Abstract
The leaves of Ficus johannis Boiss (F. johannis), commonly known as Fig tree, Anjir, and Teen, are used by the folk medicinal practitioners in Iran for controlling hyperglycemia in diabetic patients. This study investigated the pharmacological basis for antidiabetic effect of the ethanolic extract of F. johannis leaves using in vitro and in vivo experimental models. Qualitative screening of phytochemicals, estimation of total phenolic and flavonoid contents, and in vitro antioxidant and α-amylase inhibition assays were performed. Moreover, the High-performance liquid chromatography (HPLC) quantification, acute toxicity, glucose tolerance, and in vivo antidiabetic effect along with the evaluation of gene expressions involved in diabetes mellitus were carried out. Significant quantities of phenolic (71.208 ± 2.89 mgg−1 GAE) and flavonoid (26.38 ± 3.53 mgg−1 QE) were present. Inhibitory concentration (IC50) of the plant extract exhibited an excellent in vitro antioxidant (IC50 = 33.81 µg/mL) and α-amylase (IC50 = 12.18 µg/mL) inhibitory potential. The HPLC analysis confirmed the gallic acid (257.79 mgg−1) as main constituent of the extract followed by kaempferol (22.86 mgg−1), myricetin (0.16 mgg−1), and quercetin (3.22 mgg−1). Ethanolic extract displayed glucose tolerance in normo-glycemic rats. Streptozotocin-induced hyperglycemia declined dose dependently in the extract treated rats with improvement in lipid profile and liver and renal function biomarkers. The F. johannis-treated groups showed an increase in mRNA expressions of glucose transporter 4 (GLUT-4), glucokinase, insulin growth like factor 1 and peroxisomal proliferator activating receptor gamma in pancreas. However, the Glucose-6-phosphatase was downregulated. Present study suggests that the ethanolic extract of F. johannis leaves demonstrates a good anti-diabetic profile by improving insulin sensitivity, GLUT-4 translocation, and carbohydrate metabolism while inhibiting lipogenesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is an endocrinal disorder, acknowledged as long-term hyperglycemia with defective insulin production or its action and metabolic abnormalities in carbohydrates, proteins, and fats (Justino et al. 2018). International diabetes federation estimates that approximately 537 million adults are suffering from diabetes and projected a raise, up to 643 million by the year 2030. It is also estimated that 3 in 4 of these individuals live in low- or middle-income countries. The global public health abnormality is due to the decreased production of insulin or its resistance to liver and adipose tissues (Chaari et al. 2020) (Rossi 2018). Reactive oxygen species cause type 2 DM, owing to unstable nature of free radicals. Non-enzymatic glycation of proteins, glucose oxidation, and augmented lipid peroxidation generates free radicals which further damage enzymes and cellular machinery and develop insulin resistance due to oxidative stress Resistance to insulin is due to insulin receptors which are unable to respond properly to insulin production (Lugrin et al. 2014). Relative insulin deficiency and peripheral insulin resistance leads to type II DM (care 2020). Insulin is responsible for inhibition of glucose production. It does so by suppression of glucose 6 phosphatase (Nakae et al. 2001). Glucose 6 phosphatase and glucokinase act in opposite direction. They regulate free glucose intracellularly. Glucokinase activity is decreased in type II DM. Increased ratio of glucose 6 phosphatase to glucokinase, causes efflux of glucose into the blood stream (Haeusler et al. 2015). Similarly, glucose transporter type 4 (GLUT4) expressions are reduced in diabetes mellitus leading to decreased plasma glucose clearance and altering glycemic homeostasis (Passarelli and Machado 2021). Insulin like growth factor interacts with insulin receptors and mediates signaling pathways in different tissues to facilitate carbohydrate metabolism (Lewitt et al. 2014).
A large number of DM sufferers are found obese having large amount of fat in abdominal region (Sabry et al. 2019). Different treatment modalities are available for the management of diabetes. The criteria for the selection of the treatments are based upon disease prevalence, age, gender, and other associated diseases (Peters and Davidson 1996).
Plants play an essential role in the management of a number of diseases like diabetes mellitus, atherosclerosis, and hepatotoxicity (Bano et al. 2023, Barkaoui et al. 2017; Tag et al. 2012; Zhi et al. 2023). These medicinal treatment practices are being used over thousands of years around the globe (Omara et al. 2010). Generally, natural products derived from plants and herbs have low toxicity profiles and high patient compliance (Wang et al. 2017). Most of the herbal medicines and nutraceuticals have some bioactive phytochemicals which are scientifically proven for the treatment of diabetes mellitus (Giovannini et al. 2016; Mousavi et al. 2018). Human diet makes available a large number of phytochemicals with therapeutic potential. Phenolic compounds are the most abundant class of phytochemicals in edible plants, fruits, and beverages (Dias et al. 2017). The demand for natural hypoglycemic agent is increasing day by day (Patel et al. 2012); therefore, plant extracts can be considered an attractive approach for the development of diabetic treatment or disease mitigation (Chaari et al. 2020).
The genus “Ficus” comprises of 850 species, and is widely distributed in tropical and semitropical moderate zones. These plants are a significant source of food for frugivores throughout the year. About 132 species of “Ficus” are reported to be utilized for nutritional consumption. This plant Ficus johannis Boiss (F. johannis) is grown commonly in different parts of Iran, Pakistan, and Afghanistan. The leaves of F. johannis commonly known as Fig tree, Anjir, and Teen are used by the folk medicinal practitioners of Iran for controlling glucose level in diabetic patients (Baharvand-Ahmadi et al. 2016) but no scientific data is reported till now. So, we conducted this study to report its anti-diabetic activity in Pakistan. The present study was intended to evaluate the anti-hyperglycemic potential of F. johannis leaves by in vitro and in vivo models as its anti-diabetic use is reported in folklore and is used in different regions around the world.
Methods and materials
Chemicals
Streptozotocin (STZ), α-amylase, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, acarbose, and nicotinamide (NA) were purchased from Sigma-Aldrich Chemical Co. (Germany). TRIzol reagent was purchased from Advance Bioscience (Germany). All kits for analysis were purchased from Biolabs and Vivantis Technologies. All chemicals and reagents were used of analytical grade.
Collection of plant material
Fresh Ficus johannis Boiss (Moraceae) was collected from Abbottabad, Pakistan. It is located in Khyber-Pakhtunkhwa province between 34°09 ′N latitude and 73°13 ′E longitude at an altitude of 4120 feet (1260 m) (Ijaz et al. 2016). Taxonomic identification for authenticity was confirmed by Dr Zaheer-Uddin, Professor, Department of Botany, Government College University, Lahore using binocular microscope, comparing specimen in the herbarium and consulting (Baharvand-Ahmadi et al. 2016). A voucher was deposited at the herbarium of the university (GC.Herb.Bot.3540).
