Abstract
It has recently been proven that epigenetic dysregulation is importantly involved in cell transformation and therefore induces cancerous diseases. The development of molecules called epidrugs, which target specifically different epigenetic modifications to restore cellular memory and therefore the treatment, became a real challenge currently. Currently, bioactive compounds of medicinal plants as epidrugs have been can identified and explored in cancer therapy. Indeed, these molecules can target specifically different epigenetic modulators including DNMT, HDAC, HAT, and HMT. Moreover, some compounds exhibit stochastic epigenetic actions on different pathways regulating cell memory. In this work, pharmacodynamic actions of natural epidrugs belonging to cannabinoids, carotenoids, chalcones, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, tanshinones, and other chemical classes we reported and highlighted. In this review, the effects of several natural bioactive compounds of epigenetic medications on cancerous diseases were highlighted. Numerous active molecules belonging to different chemical classes such as cannabinoids, carotenoids, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, and tanshinones are discussed in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
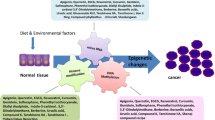
Cancer represents a complex pathology induced by several risk factors such as microbial infections, genetic mutations, environmental changes, and epigenetic variability (Yahya and Alqadhi 2021). These risk factors contribute directly or indirectly to the mechanisms of tumor transformation as well as the spread of cancer. Today with the mechanistic understanding of cellular signaling pathways, molecular evidence has shown that cancer may be the result of genetic instability leading to disruption of epigenetic pathways which lead to the transformation of cells into tumor cells (Feng and De Carvalho 2021).
Indeed, epigenetics signify all changes in gene expression that are the physical modification of the sequence of genetic material (Mancarella and Plass 2021). These modifications control transcription via the spatiotemporal regulation of transcription factors. The modifications of epigenetic include DNA methylation on CpG islands, histone acetylation and deacetylation, and chromatin remodeling (Mancarella and Plass 2021).
The disturbances of these epigenetic modifiers (loss or gain of function) often lead to major phenotypes which can predispose to a loss of cellular memory and subsequently to the mechanism of cancerization (Khan et al. 2021). Recently, therapeutic strategies that specifically target the epigenome have started to implement molecules (epidrugs) that operate in a targeted manner for one or another epigenetic modifier. Indeed, certain epidrugs have already been validated and used clinically in chemotherapy for cancer treatment (Khan et al. 2021).
Among the sources of major epidrugs, nature constitutes an inexhaustible reservoir of bioactive natural molecules acting specifically against epigenetic pathways and thus demonstrating remarkable anticancer effects (Manso et al. 2021). Indeed, natural substances such as those identified in medicinal plants showed their capacity to have epidrug activity. In this review, a systemic research was carried out on anticancer actions of bioactive compounds belonging to carotenoids, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, and tanshinones chemical classes and their action on different epigenetic ways inducing and/or involving human cancer. Therefore, we highlighted the role of these phytochemical compounds in cancer chemoprevention.
Methodology
The literature on anticancer effects with epigenetic mechanisms of carotenoids, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, and tanshinones was carried out and highlighted. The collection of data was performed by using different databases, including Google Scholar, ScienceDirect, PubMed, SpringerLink, Web of Science, Scopus, Wiley Online, and SciFinder. The used keywords are cannabinoids, carotenoids, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, and tanshinones with the screening of all published papers to select those evaluated their anticancer actions on epigenetic targets. The collected data were classified and organized in tables to facilitate and then discussed and highlighted. The structures of all highlighted natural epidrugs in this study were drawn by the ChemDraw Pro 8.0 software. Moreover, for checking the IUPAC names of these molecules, PubChem database was utilized.
