Abstract
Ethylenediaminetetraacetic acid (EDTA) washing has been used extensively to remediate heavy metal-contaminated soils. Electrochemical reduction treatment of spent washing solution is an effective method of EDTA regeneration. However, at present, these two technologies are usually regarded as two independent treatment processes. This research raised a new heavy metal-contaminated soil treatment strategy—a combination technique of coupled EDTA washing and electrochemical reduction. We speculated that the combination of EDTA washing and electroreduction treatment could improve the efficiency of Cd and Pb removal from contaminated soil. In this study, the removal performance and mechanisms of Cd and Pb under different current conditions were investigated based on a coupling of EDTA washing and electrochemical reduction. The combination technique can increase Cd and Pb removal efficiencies by 13.37–15.24% and 14.91–27.05%, respectively, compared with EDTA washing alone. Sequential extraction analysis showed that the reducible fraction improved metal removal efficiency. The percentage of metal removed increased with an increased current value and EDTA concentration. In addition, pulse current mode removed more Cd and Pb than continuous current, although the difference was not significant (p > 0.05). However, pulse current could effectively eliminate the cathodic hydrogen evolution reaction, resulting in a further heavy metal deposition at the cathode. The combination technique exhibited enhanced removal efficiency due to EDTA regeneration in the suspension and the cathodic reduction reaction. The most cost-effective treatment in 48 h was a pulse current mode of 32 min on/16 min off-32 mA-EDTA-10 mM, where 47.56% of Cd and 77.00% of Pb were removed from the soil with an electric energy consumption of 8.24 Wh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past two centuries, contamination caused by mining various heavy metals (such as Cd and Pb) has been severe and widespread (Hansen and Rojo 2007). Several remediation strategies have been developed and proposed for cleaning soils contaminated with heavy metals (Dermont et al. 2008; Yang et al. 2009; Jiang et al. 2010; Kołodyńska 2013; Zhou et al. 2014; Jelusic et al. 2014; Zhou et al. 2014; Pedersen et al. 2015; Pańczuk-Figura and Kołodyńska 2016; He et al. 2019; Kaurin et al. 2020; Liu et al. 2020).
Soil washing, which involves the separation of contaminants from the soil by solubilizing them in a washing solution, is one of the most frequently used techniques (Im et al. 2015; Wang et al. 2016; Pourfadakari et al. 2019; Kaurin et al. 2020). As a widely used washing solution, ethylenediaminetetraacetic acid (EDTA) can combine with several types of toxic metal ions in soil to form stable (Me-EDTA)(4−n)− complexes, as illustrated in Eq. (1) (Jelusic et al. 2014; Song et al. 2016, 2019a, b; Kaurin et al. 2020). Moreover, some previous studies (Im et al. 2015; Pourfadakari et al. 2019; Song et al., 2022) have demonstrated the cost-effectiveness of using EDTA. After EDTA washing, most of the available forms of metals are extracted—removed from the soil matrix and transferred to the washing solution.
Numerous strategies have been developed to remove heavy metals from the spent washing solution. Electrochemical reduction (electrodeposition) is one of the most recent technologies applied for heavy metal removal (recovery) based on the chelating effect. In addition, Nepel has confirmed that electrochemical reduction can reuse the washing solution (de Morais Nepel et al. 2020). Many studies have confirmed that the cathodic removal of heavy metals has several benefits in terms of costs, safety, and versatility (Paul Chen and Lim 2005; Peng et al. 2011). The migration of metal-chelate complexes is based on the application of a low electric field generated by an anode and a cathode, and the process potentially uses a cation exchange membrane (CEM) to separate the anode and cathode compartment (Xu et al. 2020), as follows:
-
a)
The (Me-EDTA)(4−n)− electromigrated toward the anode can be anodically degraded. As illustrated in Eqs. (2–4) (Song et al. 2019a, b), leading to the release of metals and loss of chelating materials:
$$\mathrm{EDTA}+{\mathrm{H}}_{2}\mathrm{O}\to {\mathrm{ED}}_{3}\mathrm{A}+{\mathrm{CO}}_{2}+{\mathrm{CH}}_{2}\mathrm{O}+{\mathrm{H}}^{+}+{e}^{-}$$(2)$${\mathrm{ED}}_{3}\mathrm{A}+{\mathrm{H}}_{2}\mathrm{O}\to \mathrm{EDDA}+{\mathrm{CO}}_{2}+{\mathrm{CH}}_{2}\mathrm{O}+{\mathrm{H}}^{+}+{e}^{-}$$(3)$$\mathrm{EDDA}+{\mathrm{H}}_{2}\mathrm{O}\to \mathrm{EDMA}+{\mathrm{CO}}_{2}+{\mathrm{CH}}_{2}\mathrm{O}+{\mathrm{H}}^{+}+{e}^{-}$$(4) -
b)
The complexes in the cathode compartment, on the contrary, can be electrodeposited on the cathode, which can carry out metal recovery and provide a chelating agent regenerated simultaneously, as illustrated in Eq. (5) (Song et al. 2019a, b):
$${(\mathrm{Me}-\mathrm{EDTA})}^{\left(4-n\right)-}+\left(4-n\right){e}^{-}\to \mathrm{Me}+\mathrm{EDTA}$$(5)
The application of continuous current (CC) is the most applied current mode in the electrochemical reduction processes. Several studies have reported that Cu can be removed from alkaline synthetic wastewater baths using CC (Dudek and Fedkiw 1999; Souto et al. 2011). However, there are various competing reactions at the cathode that affect the mass transport process of heavy metals. The most common competing reaction is when the H+ ions are reduced to hydrogen gas (H2), as illustrated in Eq. (6) (Paul Chen and Lim 2005; Peng et al. 2011).
