquemontii incorporated in the graphical abstract.
Author information
Authors and Affiliations
Contributions
A.M. and W.M. made experimental design, participated in data collection, analyzed the total flavonoid and phenolic compounds in the plant extract, and performed the histopathological examination. F.M. and N.F. analyzed and interpreted biochemical measurements. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Experimental protocol was planned according to laboratory animal care guidelines permitted by the Graduate studies Research Board, UAF Pakistan. The ethical certificate was issued by the institutional biosafety and bioethics committee with letter no. 1739/ORIC for the conduct of the in vivo experiment.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Awan, A.M., Majeed, W., Muhammad, F. et al. Acacia jacquemontii ethyl acetate extract reduces hyperglycemia and pro-inflammatory markers while increasing endogenous antioxidant potential in alloxan-induced diabetic rats. Environ Sci Pollut Res 29, 52605–52617 (2022). https://doi.org/10.1007/s11356-022-19493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19493-4