Preparation of plant extract
The leaves of F. johannis were separated from stems and washed with tap water to get rid of the adhering dust and foreign particles. The plant material was sliced into small pieces and shade dried. The dried plant material was powdered (700 g) and macerated in 98% ethanol (2000 mL) for 7 days. The solvent was filtered with whatman no.1 filter paper. The filtrate was concentrated under reduced pressure in a rotary evaporator at 40 °C. The extract was dried in a dry heat oven at 40 °C and stored in a refrigerator at 4 °C for further use in pharmacological evaluation (Mousavi et al. 2016).
Total phenolic content
The total phenolic content of F. johannis leaves extract was calculated by Folin-Ciocalteu’s (FC) reagent method with slight modifications and stated in mg/g gallic acid equivalent (GAE) of plant extract. F. johannis extract was diluted at different concentrations (100, 80, 60, 40, and 20 µg/mL) with methanol. Nine-milliliter distilled water and 1 mL FC reagent were added to dilutions and mixed for 5 min. Ten-milliliter 7% sodium carbonate (Na2CO3) and 4 mL distilled water were added and incubated for 90 min at 37 °C. The absorbance was determined by ultraviolet (UV) spectrophotometer at 760 nm. Linear regression model was used to determine the phenolic content using the standard calibration curve of gallic acid (Khan et al. 2020).
Total flavonoid content
The total flavonoid content of F. johannis leaves extract (100, 80, 60, 40, and 20 µg/mL) was determined by aluminium chloride (AlCl3) colorimetric method with slight modifications. The procedure involves the addition of 200 µL extract dilution, 100 µL AlCl3, 100 µL 1 M potassium acetate (CH3CO2K) solution, and 4.6 mL distilled water and incubated at 37 °C for 45 min. Same procedure was done for quercetin. The absorbance of these samples was determined by UV spectrophotometer at 415 nm. Total flavonoid content was estimated utilizing quercetin calibration curve (Zafar et al. 2021). The results were resolved as per quercetin equivalent (mg of quercetin/g of concentrate).
High-performance liquid chromatography (HPLC):
The identification of flavonoid and phenolic in F. johannis ethanolic leaves extract was carried out by high-performance liquid chromatography (HPLC). The HPLC system Shimadzu was used with a UV visible detector (280 nm), LC-10AT pump, PDA detector SPD-10AV and column (25 cm × 4.6 mm, 5 µm), and two mobile phases A (H2O: acetic acid-94:6) and B (acetonitrile 100%) with a flow rate 1 mL/min (Malik et al. 2022).
In vitro antioxidant activity by DPPH scavenging assay
Antioxidant potential of F. johannis ethanolic leaves extract was determined by DPPH scavenging assay. Briefly 1 mL of extract solution was incubated with 2 mL of DPPH solution for a time period of 30 min and absorbance was recorded at 517 nm. IC50 was calculated at different the concentrations of extract (100, 80, 60, 40, 20, and 10 µg/mL) were used to determine the percentage inhibition and later on IC50 was calculated (Abbas et al. 2020). The standard used was ascorbic acid.
α-Amylase inhibitory activity
The in vitro hypoglycemic activity of F. johannis ethanolic leaves extract was calculated by α-amylase inhibitory assay with slight modifications. Acarbose was used as a standard and dissolved in dimethylsulfoxide (DMSO) (2 mg/mL). 1 mL α-amylase solution and 2 mL 0.02 M phosphate buffer was added to 1 mL extract dilutions (100, 80, 60, 40, 20, and 10 µg/mL) and incubated at 37 °C for 30 min. 1 mL starch solution (0.5%) was added to previous solution and incubated for 15 min. 1 mL DNS reagent was added and heated at 90 °C in a water bath for 8 min. The absorbance was checked by UV spectrophotometer at 540 nm. IC50 was calculated from percentage inhibition by the following formula (Khan et al. 2020a).
Experimental animals
Colony-bred, adult male Albino Wistar rats were purchased from University of Veterinary and Animal Sciences, Lahore as experimental animals with 170–220 g body weight and aged between 10 and 12 weeks. The selected animals were kept in standard spacious polypropylene cages under standard environmental conditions such as, 25 ± 2 °C temperature with 12 h light/dark cycles. A standard pelleted diet was given to all rats ad libitum with a dietry composition of carbohydrate 58%, fat 5.7%, protein 24%, ash 8%, and fiber 6% and having free access to tap water. The rats were shifted to laboratory 1 week before the experiment to adapt the laboratory environment. The study was approved from the Institutional Research Ethics Committee (IREC-2019–87) of the University of Lahore and the study was executed accordingly (Mousavi et al. 2018).
Acute toxicity study
The acute oral toxicity study of F. johannis ethanolic leaves extract was performed according to the Organization for Economic and Co-operation and Development (OECD) guidelines. Twelve albino rats were assigned to 4 groups (n = 3) randomly. These rats were fasted over night with free access to water. Group I was given normal saline. Groups II, III, and IV were given 2000, 1000, and 500 mg/kg F. johannis extract orally via gastric gavage tube. After administration, the rats were carefully observed for behavioral and toxicological symptoms at 0.5, 4, 8, 12, 24, 48, 72 h, and 1 week (Thomson et al. 2015).
Oral glucose tolerance test (OGTT)
OGTT was performed on normal rats before the start of study. The normal rats were assigned to five groups and each group consisted of five animals (n = 5). The group I, control group was fed with normal saline. The group II was fed metformin 100 mg/kg, used as a positive control. The groups III, IV, and V were fed orally with 3 different doses of F. johannis leaves extract 125, 250, and 500 mg/kg, respectively. All treatments were given to the rats, 5 min before loading glucose (2 g/kg). After 0, 0.5, 1, 1.5, 2, 2.5, 3 h blood samples were collected from tail vein. The blood glucose levels were measured by (Accu-Check glucometer, Roche, Switzerland) (Malik et al. 2022). The hypoglycemic potential of the plant was determined by calculating AUC on GraphPad Prism 5.0.
Experimental design
The experimental animals were randomly assigned to seven groups, each containing five rats (n = 5). The division of groups was in the following manner:
-
Group I: Normal Control (NC), the normal rats given normal saline oral gavage tube for 21 days.
-
Group II: Positive Control (PC), the STZ induced diabetic rats received metformin 100 mg/kg as a standard drug orally, by oral gavage tube once daily for 21 days.