In some cases, the H2 can cause loose or spongy deposits, and therefore, interference from the H2 evolution reaction should be minimized (Paul Chen and Lim 2005). Recently, some studies have highlighted that pulse current (PC) could obtain the coating with smoothness and homogeneity composition (Baskaran et al. 2006; Yang et al. 2016). It illustrated that the variation in the current mode enables the removal of Cu with the formation from crystalline oxides to crystalline Cu in its metallic form (de Morais Nepel et al. 2020). These studies indicated that the current conditions during the electrodeposition process could influence both the heavy metal removal behavior and the mass transport process of the electroreduction.
Many studies have focused on EDTA washing and its subsequent electrochemical reduction treatments but have considered the two technologies as two independent processing steps. Few studies have attempted to combine the two treatments. In this study, we propose a treatment involving the coupling of EDTA washing and electrochemical reduction. Heavy metal-contaminated soil (target metals were Cd and Pb) collected from a mining area was used as the test soil. Laboratory experiments to treat soil suspensions were performed based on both coupling strategies under different current conditions (current value and current mode) and two individual strategies. The primary objectives of this study were to compare the target metal removal ability using a combination technique and the two processes independently to clarify the target metal transformation and migration mechanisms under different current conditions and provide essential data and reference for further development of the combination technique.
Materials and method
Chemicals, electrodes, and membrane materials
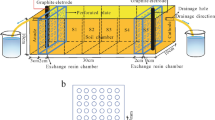
All chemicals used in this study were analytical reagent grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). A graphite electrode sheet with dimensions of 100 mm [L] × 50 mm [W] × 1 mm [H] and an ASTM 304 stainless steel sheet with the same dimensions were used as the anode and the cathode respectively. Neosepta CMB® (Astom, Japan) was selected as the cation exchange membrane material.
Experimental soil
The contaminated soil used for remediation experiments was collected from an actual mining area in Huludao City, Liaoning Provence, China. Table 1 lists the main characteristics of the soil. The collected soil was oven-dried at 40 °C. Then, the material was pulverized and sieved with a 100-mesh sieve until a homogeneous sample was obtained. The soils were then sealed and stored at 20 °C for subsequent experiments.
Experimental setup design
A schematic of the remediation setup and the conceptual diagram of current variation with time for different current modes are illustrated in Fig. 1. The experimental setup was a cuboid composed of plexiglass, with dimensions of 180 mm [L] × 60 mm [W] × 60 mm [H]. The device was separated into two parts using a CEM. One part was 120-mm-long and was used as a washing suspension compartment, and the other part, a 60-mm-long compartment, was used as the anode reservoir. The two electrodes were vertically immersed in the two compartments. The power supply (ITech, IT6322A, China) was connected to the electrode wires to provide CC and PC. Under PC, a cyclic process was obtained comprising a period with the current “ON” (i.e., application of an electric field) followed by a period with the current “OFF” (i.e., no application of an electric field) (de Morais Nepel et al. 2020). The electrical energy consumption was recorded using a host computer (ITech-IT9000).