Results and discussion
Cannabinoids as epidrug agents against cancer
Tetrahydrocannabinol (THC) is an exogenous cannabinoid isolated from the Cannabis sativa plant (Fig. 1). This compound has long been known for its anti-inflammatory efficacy and antitumor immune response suppression. In 2015, Sido et al. (2015) studied the effect of THC on the methylation of promoters of different genes involved in the activation and therefore the differentiation of MDSCs using an in vivo model (Female BL6 (wild-type) mice methylated DNA) and also using many in vitro assays such as Western blot analysis and methylation-specific PCR (MSP) analysis. Results of this study revealed that THC increases the T cell suppression in THC-induced MDSCs (myeloid-derived suppressor cells) and upregulates STAT3-associated cytokines in THC-induced MDSCs. Moreover, THC elevated the S100A8 expression, which promoted the suppressive function of MDSCs and induced hypomethylation of major key MDSC functional genes (Table 1). Very recently, a review by Griffiths and colleagues was carried out to investigate the impact of cannabidiol on epigenetic treatments and on the effectiveness of chemotherapy in oncology (Griffiths et al. 2021). Indeed, the authors have observed from the results of several research works that this compound can be very promising when it is used alone or in combination, as in the case of immunotherapy and epigenetics (Griffiths et al. 2021).
Carotenoids as epidrug agents against cancer
The carotenoids are the natural substances, known also as tetraterpenoids, and present in different vegetables and algae. Some carotenoid compounds such as astaxanthin, fucoxanthin, lycopene, and β-carotene (Fig. 2) showed remarkable effects against cancer cell lines, particularly by their ability to modulate epigenetic modifications (Table 2).
Astaxanthin and fucoxanthin
Yang et al. (2017) performed in vitro testes to evaluate the capacity of astaxanthin (AST) to reactivate Nrf2 and GSTP1 expression (in human prostate LNCaP cells) via some epigenetic modifications. During this study, several assays were conducted to demonstrate this effect such as genomic sequencing, Western blot analysis, DNMT and HDAC activity assays, and others. The findings of this investigation revealed that this compound was able to reduce the methylation of 21 CpG sites of the GSTP1 CpG island and decrease the transcription of DNMT3b, as well as significantly inhibit the enzymatic activities of DNMT and HDAC. Moreover, AST increased the expression of NQO1 mRNA in sh-mock LNCaP and also induced the expression of mRNA and certain proteins such as Nrf2 and GSTP1. However, AST did not induce effects on the methylation of Nrf2 gene promoter region.
The same authors carried out a study in 2018 on mouse skin epidermal JB6 P+ cells to investigate the epigenetic regulation of Nrf2 by AST and fucoxanthin (FX) (Yang et al. 2018). The same assays, as those performed in the previous study, were conducted in this work and it was found that AST is able to decrease colony formation in TPA-induced transformation of JB6 P+ cells and reduce DNMT activity, while it has not showed inhibitory effects against HDAC activity in JB6 P+ cells. On the other hand, the results related to the effect of FX show that this compound has the same effect as AST to decrease colony formation in TPA-induced transformation of JB6 P+ cells and to reduce DNMT activity, while it did not show inhibitory effects against HDAC activity in JB6 P+ cells. In addition, in the same cell lines, FX suppressed the methylation of Nrf2 promoter region. Moreover, in HepG2-C8 cell lines, this epidrug induced the epigenetic demethylation of CpG sites in Nrf2, increased the antioxidant response element (ARE)-luciferase, and upregulated protein and mRNA levels of Nrf2 downstream genes.
Lycopene
Lycopene is another naturally occurring compound that is abundant in tomatoes and belonging to the carotenoid family. This compound is known for its antioxidant properties and nutrient protection. In 2008, King-Batoon et al. (2008) studied the effect of lycopene on the methylation of the GSTP1 promoter and important methylation in prostatic cancer cell lines (PC3 and LNCaP cells). The results showed that this compound had no effect on the RARβ2 gene in any of the breast cancer cell lines. However, it induced demethylation of GSTP1 gene (the tumor suppressor) in MDA-MB-468 cells and restored the GSTP1 expression. Moreover, lycopene caused demethylation of RARβ2 and HIN-1 genes in non-cancer MCF10A fibrocystic breast cells. The same cells were used by Fu et al. (2014) to study the effect of lycopene on the epigenetic regulation by methylation of the main gene in these cells. A decrease in methylation levels of the GSTP1 promoter was observed, as well as an increase in protein and mRNA levels of GSTP1 in an androgen-independent PC-3 cell line. Moreover, the methylation levels of the long interspersed element (LINE-1) were reduced, as well as the short interspersed element ALU. It has also been observed that lycopene is able to downregulate the DNMT3A protein levels in PC-3 cells. However, this compound had no effect on DNMT protein expression in LNCaP cells. The last study was conducted in 2018 to investigate the mechanism of lycopene prostate cancer by targeting DNA methyltransferase (Sukirman 2018). In this study, the binding of lycopene and other ligands to the active sites (SFG and SAH) of the DNA methyltransferase was compared. Therefore, this compound has a lower binding affinity than other ligand to bind to DNMT active sites.