-
Group III: Diabetic Control (DC), the STZ induced diabetic rats given normal saline by oral gavage tube for 21 days.
-
Group IV: Normal + Ficus johannis (NFJ500), the normal rats received 500 mg/kg plant extract by oral gavage tube once daily for 21 days.
-
Group V: Ficus johannis 125 mg/kg (FJ125), the STZ induced diabetic rats received 125 mg/kg plant extract by oral gavage tube once daily for 21 days.
-
Group VI: Ficus johannis 250 mg/kg bw (FJ250), the STZ treated diabetic rats received 250 mg/kg plant extract by oral gavage tube once daily for 21 days.
-
Group VII: Ficus johannis 500 mg/kg bw (FJ500), the STZ induced diabetic rats received 500 mg/kg plant extract by oral gavage tube once daily for 21 days (Hassan et al. 2015).
Induction of diabetes
Type II diabetes was induced in 12 h fasted rats. A single dose of NA (120 mg/kg) dissolved in 0.5 mL normal saline was administered intraperitoneal. After 15 min, STZ (60 mg/kg) in a single dose, freshly dissolved in 0.5 mL 0.01 M citrate buffer (pH 4.5) was administered via intraperitoneal route (Rouhi et al. 2017). Five percent dextrose solution was administered to overnight fasted rats followed by STZ administration to prevent hypoglycemic shock (Soni et al. 2018). Glucometer was used to access the levels of blood glucose. Rats with blood glucose levels above 250 mg/dL were considered diabetic and inducted in the study (Thomson et al. 2015).
In vivo anti-diabetic activity
Post induction the rats were kept under observation for 21 days. During this period, rats were administered with the respective doses mentioned in experimental design via oral gavage tube daily. The blood glucose level was determined at days 1, 7, 14, and 21 under fasting conditions with glucometer (ACCU-CHEK, Roche, Switzerland). Moreover, we also monitored feed and water intake along with their body weights (Zafar et al. 2021).
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total ribonucleic acid (RNA) was isolated from pancreas using Trizol reagent. The RNA was reverse transcribed to single stranded complementary deoxy ribonucleic acid cDNA according to the protocol of cDNA synthesis kit (Cat no. 4368814). Species specific primers were synthesized. The cDNA was amplified in thermal cycler by given protocol of PCR kit (Cat no. 32161000). Ten-microliter PCR products, stained with ethidium bromide, were loaded in 2% agarose gel in 1 × Tris Acetate Ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.3–8.5) (Irudayaraj et al. 2016; Kim et al. 2010; Stalin et al. 2016). The electrophoretic picture was quantified by the ImageJ software and GraphPad Prism 5.0.
Biochemical analysis
At the end of the study period (21 days), rats were anesthetized using diethyl ether. Cervical dislocation was performed and blood was collected in sterile tubes through cardiac puncture. Blood sampling was performed in EDTA tubes to study the hematological variables. To study the biochemical parameters centrifugation of the blood was performed at 1500 g for a time period of 15 min at temperature 4 °C. The sera samples were separated and used to analyze triglyceride contents (TG), cholesterol contents (TC), High density lipoproteins level (HDL), low density lipoproteins level (LDL), very low-density lipoproteins level (v-LDL), alanine aminotransferase level (ALT), alkaline phosphatase level (ALP), aspartate aminotransferase level (AST), urea concentration, and creatinine contents. The samples were analyzed at the diagnostic Lab of the University of Lahore (Bano et al. 2023, Toma et al. 2015).
Histopathological investigation
After the 21 days’ study, the rats were sacrificed and their liver, kidney, and pancreas were removed. The histopathological examination was carried out by washing each tissue with ice-cold 0.9% saline and fixing the tissue sections in 10% neutral buffered formalin. The tissue sections were processed in graded series of ethanol and embedded in paraffin wax. Tissue sections were cut (5 μm thick) and stained with haematoxylin and eosin (H&E). Later, the microscopic examinations were carried out and photographed (Toma et al. 2015). Field views were selected randomly. Light microscope (optika vision lite 2.1) was used to view the histology slides for any signs of toxicity. A pathologist who was un aware of the treatment groups accessed the slides. A scoring system was devised (Table 1) to access the level of damage to the tissues. We devised a total of five grades. The grades were scored on the basis of the level of the damage ranged from 0 to 4. Hepatic alterations include inflammation, sinusoidal space dilation, and hydropic swelling ballooning hepatocytes whereas kidney injury includes fibrosis, dilation of renal tubule, and space of the glomerular capsule. For the severity of the pancreatic injury, we used the same parameters as we have accessed in one of our previous studies.
Statistical analysis
The data was presented by taking mean ± SD. GraphPad Prism 5.0 was used to determine IC50 using non-linear regression model. Furthermore, one-way analysis of variance (ANOVA) was also used to analyze the data followed by Bonferroni’s multiple comparison test.
Results
Percentage yield
The percentage yield of F. johannis ethanolic leaves extract was calculated to be 6.36%.
Preliminary phytochemical screening
The phytochemical screening confirmed the presence of some essential bioactive compounds including cardiac glycosides, flavonoids, alkaloids, phenolics, carbohydrates, proteins, saponins, and fixed oils (supplementary data Table 1).
Total phenolic and flavonoid contents
The total phenolic and flavonoid contents of F. johannis ethanolic leaves extract (100 µg/mL) was calculated as 71.208 ± 2.89 mgg−1 GAE and 26.38 ± 3.53 mgg−1 QE, respectively.
HPLC analysis
The HPLC investigation of F. johannis ethanolic leaves extract demonstrated the presence of kaempferol (22.86 mgg−1), gallic acid (257.79 mgg−1), myricetin (0.16 mgg−1), and quercetin (3.22 mgg−1) (Table 2).
DPPH radical scavenging activity
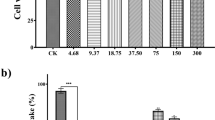
F. johannis ethanolic leaves extract showed the highest percentage inhibition at 100 µg/mL (77.98%) with IC50-33.81 µg/mL (Fig. 1A). Ascorbic acid showed IC50-20.19 µg/mL with a percentage inhibition (94.62%).
α-Amylase inhibitory assay
Concentration dependent inhibition was exhibited by F. johannis ethanolic leaves extract which was the highest at maximum dose 100 µg/mL. The percentage inhibition of F. johannis was 82.43% (IC50-28.49 µg/mL) (Fig. 1B) which was close to that of Acarbose 97.23% (IC50-12.18 µg/mL).
Acute oral toxicity
The F. johannis ethanolic leaves extract was found to be non-lethal even at highest orally administered dose 2000 mg/kg body weight. The rats were monitored for 1 week. No mortality was observed and rats did not exhibit any signs of toxicity (Table 3). The findings suggest the safety of the extract to use in experiments further.