Experimental plan
The experimental plan (three independent experiments and seven coupling experiments with a remediation time of 48 h) was conducted under the conditions listed in Table 2. The duration was designed to maintain an identical total charge transfer between CC and PC experiments under the same current (Sun et al. 2013a, b). Among them, W1 and W2 were prepared by single EDTA washing for 48 h, during which the contaminated soil suspension was prepared with 40 g contaminated soil and 200 mL extracting solution. EDTA concentrations were 10 mM and 50 mM, respectively, following a series of extraction experiments with different concentrations of leaching materials on the experimental soil (Fig. A1 details the results). T0 was a control test under experimental conditions similar to T1 but with the soil washed with deionized water (DW). Soil suspensions for T1–T7 were prepared using EDTA solution (10 mM) with a liquid/soil ratio of 5:1, which is the same as that of EDTA washing. T1 and T2 were prepared with different EDTA concentrations (10 mM and 50 mM), and the coupled electrochemical reduction process was carried out using a CC of 32 mA. T3 and T4 were carried out using different current values and similar EDTA concentrations based on CC conditions (16 mA–10 mM and 64 mA–10 mM, respectively). T5, T6, and T7 were carried out under similar current value and EDTA concentration conditions (32 mA–10 mM), with PC ratios (ton/toff) of 16 min/16 min, 32 min/16 min, and 48 min/16 min, respectively. The soil suspension and a sodium nitrate supporting electrolyte (0.1 mM, 100 mL) were added to the corresponding compartments. At 6, 12, 24, 36, and 48 h (actual power “ON” time), 5 mL of the soil suspension was taken-out of the soil washing compartment and centrifuged at 8000 rpm (RCF = 6793 g\(\times\)) for 5 min to achieve liquid–soil separation. The supernatant was filtered through a 0.45-μm membrane and stored in a 10-mL plastic colorimetric tube. The solid phase was collected by rinsing twice with purified water, completely air-dried, and preserved for later determination and analysis.
Analysis and calculation
Soil pH
Soil pH was measured by suspending 5.0 g dry soil in 25 mL distilled water. After agitation, pH was measured using a pH analyzer (Orion Star A211, Thermo Fisher Scientific, Waltham, MA, USA).
Soil heavy metal concentrations
Total Cd and Pb in the soil were digested using the HNO3-HF-HClO4 digestion method (Gao et al. 2013b, a; Tang et al. 2017) and then measured with atomic absorption spectrometry (AAS-Thermo, Ice3000 series). AAS also measured the concentration of Cd and Pb in the liquid phase. Analysis was conducted in triplicate in both cases, and mean values were used.
Removal efficiency and mass balance
The removal efficiency was calculated using Eq. (7):
where m0 refers to the mass of heavy metal in the initial soil and m1 refers to the mass of heavy metals remaining in the soil after treatment. The mass balance was defined based on the relationship between the sum of mass found in different cell parts at the end of the experiment. The initial mass was calculated based on the mean initial concentration (Sun and Ottosen 2012).
BCR sequential extraction
Modified BCR (European Community Bureau of Reference) sequential extraction was performed to monitor changes in heavy metal fractions (Yang et al. 2009). The metal fractions were classified as F1 exchangeable (EXC), F2 reducible (RED), F3 oxidizable (OXI), and F4 residual (RES).
Energy consumption
Based on voltage and current, energy consumption was calculated using Eq. (8):
where E is the energy consumption (Wh), V is the voltage between the working electrodes (V), I is the current (A), and t is the duration (h).
Electrodeposition morphology analysis
The electrodeposition coatings deposited on the cathode were further analyzed using a scanning electron microscope (SEM) (Quanta 250 FEG, USA).
Results and discussion
Variation of soil pH
Figure 2 shows the variation in soil pH. The initial soil pH was 6.9, and it decreased after EDTA washing. Similar drops in pH have also been observed in a previous study (Kaurin et al. 2020). Soil pH increased after the combination technique compared to initial values because of the OH− generated at the cathode (Yeung and Gu 2011). Due to OH− neutralization, soil pH reached about 7.0 and remained constant until the T1, T2, T3, T5, T6, and T7 experiments were finished. Since the pH variation in the T5–T7 experiments was similar to that in T1 under the same current value (32 mA), current modes had minimal effect on soil pH in the coupling condition. The high current value applied in the T4 treatment resulted in the soil pH increased to approximately 9.4. Some researchers (Zhou et al. 2014) have reported that when the applied current value reaches the limiting current density of an ion exchange membrane, water splitting occurs at the interface between CEM and the suspension. Therefore, CEM hinders the migration of H+ and OH− in the electric field, increasing soil pH. The magnitude of the current applied played a significant role in controlling soil pH during the combination process.