β-Carotene
In 2019, colon cancer stem cells were used by Kim et al. (2019) to study the mechanisms of the effects of β-carotene on microRNA expression, DNA methylation, and histone acetylation. Indeed, β-carotene is known to have anticancer effects in different cancers via numerous mechanisms, including cell cycle arrest, apoptosis, cell growth regulation, immune system modulation, inhibition of cell proliferation, and antioxidant activity. The results obtained in this study were more in-depth because they showed that this constituent reduced cell proliferation and sphere formation in CD44+CD133+ colon CSCs. However, treatment with β-carotene elevated acetylation levels of histones H3 and H4. Further, after miRNA sequencing array analysis, it was found that β-carotene is able to regulate the expression of miRNAs associated with histone acetylation. In addition, this compound downregulated the expression of DNMT3A mRNA and the methylation of DNA in colon CSCs.
Chalcones as epidrugs against cancer
Some natural chalcones may also play a role in epigenetic targeting of cancerous diseases, such as isoliquiritigenin (ISL) and phloretin (Fig. 3), belonging to the chalcone family. Indeed, Lee and colleagues (Lee et al. 2015) carried out an investigation to assess the antitumor and antiviral effects of ISLa against EBVaGC. The results revealed that this molecule induces apoptosis in SNU719 cells, affects cell cycle progression of SNU719, induces the transduction of signal, stimulates apoptosis, and induces EBV (Epstein–Barr virus) gene transcription. In addition, ISL eliminated DNMT1 and DNMT3A expressions. Moreover, it decreased the use of F promoter and increased frequency of use of Q promoter, which supports the functional role of this molecule in EBV latency establishment. In the same year, a mouse mammary tumor model was used by Wang et al. (2015) to demonstrate the effects of ISL treatment on breast cancer. Analysis in this study showed that ISL suppressed the mammary hyperplasia and breast cancer initiation in mice and inhibited lung metastasis and breast cancer growth in these animals. Furthermore, molecular docking analysis identified the Wnt inhibitory factor-1 (WIF-1) as the primary target of ISL in limiting breast CSC. Wherefore, the ISL increased the WIF1 expression by demethylation of the promoter through inhibiting DNMT1 methyltransferase and limited the self-renewal ability of human breast CSCs in mice (Table 3).
Regarding phloretin, Paluszczak et al. (2010) evaluated the effect of this molecule on the epigenetic modifications in MCF7 breast cancer cells. The results show that phloretin inhibited DNMT activity and increased the level of DNMT1 transcription. Phloretin did not cause any effect on the methylation pattern or expression of RASSF1A, GSTP1, or HIN-1 in MCF7 cells. In addition, it did not affect histone H3 methylation.
Fatty acids as epidrugs against cancer
Fatty acids are essential metabolites in vegetables and contain molecules with important biological effects. Recent data showed that several fatty acids isolated from vegetables such as linoleic acid, docosahexaenoic acid, eicosatetraenoic acid, butyrate, and butyric acid (Fig. 4) exhibit anticancer properties with different mechanisms, including their action on epigenetic pathways involved in cancer inducing and promotion (Table 4).
Butyrate
It is a fatty acid presents in several natural sources, including medicinal plants. Several studies have shown the importance of honey, alone and in combination with other compounds, in the treatment of various types of cancer. Cho et al. (2014) used HCT-116 cells to demonstrate the effect of butyrate on the epigenetic regulation of apoptosis-related genes in these cells. Moreover, this molecule can also affect DNA methylation of apoptosis-related genes; the transcription of Cideb, Dapk1, and Tnfrsf25; and the modification of histone acetylation.