Oral glucose tolerance test (OGTT)
Remarkable hypoglycemic activity was observed by F. johannis ethanolic leaves extract compared to metformin. FJ500 exhibited (21.49%) (Fig. 2) inhibition factor which is comparable to standard drug (27.53%).
Anti-hyperglycemic potential of F. johannis
F. johannis ethanolic leaves extract exhibited significant hypoglycemic potential in STZ-NA loaded rats. The glucose levels of FJ500 and PC on day 1 were 338 ± 11.2 and 345 ± 9.45 mg/dl respectively. These levels declined significantly (p < 0.05) to 133 ± 6.62 and 120 ± 7.44 mg/dl on day 21. The results were compared with disease control group (Fig. 3). However, the glucose level of DC on day 1 and day 21 was 350 ± 7.42 and 394 ± 6.96 mg/dl, respectively.
Effect of the treatments on the animal blood glucose levels during anti-hyperglycemic study. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and is expressed as “a” (p < 0.05), “b” (p < 0.01), and “c” (p < 0.001) when compared to the disease control group. Abbreviations: NC; normal control, PC; positive control, NFJ; normal rats treated with F. johannis, FJ; F. johannis; and DC; diabetic control
Effect on dietary intake and weight alterations
Weight gain, feed, and water intake were observed on days 1, 7, 14, and 21. Significant improvement in weight gain was observed (p < 0.05) compared to the normal group (Table 4). Diabetic rats consumed higher amounts of feed and water. The administration of F. johannis ethanolic leaves extract significantly reduced (p < 0.05) the quantity of food and water consumed by diabetic rats (Tables 5, 6).
Evaluation of proliferation
Gene expression of KI67 decreased in the disease control group, upon treatment with NFJ500 expression of KI67 increased. Similarly, peroxisomal proliferator activating receptor gamma (PPAR-γ), topoisomerase 2 alpha (TOP2A), and proliferating cell nuclear antigen (PCNA) gene expressions are also increased in dose-dependent manner compared to disease control group. Post treatment, the levels of gene expression were found in the following descending order NFJ500 > NC > PC > FJ500 > FJ250 > FJ125 > DC (Fig. 4). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal control and remained insignificant for all groups.
Effect of the treatments on relative mRNA expression of genes related to cell proliferation. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and is expressed as “a” (p < 0.05), “b” (p < 0.01), and “c” (p < 0.001) when compared to the disease control group. Abbreviations: NC; normal control, PC; positive control, NFJ; normal rats treated with F. johannis, FJ; F. johannis; and DC; diabetic control
Evaluation of apoptosis
Gene expression of apoptotic markers exhibited upregulation in diseased control group. Expression levels of BCL2 associated X (BAX) and P53 genes were decreased upon treatment with extracts of F. johannis. Post treatment, the levels of gene expression were found to be in the following order NC < NFJ500 < PC < FJ500 < FJ250 < FJ125 < DC (Fig. 5). GAPDH served as internal control and remained insignificant for all groups.
Effect of treatments on relative mRNA expression of genes related to apoptosis. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and is expressed as “a” (p < 0.05), “b” (p < 0.01), and “c” (p < 0.001) when compared to the disease control group. Abbreviations: NC; normal control, PC; positive control, NFJ; normal rats treated with F. johannis, FJ; F. johannis; and DC; diabetic control
Evaluation of functional genes
Glucose-6-Phosphatase (G6P) expression in diabetic control were increased compared to normal control. Gene expressions of G6P were significantly decreased when the rats were treated with F. johannis extracts. The results were comparable to positive control (STZ + 100 mg/kg metformin). On the contrary, the expression of glucokinase (GCK), glucose transporter 4 (GLUT4), and insulin growth like factor (IGF) was decreased in the diseased control groups. Treatment with NFJ500 (normal + 500 mg/kg extract) resulted in increased expressions of the abovementioned functional genes of diabetes (Fig. 6). GAPDH served as an internal control and remained significant in all groups (Table 7).
Effect of treatments on relative mRNA expression of functional genes. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and is expressed as “a” (p < 0.05), “b” (p < 0.01), and “c” (p < 0.001) when compared to the disease control group. Abbreviations: NC; normal control, PC; positive control, NFJ; normal rats treated with F. johannis, FJ; F. johannis; and DC; diabetic control
Biochemical analysis
The liver function tests alanine aminotransferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) of blood samples revealed the significant improvement in the plasma samples of F. johannis ethanolic leaves extract treated rats. The increased values of enzymes due to induction with STZ were restored close to normal (Fig. 7A) by administration of F. johannis ethanolic leaves extract (125, 250, and 500 mg/kg). Lipid profile (LDL, HDL, TC, and TG) levels at 21st day of the study was reinstated close to normal (Fig. 7B, C). Atherogenic index calculated from lipid profile parameters exhibited restoration of index up to (1.41) in FJ500 group compared to DC (7.04). Treatment with ethanolic leaves extract of F. johannis helped in decreasing the enhanced concentration of urea in diabetic rats. However, creatinine level was not altered (Table 8). Glycosylated hemoglobin (HbA1c) was also improved in FJ500 group (5.56 ± 0.20 mg/dL), upon treatment with F. johannis ethanolic leaves extract (Fig. 7D) compared to DC group (7.36 ± 0.18 mg/dL).
Effect of treatment on A liver function tests, B and C lipid profile, and D HbA1C. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and represented as “a” p < 0.05, “b” p < 0.01, and “c” p < 0.001 compared to the normal control group. Abbreviations: NC; normal control, PC; positive control, NFJ; normal rats treated with F. johannis, FJ; F. johannis, DC; diabetic control, ALT; alanine aminotransferase, AST; Aspartate transaminase, ALP; alkaline phosphatase, HDL; high-density lipoproteins, LDL; low-density lipoprotein, VLDL; very low-density lipoprotein
Histopathological investigations
Significant histology changes presented in diabetic control group were reversed by F. johannis ethanolic leaves extract justifying its protective effect in pancreas, liver, and kidney sections (Fig. 8A, B, C). Control group exhibited no change in structure of pancreas. Inflamed acinar cells were observed after administration of STZ (Fig. 9A). On 21st day of the study, the administration of F. johannis ethanolic leaves extract resulted in repair of pancreatic tissue architecture exhibiting a decrease in inflammation and restoration of acinar cells. Hepatic inflammation, sinusoidal space dilation, and hydropic ballooning hepatocytes were not present in normal rats. Hepatic inflammation, sinusoidal space dilation, and ballooning hepatocytes were seen in diseased rats. Hydropic swelling and ballooning in hepatocytes was greater in the diseased rats when compared to other groups. The treatment with F. johannis ethanolic leaves extract exhibited a decreasing trend in above mentioned parameters (Fig. 9B). Similarly, no change in histological structure of renal tissues was observed in control rats. STZ administration resulted in altered structure of renal tissues manifested as fibrosis, dilation of renal tubule, and space in glomerular capsule (Fig. 9C). The treatment with F. johannis ethanolic leaves extract resulted in restoration of renal tissue with significant reductions in the scores of fibrosis, dilation of renal tubule, and space of the glomerular capsule.