Target metal removal
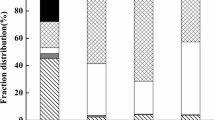
Figure 3 shows the variation in target metal removal efficiency under separate processes and coupled processes during the remediation. It illustrates that target metals were further removed from the soil suspension due to the combination technique, although with varying removal efficiencies. Cd was removed from the soil matrix in the following order of efficiency: T2 (CC–32 mA–50 mM): 54.71% > T4 (CC–64 mA): 52.12% > T6 (PC–32 min/16 min–32 mA): 47.56% > T5 (PC–16 min/16 min–32 mA): 47.25% > T1 (CC–32 mA–10 mM): 46.60% > T7 (PC–48 min/16 min–32 mA): 46.01% > W2 (EDTA–50 mM): 41.43% > T3 (CC–16 mA): 37.75% > W1 (EDTA–10 mM): 31.36% > T0 (DW): 5.98%. Conversely, Pb was removed from the soil matrix in the following order of efficiency: T2 (CC–32 mA–50 mM): 80.93% > T4 (CC–64 mA): 80.50% > T6 (PC–32 min/16 min–32 mA): 77.00% > T1(CC–32 mA–10 mM): 76.93% = T7 (PC–48 min/16 min–32 mA): 76.93% > T5 (CC–16 min/16 min–32 mA): 75.60% > T3 (CC–16 mA): 66.23% > W2 (EDTA–50 mM): 66.02% > W1 (EDTA–10 mM): 49.88% > T0 (DW): 6.47%. Furthermore, Fig. 4 depicts the changes in the distributions of target metal contents in soil suspension (in the soil or liquid phases) of all experiments. The mass of the target metals in the soil phase of the soil suspension decreased obviously after treatment compared to their initial values. Figure 5 illustrates BCR fractionation before and after all experiments. The target metal recoveries of BCR ranged from 98 to 108%.
Metal removal: comparison of combination technique and separate processes
Figure 3 illustrates that the target metals were not significantly removed from the soil phase through a single electrochemical reduction process in the T0 (control) treatment. The reason was that deionized water was used as the washing solution, and the hydroxide ions generated by electrolysis precipitated heavy metals, decreasing metal removal efficiency (Jensen et al. 2006). According to the results, EDTA played a key mediating role between metal desorption from soil particle surfaces and electrodeposition. In addition, EDTA can inhibit cathodic electrolysis reactions and makes electrochemical reduction reactions more efficient.
Concerning the effect of the electrochemical reduction process, under similar EDTA concentrations, the combination technique yielded higher metal removal efficiencies than single EDTA washing. Figure 3 illustrates that removal efficiency under coupling increases with increased current values (T1 vs. T3, T4), implying that a higher current facilitates heavy metal removal from the soil matrix. In addition, the current mode (CC and PC) at the same value (32 mA) achieved almost the same effect of improving heavy metal removal efficiency during the remediation experiments (T1 vs. T5, T6, T7). Compared with separate processes, the efficiency of target metal removal from the soil phase under the combination technique increased in the cases of Cd and Pb by 6.39–20.76% and 16.35–30.62%, respectively. However, Cd removal from the soil phase was lower than Pb. The main reason was that the stability constant (K) of the PbEDTA2− complex is more considerable than that of CdEDTA2− (Begum et al. 2013). Compared with the original soil, the exchangeable and reducible Pb decreased more than Cd. In addition, Cd mainly exists in the form of residue, which is more closely bound to soil particles and is more difficult to remove from the soil.
It has been confirmed that the extraction efficiency of EDTA decreases and then increases with the increase of pH (Begum et al. 2012). In this study, the extraction efficiency of W2 was more remarkable than W1, which may be caused by the superposition of two factors, the increase of EDTA concentration and the decrease of pH of the soil suspension. A similar study (Begum et al. 2013) also found the difference in extraction efficiency caused by different concentrations of EDTA. After W1 and W2 achieved the optimal extraction effect, the target metal removal efficiencies under the coupled process increased continuously during the remediation, confirming the combination technique’s synergistic enhancement effect.
Interestingly, it can be seen from Fig. 2 that the pH of T5–T7 after treatment was less than T2; however, the removal efficiency of T5–T7 for heavy metals was higher than T2. Theoretically, a high pH means a high chelating ability of EDTA, which has been confirmed that the corresponding complexes of the ML systems (M = Cd2+,Pb2+, L = EDTA) have the following log sequences: (a) Cd2+: log KML (pH = 7) < log KML (pH = 8), (b) Pb2+: log KML (pH = 7) < log KML (pH = 8) (Begum et al. 2013). Such an observation indicated that except for pH, other factors affect the removal efficiency of Cd and Pb in the soil. On the one hand, when the electrochemical reduction occurs at the cathode, thereby facilitating EDTA regeneration, which could further extract the heavy metals on soil particle surfaces; this was proved by a previous study as a synergistic effect of coupling strategy (Song et al. 2022). On the other hand, enhancement of target metal removal efficiency under the coupled condition could be related to Cd and Pb fractionation, which were weakly bound to the soil.