The authors found also that butyrate increases the colonocyte apoptosis. In addition, the treatment of HCT-116 cells with butyrate combined with docosahexaenoic acid importantly decreased the methylation of the proapoptotic genes (Cideb, Dapk1, Bcl2l11, Ltbr, and Tnfrsf25) compared to untreated control cells. In the same year, Zheng et al. (2014) studied the effect of butyrate on aberrant epigenetic alteration in Eca9706 cells. Consequently, in a dose-dependent manner, butyrate suppressed the human esophageal 9706 cancer cell growth. Moreover, treatment of Eca9706 cells with butyrate in combination with quercetin inhibited Eca9706 cell proliferation more than that induced by the compound alone. Also, using the immunoblotting assay, it was shown that butyrate in combination with quercetin could downregulate the reverse expressions of global DNMT1, NF-κB p65, HDAC1, and cyclin D1, whereas it upregulated the expressions of caspase-3 and p16INK4α. In addition, the treatment of Eca9706 cells with butyrate in combination with quercetin displayed an inverse effect targeting both altered DNA methylation and histone acetylation, acting as an HDAC inhibitor mediated by the epigenetic-NF-κB cascade signaling. Saldanha et al. (2014) demonstrated that the treatment of colon cancer cells with sodium butyrate (NaB) in combination with epigallocatechin gallate (EGCG) induced apoptosis and cell cycle arrest in RKO, HCT-116, and HT-29 colorectal cancer cells; this arrested mainly in the G2/M phase and for HT-29 CRC cells in the G1 phase. Additionally, real-time PCR analysis showed an increase in p21, NF-κB p65, and HDAC1 and a decrease in DNMT1 and survivin when RKO CRC cells were treated with the two molecules combined. While the combined treatment inhibited HDAC1, DNMT1, and survivin in the three CRC cells tested. Further assays from this study, conducted to clarify the combination treatment mechanism, showed that this combination inhibits DNMT3A and DNMT3B and induces p21 through a p53-dependent mechanism in RKO CRC cells. In addition, the Western blot assay showed that the combined treatment significantly decreased the percentage of CpG methylation.
Two years later, a study was carried out by Fialova et al. (2016) to evaluate the role of NaB on epigenetic modulation of androgen receptors (AR) gene expression in DU145 prostate cancer cells. The results of this study showed that NaB combined with 5′-aza-2′-deoxycytidine (Aza-dC) induced both AR gene expression at the mRNA level and the increase in histone H4 acetylation in AR gene promoter. In addition, the combination of the two molecules activated and maintained the G2/M cell cycle arrest with better survival in normal cells compared to prostate cancer DU145 cells. One of the reasons for combining two compounds is that one of the compounds reduces the toxicity of the other. In this study, it was found that the combination of NaB with Aza-dC reduces the toxicity of NaB.
In 2017, CHO DP-12 cells were used by Wippermann et al. (2017) to analyze genome-wide butyrate-induced several changes in the methylation of numerous gene expression and affected therefore their expression. The results reported that butyrate affects the cell cycle, apoptosis, central energy metabolism, and protein biosynthesis in the CHO DP-12 cell line. On the other hand, the authors found that differential methylated regions contain binding-site motifs of transcription factors.
Butyric acid
This acid is a short-chain fatty acid (CH3 CH2 CH2CO2 H) formed when the good bacteria in our gut breakdown dietary fiber. Tiwari and Gupta (2014) studied in vivo the chemopreventive effect of butyric acid alone or in combination with nicotinamide and calcium glucarate. After 16 weeks of treatment, butyric acid was shown to prevent tumor development, but protection was greatly improved when combined with nicotinamide and calcium glucarate. Molecular analyses revealed that butyric acid had an antitumor effect against 7,12-dimethylbenz[a]anthracene (DMBA)-induced tumor by downregulating miR-203 levels at 16 weeks and upregulating HDAC, DNMT, and DNA methylation promoter of miR-203 at 4 or 16 weeks. In addition, the co-administration of butyric acid combined with nicotinamide and calcium glucarate prevented the effect of altered gene expression (after 16 weeks) more than that of the single compound through regulation of miR-203 status via epigenetic modifications.