1A The examination of the submitted sample of pancreas exhibited no pathological changes, Islets of Langerhans were normal in size. Insulin producing beta cells were normal. 1B Degenerative changes were observed. The acinar cells were undergoing Hydropic degeneration. Lumen of blood vessels had fat lobules. 1C No inflammatory cells were present. Acinar cells were normal. 2A The examination of the submitted sample of liver exhibited no pathological changes. Most of the hepatocytes were normal. 2B Most of the hepatocytes were undergoing coagulative necrosis and nucleus are not seen. Severe degenerative changes were observed. The hepatocytes have more eosinophilic and granular cytoplasm. 2C Most hepatocytes were normal. Few hepatocytes had undergone necrotic changes. 3A Renal tubules were normal. Glomerulus and Bowman’s capsule were normal. No pathological changes were observed in the kidney Section 3B The epithelial cells of renal tubules were undergoing severe coagulative necrosis. 3C The renal tubules were almost normal. The glomerulus and Bowman’s capsule were normal
Effect of F.johannis leaf extract on histological scores of A pancreas, B liver, and C kidney. Data is presented as mean ± SD. The level of significance was estimated by one-way ANOVA followed by Fisher’s LSD test and is expressed as “a” (p < 0.05), “b” (p < 0.01), and “c” (p < 0.001) when compared to the normal control group. Abbreviations: FJLE; F. johannis leaves extract
Discussion
Diabetes Mellitus is an endocrinal metabolic disease, defined as persistent hyperglycemia resulting by an absolute deficiency of insulin secretion or resistance to the hormone (Rossi 2018). Present study was conducted to assess the antidiabetic and antioxidant action of Ficus johannis Boiss. (Moraceae).
HPLC analysis confirmed the presence of kaempferol, gallic acid, myricetin, and quercetin in F. johannis ethanolic leaves extract. Previously phenolic compounds have been found to increase glucose uptake, glycogen synthesis, improved glucose balance, and lipid profiles leading to decrease in complications associated with different diseases like diabetes mellitus, obesity, and cardiovascular disorders (Vinayagam et al. 2016). Different studies have suggested that phenolic compounds decrease the chances of degenerative disorders, by providing protection against oxidative stress and imparting their role as antioxidants. The high phenolic and flavonoid contents in F. johannis ethanolic leaves extract elaborate its role as an antioxidant (Rice-Evans et al. 1997; Saibabu et al. 2015; Zulfqar et al. 2020).
α-amylase inhibitory potential was also observed. Phenolics such as quercetin, and gallic acid can bind themselves with proteins to inhibit glucoside hydrolases (Malik et al. 2022). FJ500 group exhibited significant inhibition of hyperglycemia (21.49%) in normal rats, an effect comparable to the one observed by metformin. Reduction in Area under curve (AUC) reflected a change in absorption process of glucose and it is attributed to the inhibited sodium glucose transporter (SGLT 1) expressions. The results corroborate the previous findings (Amuri et al. 2017).
STZ damages pancreatic β-cells, resulting in hypo-insulinemia and hyperglycemia (Graham et al. 2011). STZ is a nitrosourea alkylating agent which is selectively taken into the pancreatic β-cells due to resemblance with structure of glucose (Papich 2007). Dose-dependent hypoglycemic effect was seen by the treatment with F. johannis ethanolic leaves extract. Decrease in glucose levels may be attributed to phenolic and flavonoid compounds present in F. johannis ethanolic leaves extract. Quercetin exhibits protective effects in experimental diabetes as it decreases the oxidative stress and preserves the integrity of pancreatic β-cells (Yelumalai et al. 2019).
Irrespective of the high feed and water consumption in the untreated rats, the body weight decreased compared to F. johannis ethanolic leaves extract treated groups. The improved consumption of food and water in the F. johannis-treated rats may have resulted in enhanced metabolism. The results are comparable with previous observations which have also reported weight loss in diabetic rats. This evidently indicates that F. johannis ethanolic leaves extract amplified glucose metabolism and has preventive effects on degradation of structural proteins which increased body weights in the rats (Soni et al. 2018).
Levels of liver biomarkers (ALT, AST, and ALP) were restored upon treatment with F. johannis ethanolic leaves extract in a dose-dependent fashion. The effect can be attributed to the amelioration of hepatic phosphatases and transaminases related with decreased oxidation (Malik et al. 2022). Decrease in ALT upon administration of F. johannis ethanolic leaves extract could be attributed to improved insulin activity which results due to decrease of transcription of ALT associated gluconeogenesis (Farhangi et al. 2016). Elevated urea levels were restored near to normal after treatment with F. johannis ethanolic leaves extract. However, no effect was observed on creatinine level in diabetic rats(Chandran et al. 2016).