According to Fig. 5, the exchangeable and residual fractions were the dominant Cd BCR fractions in the contaminated soil in the initial state, accounting for 26% and 40% of the fractions, respectively. Conversely, Pb contained more reducible and exchangeable fractions, which accounted for 32% and 35%, respectively. The target metals in the residual fraction were the major contaminants remaining in the soil phase after remediation. They were strongly bound to the soil and considerably immobile during treatment (Ryu et al. 2009). After EDTA washing (W1 and W2), the mass of various forms of Cd and Pb in the soil decreased, and the exchangeable heavy metals largely dropped. In addition, the reducible Cd and Pb percentages in soil decreased in the T1–T7 treatments and accounted for significant proportions of total metal removals from the soil phase. Numerous researchers have reported similar observations (Gao et al. 2013b, a; Wang et al. 2016). The results indicate that the coupling strategy can effectively remove the reducible heavy metals from the soil.
Metal removal: comparison of CC and PC modes in the combination technique
Figure 4 shows barely any difference in the mass of target metal remaining in the soil based on different current modes under similar current conditions (T1 vs. T5, T6, T7). A similar study observed no significant improvement under PC in Cu-contaminated soil (Sun et al. 2012). Figure 5 also indicates that the target metals in the exchangeable and reducible fractions decreased. In contrast, the oxidizable and residual fractions increased following a coupling strategy based on both CC and PC. However, according to the mass balance (Table 3), Cd in the supernatant decreased from 0.186 mg to 0.059–0.090 mg, whereas Pb content dropped from 5.163 mg to 1.226–1.991 mg. Considering the almost similar speciation fractions and different electrodeposition mass following the coupling strategy, PC did not influence soil fractionation and may have only influenced the mass transport process between the suspension and the cathode.
Figures 6 and 7 show the surface morphologies of the target metal deposits obtained under CC and PC. From Fig. 6, it was evident that there were many apparent pits on the deposit surfaces based under CC. In addition, higher current values increased the number and size of pits on the surface. Loose and spongy deposits have been attributed to the existence or formation of some interfacial inhibitors, which influence the surface morphology of electrodepositions, such as H2 generation during the deposition process (Paul Chen and Lim 2005). However, the deposit surfaces under PC (Fig. 7) were smooth, excluding T7, which contained some minor pits. Compared to the process under PC, the limiting factor of the poor deposition effect under CC is mainly due to a cathodic H2 evolution reaction (El-Sherik et al. 1997; Paul Chen and Lim 2005), which played a significant role in electrodeposition morphology. Such pits resulted from H2 bubbles that remained attached to cathode surfaces for extended periods. The different rates of the electrode reactions and mass transport processes resulted in significant drops in the concentrations of (Me-EDTA)(4−n)− at the cathode surface diffusion layer, leading to a competing reaction, in this case, H2 evolution, as illustrated in Eq. (6). Therefore, the H2 barrier obstructed the mass transport process between the suspension supernatant and cathode surface. A short period in “OFF” for the diffusion phenomenon can be used during the interval of the off-time so that the diffusion gradients (produced during the “ON” time) can be diminished (Hansen and Rojo 2007) and (Me-EDTA)(4−n)− at the cathode surface replenished adequately. Hence, the H2 evolution reaction was effectively shielded (Paul Chen and Lim 2005), and the mass transport barrier caused by H2 was broken. Considering the relatively more uniform deposits obtained from the T5–T7 treatments, it can be concluded that PC can improve the grain structure of the deposited coating with refined crystalline grains and homogeneity (Baskaran et al. 2006; Palacios-Padrós et al. 2010; Caballero-Briones et al. 2011). However, in the PC mode, it is also necessary to avoid setting an unreasonable pulse ratio, which may cause H2 evolution reactions like in the T7 treatment.
The most intriguing aspect of the results is that the mass of target metal in suspension increased during the power-off period and dropped during the subsequent power-on period (Fig. 8). It implied that the treatment process in the washing suspension could increase the redox potential in the soil suspension through aeration (Sun et al. 2013a, b) and provide a basis for the oxidation of heavy metals that have been deposited on the cathode. The re-oxidized metal ions are pulled back into the soil suspension by a large amount of regenerated EDTA, as illustrated in Eq. (5), resulting in a rise of mass in suspension. An automatic lifting device that matches the pulse frequency could be installed to address such a shortcoming. When the PC is off during the cycle, the automatic lifting device lifts the cathode out of the washing suspension, which protects the metal coating deposited on the cathode from desorption during the power-off period.