Docosahexaenoic acid (DHA)
It is an omega-3 fatty acid present in different vegetables. Some studies demonstrated the activity of this acid against various cancer cells through their epigenetic regulation. Cho et al. (2014) conducted a study to illustrate the apoptosis effect of DHA combined with butyrate. The results of this study were previously reported in the butyrate section. In addition to this, the acid decreased Cideb, Dapk1, and Tnfrsf25 methylation promoters. In 2018, Sarabi and Naghibalhossaini (2018) studied the effect of some polyunsaturated fatty acids, including DHA, on DNA methylation and DNMT expression in HCT116, HT29/219, SW742, LS180, and Caco2 cell lines (human colorectal cancer cells). After 6 days of treatment with DHA, cell-type-specific differences in the expression of the DNMT1, DNMT3a, and 3b genes were observed. Moreover, this acid induced global hypermethylation in HT29/219 and HCT116 cells, while reducing methylation in Caco2 cells were observed. In addition, the demethylation of the Cox2 promoter in the HT29/219, p14, and PPARγ promoters in HCT116 cells and the ECAD promoter in SW742 cells was induced when the cells were treated with DHA.
Eicosapentaenoic acid
Eicosapentaenoic acid (EPA) is also an omega-3 fatty acid. The mechanism of action of DNA demethylation induced by EPA in carcinoma cells revealed that this molecule increases 5-hydroxymethylcytosine (5hmC) on DNA, inducing p21WAF1/CIP1 gene expression, which slows the progression cycle of cancer cells, as well as the simultaneous binding to the peroxisome proliferator-activated receptor γ (PPARγ) and retinoid X receptor α (RXRα), thereby promoting their heterodimer and inducing a PPARγ-ten-eleven translocation (TET1) interaction (Ceccarelli et al. 2018). In addition, these results indicate that eicosapentaenoic acid exerts its anticancer activity by regulating DNA methylation levels allowing TET1 to perform its function. In the same year, a study carried by Sarabi and Naghibalhossaini (2018), for the evaluation of the effect of EPA on DNA methylation and DNMT expression in human colorectal cancer cells, showed results identical to those previously obtained by docosahexaenoic acid.
A recent study aimed to demonstrate the molecular mechanism of EPA in inhibiting the expression of HDAC1 and DNMT in carcinoma cells (Ceccarelli et al. 2020). The results of this work reported that this acid inhibits HDAC1 and DNMT expression and thus promotes the expression of the tumor suppressor gene. This mechanism is produced by the binding of EPA to PPARγ and activates it in hepatocellular carcinoma (HCC) cells, thereby downregulating HDAC1, which sequentially reduces the expression of DNMT1, 3A, and 3B. Moreover, EPA bound to PPARγ induced re-expression of the tumor suppressor gene Hic-1.
Omega-3 polyunsaturated fatty acids (omega-3 PUFAs)
In 2019, colorectal cancer (CRC) model rats used by Huang et al. (2019) to investigate the antitumor effect of omega-3 PUFAs on DNA demethylation pathways. From the results, it was found that the group treated with omega-3 PUFAs had a lower incidence and tumor size than the control group. In addition, a significant increase in the 5hmC percentage was observed in the omega-3 PUFA-treated group compared to the control group. In summary of this study, a positive correlation was noted between the anticancer effect of the omega-3 PUFAs and global 5hmC accumulation and TET1 expression.
Linoleic acid
A study by Sarabi and Naghibalhossaini (2018) to test the antitumor activity of polyunsaturated fatty acids, including linoleic acid, on DNMTs in HCT116, HT29/219, SW742, LS180, and Caco2 cell lines. After 6 days of treatment with linoleic acid, the authors recorded cell-type-specific differences in the expression of the DNMT1, DNMT3a, and 3b genes.
Squamocin
In 2011, Lee et al. (2011) studied the possible antitumor mechanism of squamocin on different cell lines (GBM8401, Huh-7, and SW620). It was found that squamocin inhibited the proliferation of cells tested by inducing changes in apoptosis and cell cycle. Moreover, squamocin also affects histone H3 phosphorylation, which is associated with the expressions of these histone-modifying enzymes. Moreover, squamocin caused an arrest in the cell cycle at the G1 phase and induced apoptosis via extrinsic and intrinsic pathways. In addition, the results showed that squamocin affects the epigenetic pathways in S10 and S28 cells by modulating histone H3 phosphorylation with downregulation of pMSK1 and aurora B expressions.