The expressions of GLUT4, GCK, and IGF-1 were improved whereas expressions of G6P were declined upon treatment with F. johannis ethanolic leaves extract. GLUT4 is responsible for glucose homeostasis however, GLUT4 translocation is disturbed by insulin deficiency during diabetes. As a result, it remains inside the cells, non-functional, causing an elevation of blood glucose level (Balakrishnan et al. 2019). Previously, Ficus carica was found to improve glucose utilization by restoring GLUT4 translocation to plasma membrane. F. johannis ethanolic leaves extract up-regulated the gene expression of GLUT4 in diabetic rats correlating with the previous study (Irudayaraj et al. 2016). Gallic acid has the ability to initiate adipogenesis and fat accumulation by activating PPAR-γ and C/EBPα. It also has the ability to translocate GLUT-4 leading to an increase uptake of glucose. Myricetin also exhibits anti-hyperglycemic properties, mediated by the regulation of glucose transport through GLUT2. Myricetin administration was also linked with increased insulin sensitivity and inhibition of pancreatic β-cell apoptosis (Lalitha et al. 2020). The increased activities of G6P in diabetic rats are attributed to the increased synthesis of the enzymes involved in glucose production during diabetes by liver. Kaempferol ameliorates glucose intolerance, fasting hyperglycemia, and insulin resistance. These effects are linked with the ability of kaempferol to inhibit G6P activity and hepatic gluconeogenesis. F. johannis treatment significantly decreased blood glucose levels and G6P gene expression leading to decrease gluconeogenesis probably by reducing the activities of G6P, thereby reducing the endogenous production of glucose (Hemmati et al. 2017). F. johannis ethanolic leaves extract improved the gene expression of GCK improving carbohydrate metabolism in comparison to diabetic rats. It can be suggested that F. johannis exhibits protective effects against oxidative stress in rats and its hypoglycemic effect could be attributed to increase in insulin level, resulting in glycolysis, gluconeogenesis pathways regulation (Pari and Chandramohan 2017). Kaempferol increases the GCK activity and improves hepatic glucose metabolism. These findings suggest, kaempferol as an effective antidiabetic natural compound which regulates hepatic gluconeogenesis and improves insulin sensitivity (Alkhalidy et al. 2018). It is suggested that F. johannis ethanolic leaves extract have the potential to be used for the treatment of type II DM acting on multiple targets attributed to the presence of kaempferol and quercetin (Al-Nour et al. 2019). Treatment with F. johannis ethanolic leaves extract, significantly decreased blood glucose levels by up regulation of IGF-1 gene expression probably by improving lipogenesis and insulin sensitivity and overcoming insulin resistance (Kang et al. 2016).
Expressions of KI67, PPAR-γ, PCNA, and topoisomerase II enzyme (TOP2A) were increased whereas BAX expressions were decreased upon treatment with F. johannis ethanolic leaves extract. The mRNA expression of KI67 was significantly increased by the antioxidant activity of F. johannis ethanolic leaves extract causing the preservation of pancreatic β-cell integrity and maintaining tissue homeostasis and growth (Bologna-Molina et al. 2013). The gene expression of PPAR-γ was significantly increased in F. johannis-treated groups in a dose-dependent fashion, thus improving glucose homeostasis by activation of GCK and GLUT2 in liver and pancreas. Improvement in gene expression levels of PPAR-γ is also linked with increased sensitization of insulin in peripheral tissues by stimulating its secretion and controlling blood glucose by increased sensitivity in β-cells of pancreas (Assaei et al. 2016). The treatment with F. johannis ethanolic leaves extract significantly increased the gene expression level of PCNA in diabetic rats indicating normal cell proliferation and preservation of pancreatic β-cells (Bodduluru et al. 2016). The mRNA expression of topoisomerase II enzyme (TOP2A) was increased significantly in STZ-NA diabetic rats upon treatment with F. johannis ethanolic leaves extract in comparison of the diabetic group. It exhibits proliferation and normal functioning of pancreatic β-cells due to the antioxidant activity of extract (Harkin et al. 2016). F. johannis reduced apoptosis and increases cell survival in pancreatic β-cells as the P53 expression was down regulated after treatment with extract indicating a decline in oxidative stress which was previously reported. P53 gene expression was increased in diabetic rats (Gharib et al. 2018). BAX expression increases in diabetes at mRNA and protein levels due to apoptosis. Previously experimental models support in vitro and in vivo over expression of BAX in apoptosis (Duchen 2004). The gene expression of BAX was decreased significantly in F. johannis-treated diabetic rats compared to normal control group indicating a decrease in progression of diabetes linked with apoptosis.
Type II DM is associated with hyperlipidemia. Hyperlipidemia is associated with the hindrance of glucose which in turn leads to the lipid availability. LDL deposition on the arteries wall leads to the transportation of the plaques into the liver via HDL. Hence an increase in level of HDL and lowering of cholesterol and triglycerides can be a useful therapeutic alternative (Mooradian 2009). Treatment with F. johannis ethanolic leaves extract effectively improved dyslipidemia in a dose-dependent manner by improvement in insulin sensitivity. Results are in concurrence with a study where the findings suggested that flavonoids are responsible for producing hypo-lipidemia (El-Hadary and Ramadan 2019). Marked improvement observed in atherogenic index after administration of FJ500 is attributed with the reduction in the risk of infarction, diabetes mellitus, and atherosclerotic conditions (Zhang et al. 2016).
The histopathological examinations showed dose-dependent improvement in liver, kidney, and pancreas of STZ-induced diabetic rats when treated with F. johannis ethanolic leaves extract compared to the disease control group. The findings of the current study corroborated the earlier investigation on herbal drugs suggesting their role in the amelioration of oxidative damage in organs and prevention of diabetic complications (Wang et al. 2017; Zafar et al. 2021).
Conclusion
Present study confirms that F. johannis leaves ethanolic extract possess remarkable antidiabetic activity based upon both in vitro and in vivo analysis. It does so by modulation of pancreas function and also evident from cellular morphology of pancreas in STZ induced diabetic rats. Biophenol (Gallic acid) and flavonoids (kaempferol, myricetin, and quercetin) were quantified and considered to be responsible for the in vitro and in vivo antidiabetic potential. These macromolecules present in the F. johannis leaves ethanolic extract has the potential to regulate the concentration of glucose by modulation of GLUT4, G6P, IGF-1, and GCK in T2DM rats.
Data availability
The data that support the findings of this study are available and submitted in form of supplementary file along with the manuscript.
Abbreviations
- ALT:
-
Aalanine aminotransferase
- ALP:
-
Alkaline phosphatase
- AlCl3:
-
Aluminium chloride
- AST:
-
Aspartate aminotransferase
- DC:
-
Diabetic control
- DM:
-
Diabetes mellitus
- DMSO:
-
Dimethylsulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid
- F. johannis :
-
Ficus johannis Boiss.