Electrical energy consumption
Energy consumption is one of the most important considerations when considering electrochemical reduction to apply to treat contaminated soils. Figure 9 shows that electric energy consumption increased with processing time. The T4 treatment achieved a maximum total energy consumption of 18.40 Wh, whereas 7.01 Wh (T3) was the minimum value in all the experiments. Under similar current values and EDTA concentrations, the combination technique based under CC required more electric energy than those based on PC (T1 > T7 > T5 > T6), which confirmed that PC effectively alleviated the excess energy losses caused by mass transport obstacles. Table 3 lists the energy consumed to remove 1% of target metals from soil. Under similar removal efficiencies from the soil, the cost increased with the applied current value. The PC consumed lower electrical energy compared with CC. Considering electrical energy consumption and the mass of heavy metals removed, T6 (32 min on/16 min off‒32 mA) was the most cost-effective treatment for removing heavy metals, demonstrating the advantages of the PC in reducing energy consumption.
Conclusion
This study investigated the Cd and Pb removal behavior and mechanisms following the coupling of EDTA washing and electrochemical reduction. The removal efficiencies and mechanisms were studied under varying soil pH and current conditions, the target metal removal efficiencies, and energy consumption were compared among different treatments. The following conclusions were obtained:
EDTA played a critical mediating role in the mass transfer between metal desorption from soil. The combination technique coupling of EDTA washing and electrochemical reduction improved target metal (Cd and Pb) removal efficiencies under the present experimental conditions. The combination technique can increase the pH of the soil and improves the extraction efficiency of EDTA. The synergistic enhancement could regenerate EDTA in the suspension during remediation to further extract heavy metals from soil and promote further removal of reducible fractions of heavy metals from the soil. Compared with the CC current mode, the PC condition did not improve target metal (Cd and Pb) removal efficiency from the soil based on the coupled approach; furthermore, the PC condition could not alter the speciation fractions of heavy metals. However, the dissolved metals under CC conditions mainly stayed in the liquid phase of the suspension, which required more remediation time or further treatment of the lixiviant using other methods. In contrast, the PC mode could alleviate the mass transport obstacles caused by cathodic H2 evolution, promote mass transfer between suspension supernatant and the cathode surface, improve the grain structure of the deposited coating, and reduce energy consumption.
In brief, EDTA washing and electrochemical reduction coupling is an innovative remediation strategy for enhanced heavy metal removal from the soil in mining areas.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary material.
References
Baskaran I, Narayanan TSNS, Stephen A (2006) Pulsed electrodeposition of nanocrystalline Cu–Ni alloy films and evaluation of their characteristic properties. Mater Lett 60:1990–1995. https://doi.org/10.1016/j.matlet.2005.12.065
Begum ZA, Rahman IMM, Tate Y, Sawai H, Maki T, Hasegawa H (2012) Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants. Chemosphere 87:1161–1170. https://doi.org/10.1016/j.chemosphere.2012.02.032
Begum ZA, Rahman IMM, Sawai H, Mizutani S, Maki T, Hasegawa H (2013) Effect of extraction variables on the biodegradable chelant-assisted removal of toxic metals from artificially contaminated European reference soils Water, Air, & Soil Pollution 224https://doi.org/10.1007/s11270-012-1381-4
Caballero-Briones F, Palacios-Padrós A, Sanz F (2011) CuInSe2 films prepared by three step pulsed electrodeposition. Deposition mechanisms, optical and photoelectrochemical studies. Electrochim Acta 56:9556–9567. https://doi.org/10.1016/j.electacta.2011.06.024
de Morais Nepel TC, Landers R, Vieira MGA, de Almeida Neto AF (2020) Metallic copper removal optimization from real wastewater using pulsed electrodeposition. J Hazard Mater 384:121416. https://doi.org/10.1016/j.jhazmat.2019.121416