Inositol hexaphosphate
Pandey and Gupta (2010) evaluated in vivo the antitumor effect of inositol hexaphosphate (IP6) in mouse lungs before tumor development, using female albino Swiss mice made diabetic by intravenous injection of ethylnitrosourea (120 mg/kg). After 3 months of treatment, IP6 reduced the expressions of DNMT1, MeCP2, MBD1, and HDAC1 through the reduction of the upregulation of these genes; it thus reduced the expression of COX-2 (14%) and MLH1 (21%). IP6 also reduced the downregulation of the tumor suppressor gene p16 (6%).
Furthermore, the anticancer and antiproliferative effects of this molecule have been studied by Yu et al. (2017) in rats with CRC. Consequently, it inhibited tumors, in terms of incidence, number, weight, and volume. In terms of antitumor mechanism, IP6 decreased the Akt and c-Myc mRNA levels through downregulation of Akt, pAkt, pGSK-3β, and c-Myc protein expression, as well as upregulation of the expression of the pβ-catenin protein. These results revealed that IP6 may have an antiproliferative effect against colorectal cancer by crosstalk between the PI3K/Akt and Wnt pathways.
Lignans as epidrugs against cancer
Lignans are phenolic compounds belonging to phytoestrogen metabolism. Recent findings revealed the anticancer effects of some lignan’s compounds such as honokiol, peperomin E, and nordihydroguaiaretic acid (Table 5 and Fig. 5).
Honokiol
Honokiol is a poly-phenolic compound found in species of the genus Magnolia. Prasad et al. (2017) examined the effects of this compound on UVB-induced immunosuppression by utilizing contact hypersensitivity (CHS) in C3H/HeN mice. Topical application of this molecule to the skin at a dose of 0.5 and 1.0 mg/cm2 inhibited the UVB-induced suppression of the CHS response in mice and inhibited the COX-2 expression and the PGE2 production in the UVB-exposed skin. In addition, the global DNA methylation analysis shows that the honokiol prevented the UVB-induced DNA hypermethylation in mouse skin by increasing the levels of TET enzyme, which is responsible for this effect. A year later, Prasad and Katiyar (2018) studied the effect of honokiol in vitro on DNA methylation using two cell lines, PANC-1 and AsPC-1. The results show that treatment of these cells with honokiol for 5 days decreased the global DNA methylation levels (60–80%) compared to control cells. This molecule also increased the 5-hmC levels in a dose-dependent manner as well as the levels of TET activity and protein expression in pancreatic cancer cell lines. Moreover, cells treated with honokiol reactivated the tumor suppressor genes/proteins levels, such as p16INK4a and RASSF1A in cancer cells.
Nordihydroguaiaretic acid
Nordihydroguaiaretic acid (NDGA) is a lignan present in large amounts in Larrea tridentate. Cui et al. (2008a) carried out two studies to demonstrate the prevention mechanism that NDGA plays in the epigenetic modifications in human breast cancer cells, using two cancer cell lines, T47D and RKO. NDGA was able to inhibit the growth of cancer cells by arresting the cell cycle in the G1 phase. Moreover, NDGA induced a partial demethylation of the 5′ region of the p16INK4a gene in both cell lines, which restored the expression of p16INK4a mRNA. The other study was performed in vitro on SKBR3 and MDA-MB-435 cancer cell lines, also in vivo on T cell-deficient nude (nu/nu) mice. The in vitro results show that NDGA limited the growth of human breast cancer cell lines SKBR3 (IC50 = 31.09 ± 1.6 μmol/L) and MDA-MB-435 (IC50 = 38.8 ± 2.1 μmol/L). In addition, in both models, it was shown that NDGA inhibits the growth of human breast carcinoma, as well as reactivates the methylation-silenced E-cadherin gene (Cui et al. 2008b).