- FJ 125:
-
Ficus johannis 125 Mg/kg
- FJ 250:
-
Ficus johannis 250 Mg/kg
- FJ 500:
-
Ficus johannis 500 Mg/kg
- FC:
-
Folin-Ciocalteu’s
- G6P:
-
Glucose-6-Phosphatase
- GCK:
-
Glucokinase
- GLUT4:
-
Glucose transporter 4
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GAE:
-
Gallic acid equivalent
- HDL:
-
High density lipoproteins
- H&E:
-
Haematoxylin and eosin
- HPLC:
-
High-performance liquid chromatography
- IC:
-
Inhibitor concentration
- IREC:
-
Institutional Research Ethics Committee
- IGF:
-
Insulin growth like factor
- LDL:
-
Low density lipoproteins
- NIDDM:
-
Non-insulin-dependent diabetes mellitus
- NA:
-
Nicotinamide
- NC:
-
Normal control
- NFJ:
-
Normal + Ficus johannis
- OGTT:
-
Oral glucose tolerance test
- OECD:
-
Organization for Economic and Co-operation and Development
- PPAR-γ:
-
Peroxisomal proliferator activating receptor gamma
- PC:
-
Positive control
- CH3CO2K:
-
Potassium acetate
- PCNA:
-
Proliferating cell nuclear antigen
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- PPN:
-
Parts per million
- RNA:
-
Ribonucleic acid
- Na2CO3 :
-
Sodium carbonate
- STZ:
-
Streptozotocin
- TOP2A:
-
Topoisomerase 2 alpha
- TC:
-
Total cholesterol level
- TG:
-
Triglyceride level
- UV:
-
Ultraviolet
- v-LDL:
-
Very low-density lipoproteins
- WHO:
-
World Health Organization
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
References
Abbas A, Hassan SS, Sharif A, Ahmed S (2020) Evaluation of the antioxidant and anti-inflammatory activities of solvent extracts of Tricholepis chaetolepis (Boiss) Rech. f. whole plant. Nat Prod Res 34:575–579
Alkhalidy H, Moore W, Wang A, Luo J, McMillan RP, Wang Y, Zhen W, Hulver MW, Liu D (2018) Kaempferol ameliorates hyperglycemia through suppressing hepatic gluconeogenesis and enhancing hepatic insulin sensitivity in diet-induced obese mice. J Nutr Biochem 58:90–101
Al-Nour MY, Ibrahim MM, Elsaman T (2019) Ellagic acid, Kaempferol, and Quercetin from Acacia nilotica: promising combined drug with multiple mechanisms of action. Curr Pharmacol Rep 5:255–280
Amuri B, Maseho M, Simbi L, Okusa P, Duez P, Byanga K (2017) Hypoglycemic and antihyperglycemic activities of nine medicinal herbs used as antidiabetic in the region of Lubumbashi (DR Congo). Phytother Res 31:1029–1033
Assaei R, Mokarram P, Dastghaib S, Darbandi S, Darbandi M, Zal F, Akmali M, Omrani GHR (2016) Hypoglycemic effect of aquatic extract of Stevia in pancreas of diabetic rats: PPARγ-dependent regulation or antioxidant potential. Avicenna J Med Biotechnol 8:65
Association AD (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care 44:S15–S33
Baharvand-Ahmadi B, Bahmani M, Tajeddini P, Naghdi N, Rafieian-Kopaei M (2016) An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J Nephropathol 5:44
Balakrishnan BB, Krishnasamy K, Mayakrishnan V, Selvaraj A (2019) Moringa concanensis Nimmo extracts ameliorates hyperglycemia-mediated oxidative stress and upregulates PPARγ and GLUT4 gene expression in liver and pancreas of streptozotocin-nicotinamide induced diabetic rats. Biomed Pharmacother 112:108688
Bano S, Sharif A, Akhtar B, Abdel-Daim MM, Akhtar MF, Ali FL (2023) Mechanistic insights on the possible protective role of polyphenols extracted from Tamarix aphylla aerial parts against sodium arsenite-induced hepatotoxicity in rats. Environ Sci Pollut Res Int 30:16565–16578
Barkaoui M, Katiri A, Boubaker H, Msanda F (2017) Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J Ethnopharmacol 198:338–350
Bodduluru LN, Kasala ER, Madhana RM, Barua CC, Hussain MI, Haloi P, Borah P (2016) Naringenin ameliorates inflammation and cell proliferation in benzo (a) pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int Immunopharmacol 30:102–110
Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sánchez-Acuña G (2013) Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumor. Medicina Oral Patologia Oral y Cirugia Bucal 18:e174
Chaari A, Abdellatif B, Nabi F, Khan RH (2020) Date palm (Phoenix dactylifera L.) fruit’s polyphenols as potential inhibitors for human amylin fibril formation and toxicity in type 2 diabetes. Int J Biol Macromol 164:1794–1808
Chandran R, Parimelazhagan T, Shanmugam S, Thankarajan S (2016) Antidiabetic activity of Syzygium calophyllifolium in Streptozotocin-Nicotinamide induced Type-2 diabetic rats. Biomed Pharmacother 82:547–554
Dias TR, Alves MG, Casal S, Oliveira PF, Silva BM (2017) Promising potential of dietary (poly) phenolic compounds in the prevention and treatment of diabetes mellitus. Curr Med Chem 24:334–354
Duchen MR (2004) Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 25:365–451
El-Hadary AE, Ramadan MF (2019) Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J Food Biochem 43:e12803
Farhangi MA, Javid AZ, Dehghan P (2016) The effect of enriched chicory inulin on liver enzymes, calcium homeostasis and hematological parameters in patients with type 2 diabetes mellitus: a randomized placebo-controlled trial. Prim Care Diabetes 10:265–271
Gharib E, Kouhsari SM, Izad M (2018) Punica granatum L. fruit aqueous extract suppresses reactive oxygen species-mediated p53/p65/miR-145 expressions followed by elevated levels of irs-1 in alloxan-diabetic rats. Cell Journal (Yakhteh) 19:520
Giovannini P, Howes M-JR, Edwards SE (2016) Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: a review. J Ethnopharmacol 184:58–71
Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman H-J (2011) The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med 61:356–360
Haeusler RA, Camastra S, Astiarraga B, Nannipieri M, Anselmino M, Ferrannini E (2015) Decreased expression of hepatic glucokinase in type 2 diabetes. 4:222–226
Harkin LF, Gerrelli D, Gold Diaz DC, Santos C, Alzu’bi A, Austin CA, Clowry GJ (2016) Distinct expression patterns for type II topoisomerases IIA and IIB in the early foetalHaeusler human telencephalon. J Anat 228:452–463
Hassan SK, El-Sammad NM, Mousa AM, Mohammed MH, Hashim ANE, Werner V, Lindequist U, Nawwar MAE-M (2015) Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 5:462–471
Hemmati M, Serki E, Gholami M, Hoshyar R (2017) Effects of an ethanolic extract of Berberis vulgaris fruits on hyperglycemia and related gene expression in streptozotocin-induced diabetic rats. Clin Phytoscience 2:3
Ijaz F, Iqbal Z, Rahman IU, Alam J, Khan SM, Shah GM, Khan K, Afzal A (2016) Investigation of traditional medicinal floral knowledge of Sarban Hills, Abbottabad, KP, Pakistan. J Ethnopharmacol 179:208–233
Irudayaraj SS, Stalin A, Sunil C, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S (2016) Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem Biol Interact 256:85–93