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31. https://doi.org/10.1016/j.jhazmat.2007.10.043
Dudek DA, Fedkiw PS (1999) Electrodeposition of copper from cuprous cyanide electrolyte: I. Current distribution on a stationary disk. J Electroanal Chem 474:16–30. https://doi.org/10.1016/S0022-0728(99)00299-5
El-Sherik AM, Erb U, Page J (1997) Microstructural evolution in pulse plated nickel electrodeposits. Surf Coat Technol 88:70–78. https://doi.org/10.1016/S0257-8972(96)02928-3
Gao J, Luo Q, Zhang C, Li B, Meng L (2013a) Enhanced electrokinetic removal of cadmium from sludge using a coupled catholyte circulation system with multilayer of anion exchange resin. Chem Eng J 234:1–8. https://doi.org/10.1016/j.cej.2013.08.019
Gao J, Luo Q-S, Zhu J, Zhang C-B, Li B-Z (2013b) Effects of electrokinetic treatment of contaminated sludge on migration and transformation of Cd, Ni and Zn in various bonding states. Chemosphere 93:2869–2876. https://doi.org/10.1016/j.chemosphere.2013.08.079
Hansen HK, Rojo A (2007) Testing pulsed electric fields in electroremediation of copper mine tailings. Electrochim Acta 52:3399–3405. https://doi.org/10.1016/j.electacta.2006.07.064
Im J, Yang K, Jho EH, Nam K (2015) Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 138:253–258. https://doi.org/10.1016/j.chemosphere.2015.06.004
Jelusic M, Vodnik D, Macek I, Lestan D (2014) Effect of EDTA washing of metal polluted garden soils. Part II: can remediated soil be used as a plant substrate? Sci Total Environ 475:142–152. https://doi.org/10.1016/j.scitotenv.2013.11.111
Jensen PE, Ottosen LM, Pedersen AJ (2006) Speciation of Pb in industrially polluted soils. Water Air Soil Pollut 170:359–382. https://doi.org/10.1007/s11270-005-9008-7
Jiang C-a, Wu Q-T, Sterckeman T, Schwartz C, Sirguey C, Ouvrard S, Perriguey J, Morel J-L (2010) Co-planting can phytoextract similar amounts of cadmium and zinc to mono-cropping from contaminated soils. Ecol Eng 36:391–395. https://doi.org/10.1016/j.ecoleng.2009.11.005
Kaurin A, Gluhar S, Tilikj N, Lestan D (2020) Soil washing with biodegradable chelating agents and EDTA: effect on soil properties and plant growth. Chemosphere 260:127673. https://doi.org/10.1016/j.chemosphere.2020.127673
Kołodyńska D (2013) Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ Sci Pollut Res 20:5939–5949. https://doi.org/10.1007/s11356-013-1576-2
Liu L, Luo D, Yao G, Huang X, Wei L, Liu Y, Wu Q, Mai X, Liu G, Xiao T (2020) Comparative activation process of Pb, Cd and Tl using chelating agents from contaminated red soils. Int J Env Res Pub He 17:497. https://doi.org/10.3390/ijerph17020497
Palacios-Padrós A, Caballero-Briones F, Sanz F (2010) Enhancement in as-grown CuInSe2 film microstructure by a three potential pulsed electrodeposition method. Electrochem Commun 12:1025–1029. https://doi.org/10.1016/j.elecom.2010.05.015
Pańczuk-Figura I, Kołodyńska D (2016) Biodegradable chelating agent for heavy metal ions removal. Sep Purif Technol 51:2576–2585. https://doi.org/10.1080/01496395.2016.1210642
Chen JP, Lim LL (2005) Recovery of precious metals by an electrochemical deposition method. Chemosphere 60:1384–1392. https://doi.org/10.1016/j.chemosphere.2005.02.001
Pedersen KB, Ottosen LM, Jensen PE, Lejon T (2015) Comparison of 2-compartment, 3-compartment and stack designs for electrodialytic removal of heavy metals from harbour sediments. Electrochim Acta 181:48–57. https://doi.org/10.1016/j.electacta.2014.12.003
Peng C, Liu Y, Bi J, Xu H, Ahmed A-S (2011) Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. J Hazard Mater 189:814–820. https://doi.org/10.1016/j.jhazmat.2011.03.034
Pourfadakari S, Ahmadi M, Jaafarzadeh N, Takdastan A, Ghafari S, Jorfi S (2019) Remediation of PAHs contaminated soil using a sequence of soil washing with biosurfactant produced by Pseudomonas aeruginosa strain PF2 and electrokinetic oxidation of desorbed solution, effect of electrode modification with Fe3O4 nanoparticles. J Hazard Mater 379:120839. https://doi.org/10.1016/j.jhazmat.2019.120839
Ryu B-G, Park S-W, Baek K, Yang J-S (2009) Pulsed electrokinetic decontamination of agricultural lands around abandoned mines contaminated with heavy metals. Sep Sci Technol 44:2421–2436. https://doi.org/10.1080/01496390902983778
Song Y, Ammami M-T, Benamar A, Mezazigh S, Wang H (2016) Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ Sci Pollut Res 23:10577–10586. https://doi.org/10.1007/s11356-015-5966-5