Justino AB, Miranda NC, Franco RR, Martins MM, Silva NMd, Espindola FS (2018) Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed Pharmacother 100:83–92
Kang HS, Cho H-C, Lee J-H, Oh GT, Koo S-H, Park B-H, Lee I-K, Choi H-S, Song D-K, Im S-S (2016) Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states. Sci Rep 6:1–13
Khan D, Sharif A, Zafar M, Akhtar B, Akhtar MF, Awan S (2020) Delonix regia a folklore remedy for diabetes; attenuates oxidative stress and modulates type ii diabetes mellitus. Curr Pharm Biotechnol 21(11):1059–1069
Kim HM, Kang JS, Kim JY, Park S-K, Kim HS, Lee YJ, Yun J, Hong JT, Kim Y, Han S-B (2010) Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int Immunopharmacol 10:72–78
Lalitha N, Sadashivaiah B, Talahalli RR, Singh SA (2020) Lectin rich horsegram protein and myricetin activates insulin signaling–A study targeting PTP1β. J Funct Foods 67:103845
Lewitt MS, Dent MS, Hall K (2014) The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J Clin Med 3:1561–1574
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230
Malik M, Sharif A, Hassan SU, Muhammad F, Khan HM, Akhtar B, Saeed M (2022) Amelioration of hyperglycaemia and modulation of pro-inflammatory cytokines by Tamarix gallica fractions in alloxan induced diabetic rats. Archives of Physiologyand Biochemistry 128:1666–1675
Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol 5:150–159
Mousavi L, Salleh RM, Murugaiyah V, Asmawi MZ (2016) Hypoglycemic and anti-hyperglycemic study of Ocimum tenuiflorum L. leaves extract in normal and streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 6:1029–1036
Mousavi L, Salleh RM, Murugaiyah V (2018) Phytochemical and bioactive compoundsTherapeutic Potential of Dietary Phenolic Acids. Advances identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. Int J Food Prop 21:2390–2399
Nakae J, Kitamura T, Silver DL, Accili D (2001) The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367
Omara EA, Kam A, Alqahtania A, Li KM, Razmovski-Naumovski V, Nammi S, Chan K, Roufogalis BD, Li GQ (2010) Herbal medicines and nutraceuticals for diabetic vascular complications: mechanisms of action and bioactive phytochemicals. Curr Pharm Des 16:3776–3807
Papich MG (2007) Saunders handbook of veterinary drugs. Elsevier
Pari L, Chandramohan R (2017) Modulatory effects of naringin on hepatic key enzymes of carbohydrate metabolism in high-fat diet/low-dose streptozotocin-induced diabetes in rats. Gen Physiol Biophys 36:343–352
Passarelli M, Machado UF (2021) AGEs-induced and endoplasmic reticulum stress/inflammation-mediated regulation of GLUT4 expression and atherogenesis in diabetes mellitus. Cells 11:104
Patel D, Kumar R, Laloo D, Hemalatha S (2012) Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed 2:411–420
Peters AL, Davidson MB (1996) Maximal dose glyburide therapy in markedly symptomatic patients with type 2 diabetes: a new use for an old friend. J Clin Endocrinol Metab 81:2423–2427
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Rossi G (2018) Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69
Rouhi SZT, Sarker MMR, Rahmat A, Alkahtani SA, Othman F (2017) The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. BMC Complement Altern Med 17:156
Sabry MM, Mahmoud MM, Shoukry HS, Rashed L, Kamar SS, Ahmed MM (2019) Interactive effects of apelin, renin–angiotensin system and nitric oxide in treatment of obesity-induced type 2 diabetes mellitus in male albino rats. Arch Physiol Biochem 125:244–254
Saibabu V, Fatima Z, Khan LA, Hameed S (2015) Therapeutic potential of dietary phenolic acids. Advances in pharmacological sciences 2015:823539
Soni LK, Dobhal MP, Arya D, Bhagour K, Parasher P, Gupta R (2018) In vitro and in vivo antidiabetic activity of isolated fraction of Prosopis cineraria against streptozotocin-induced experimental diabetes: a mechanistic study. Biomed Pharmacother 108:1015–1021
Stalin A, Irudayaraj SS, Gandhi GR, Balakrishna K, Ignacimuthu S, Al-Dhabi NA (2016) Hypoglycemic activity of 6-bromoembelin and vilangin in high-fat diet fed-streptozotocin-induced type 2 diabetic rats and molecular docking studies. Life Sci 153:100–117
Tag H, Kalita P, Dwivedi P, Das A, Namsa ND (2012) Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. J Ethnopharmacol 141:786–795
Thomson M, Al-Qattan KK, Divya J, Ali M (2015) Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med 16:17
Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S (2015) Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med 15:242
Vinayagam R, Jayachandran M, Xu B (2016) Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother Res 30:184–199
Wang J, Wang C, Li S, Li W, Yuan G, Pan Y, Chen H (2017) Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother 95:1669–1677
Yelumalai S, Giribabu N, Kamarulzaman Karim SZO, Salleh NB (2019) In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Arch Med Sci: AMS 15:240
Zafar M, Sharif A, Khan D, Akhtar B, Muhammad F, Akhtar MF, Fatima TJTR (2021) Preventive effect of Euphorbia royleana Boiss on diabetes induced by streptozotocin via modulating oxidative stress and deoxyribonucleic acid damage. 40:777–790
Zhang Y, Hu T, Zhou H, Zhang Y, Jin G, Yang Y (2016) Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int J Biol Macromol 83:126–132
Zhi W, Liu Y, Wang X, Zhang H (2023) Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis. J Ethnopharmacol 301:115749
Zulfqar F, Akhtar MF, Saleem A, Akhtar B, Sharif A, Saleem U (2020) Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (Pine nuts) extracts. J Food Biochem 44:e13199
Author information
Authors and Affiliations
Contributions
Afshan Asghar performed most of the experimental work and participated in data interpretation. Bushra Akhtar and Muhammad Furqan Akhtar participated in the data analysis and statistical analysis. Ali Sharif and Sana Javaid Awan conceived the study and carried out the experimental design and data interpretation. Ali Sharif, Shahnaz, and Sajid Ali prepared and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted followed by the approval of Animal Ethical Committee (IREC-2019–87) in accordance with the NC3Rs ARRIVE Guidelines, adhere to ethical guidelines of The Basel Declaration, the International Council for Laboratory Animal Science (ICLAS) ethical guidelines, and Directive 2010/63/EU.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asghar, A., Sharif, A., Awan, S.J. et al. “Ficus johannis Boiss. leaves ethanolic extract ameliorate streptozotocin-induced diabetes in rats by upregulating the expressions of GCK, GLUT4, and IGF and downregulating G6P”. Environ Sci Pollut Res 30, 49108–49124 (2023). https://doi.org/10.1007/s11356-023-25765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25765-4