Song Y, Cang L, Xu H, Wu S, Zhou D (2019a) Migration and decomplexation of metal-chelate complexes causing metal accumulation phenomenon after chelate-enhanced electrokinetic remediation. J Hazard Mater 377:106–112. https://doi.org/10.1016/j.jhazmat.2019.05.055
Song Y, Sun T, Cang L, Wu S, Zhou D (2019b) Migration and transformation of Cu (II)-EDTA during electrodialysis accompanied by an electrochemical process with different compartment designs. Electrochim Acta 295:605–614. https://doi.org/10.1016/j.electacta.2018.10.162
Song Y, Gao S, Yuan X, Sun R, Wang R (2022) Two-compartment membrane electrochemical remediation of heavy metals from an aged electroplating-contaminated soil: a comparative study of anodic and cathodic processes. J Hazard Mater 423:127235. https://doi.org/10.1016/j.jhazmat.2021.127235
Souto RM, Ricci F, Szpyrkowicz L, Rodriguez JL, Pastor E (2011) FTIR characterization of surface interactions of cyanide and copper cyanide with a platinum electrode in alkaline solution. J Phys Chem C 115:3671–3677. https://doi.org/10.1021/jp108571c
Sun TR, Ottosen LM (2012) Effects of pulse current on energy consumption and removal of heavy metals during electrodialytic soil remediation. Electrochim Acta 86:28–35. https://doi.org/10.1016/j.electacta.2012.04.033
Sun TR, Ottosen LM, Jensen PE (2012) Pulse current enhanced electrodialytic soil remediation—comparison of different pulse frequencies. J Hazard Mater 237:299–306. https://doi.org/10.1016/j.jhazmat.2012.08.043
Sun TR, Ottosen LM, Jensen PE, Kirkelund GM (2013a) Effect of pulse current on acidification and removal of Cu, Cd, and As during suspended electrodialytic soil remediation. Electrochim Acta 107:187–193. https://doi.org/10.1016/j.electacta.2013.05.138
Sun TR, Ottosen LM, Mortensen J (2013b) Electrodialytic soil remediation enhanced by low frequency pulse current–overall chronopotentiometric measurement. Chemosphere 90:1520–1525. https://doi.org/10.1016/j.chemosphere.2012.08.038
Tang J, He J, Liu T, Xin X, Hu H (2017) Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 189:599–608. https://doi.org/10.1016/j.chemosphere.2017.09.104
Wang G, Zhang S, Xu X, Zhong Q, Zhang C, Jia Y, Li T, Deng O, Li Y (2016) Heavy metal removal by GLDA washing: optimization, redistribution, recycling, and changes in soil fertility. Sci Total Environ 569:557–568. https://doi.org/10.1016/j.scitotenv.2016.06.155
Xu H, Song Y, Cang L, Zhou D (2020) Ion exchange membranes enhance the electrokinetic in situ chemical oxidation of PAH-contaminated soil. J Hazard Mater 382:121042. https://doi.org/10.1016/j.jhazmat.2019.121042
Yang J-S, Lee JY, Baek K, Kwon T-S, Choi J (2009) Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. J Hazard Mater 171:443–451. https://doi.org/10.1016/j.jhazmat.2009.06.021
Yang Y, Li Y, Pritzker M (2016) Control of Cu2O film morphology using potentiostatic pulsed electrodeposition. Electrochim Acta 213:225–235. https://doi.org/10.1016/j.electacta.2016.07.116
Yeung AT, Gu Y-Y (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mater 195:11–29. https://doi.org/10.1016/j.jhazmat.2011.08.047
Zhou M, Zhu S, Liu F, Zhou D (2014) Pulse-enhanced electrokinetic remediation of fluorine-contaminated soil. Korean J Chem Eng 31:2008–2013. https://doi.org/10.1007/s11814-014-0137-9
Acknowledgements
All authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. U1906221) and the National Natural Science Foundation of China (No. 41601333).
Funding
This study was financially supported by the “National Natural Science Foundation of China” (No. U1906221) and the “National Natural Science Foundation of China” (No. 41601333).
Author information
Authors and Affiliations
Contributions
SG and RS contributed to the study conceptualization and methodology. SG, YW, and ZW performed the data collection and formal analysis. SG wrote the first draft, and YW, ZW, XT, and RS reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, S., Wang, Y., Wang, Z. et al. Removal behavior and mechanisms of cadmium and lead by coupled ethylenediaminetetraacetic acid washing and electrochemical reduction: influence of current conditions. Environ Sci Pollut Res 29, 29818–29829 (2022). https://doi.org/10.1007/s11356-021-18480-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18480-5