Peperomin E
The antitumor effects of peperomin E and its possible mechanisms have been evaluated (in vitro and in vivo) by Wang et al. (2016a). This molecule is a natural secolignan isolated from Peperomia dindygulensis. The assays applied have shown that this molecule inhibits the proliferation of BEAS-2B, A549, H1229, and NCI-H460 cells in a dose-dependent manner and induces apoptosis and cell cycle arrest in non-small-cell lung cancer (NSCLC) cell lines of the same way. In vivo treatment of A549 xenograft in BALB/c nude mice model with peperomin E inhibited the tumor growth without side effects. Furthermore, using an in silico target fishing method, it was found that peperomin E inhibits DNMT1 by the interaction with this active domain of DNMT1, which importantly affect genome methylation status. In addition, other results showed that this molecule decreased global methylation and reactivated silenced tumor suppressor genes via epigenetic modulators such as RASSF1A, APC, RUNX3, and p16INK4DNMT1 activity and expression, through decreased DNMT1 activity and its expression. Two years later, the same authors (Wanga et al. 2018) studied the same in vitro activity of peperomin E on GC cancer cell and on an orthotopic xenograft mouse model in vivo. Peperomin E, in a dose-dependent manner, suppressed the invasion and migration of poorly differentiated gastric cancer cells (in vitro and in vivo). This molecule also exhibited a covalent bond with the catalytic domain of DNMT1 and inhibited the DNMT1 activity with an IC50 value of 3.61 μM by downregulating the DNMT1, 3a, and 3b mRNA and protein expression in gastric cancer cells. The inhibition mechanism that peperomin elicits on DNMT1activity was caused by the induction of relative global DNA hypomethylation.
Polysaccharides as epidrugs against cancer
Fucoidan is a natural sulfated polysaccharide substance (Fig. 6) of marine origin located in brown algae cell membrane. Yan et al. (2015) revealed that fucoidan may have an antitumor effect against hepatocellular carcinoma (HCC) by upregulating miR-29b of human HCC cells and suppressing DNMT3B, which led to the MTSS1 upregulation. On the other hand, the signaling pathway of HCC cells for TGF-β receptors and Smad signaling of HCC cells were also “downregulated.” These findings led to the reduction of EMT and the prevention of degradation of the extracellular matrix, which reduced HCC cell invasion activity (Table 6).
Another study by Liao et al. (2019) proved the inhibitory effect of oligo-fucoidan (OF) which clearly inhibited the proliferation of malignant glioma cells but only partially influenced that of SVGp12 cells, the results showed. Supplemented by strong induction of mRNA differentiation marker (MBP, OLIG2, S100β, GFAP, NeuN, and MAP2) in U87MG and GBM8401 cells, OFs inhibited the expression of DNMT1, 3A, and 3B protein expression, as a consequence, OF reduced the methylation of p21, a DNMT3B target gene. OF could also establish synergies between growth inhibition and MBP induction in U87MG cells in combination with the clinical inhibitor of DNMT, decitabine.
Saponins as epidrugs against cancer
Polyphyllin I
Polyphyllin I (PPI), an active compound (Fig. 7) belongs to saponin subclass, isolated from Parispolyphylla. Several studies were undertaken to discuss its inhibitory effect on tumor growth. Li et al. (2016) tested this compound on NSCLC cells. The results showed growth inhibition and cell cycle arrest in a dose-dependent manner, with increased phosphorylation of SAPK/JNK signaling cascades, decreased the expression of p65 and DNMT1 proteins, and decreased the activity of EZH2 protein, mRNA, and promoter. In addition, PPI was shown to have a decreasing effect on tumor growth, protein expression levels of p65, DNMT1 and EZH2, with increased phosphorylation of SAPK/JNK in vivo. A study by Xiang et al. (2018) aimed to assess the effect of this compound on castration-resistant prostate cancer (CRPC) cells. The findings revealed that PPI exerts an inhibitory effect on growth and induces cell cycle arrest, limiting migration and invasion in this type of cell, with down-expressing proteins EZH2 and DNMT1 and lowering levels of HOTAIR mRNA (Table 7).
Compound K
In 2013, a study by Kang et al. (2013) was performed to determine whether this compound has the capacity to reactivate tumor suppressor genes in human colorectal cancer HT-29 cells by reducing DNMT activity. The results revealed that this compound inhibits growth of the HT-29 cell at a dose- and time-dependent manner. In addition, compound K inhibited the expression and activity of DNMT1, which led to reverse the methylation and reactivated the tumor suppressor gene RUNX3. It was revealed that this compound induces also the expression of Smad4 and Bim as well as the upregulation of extracellular signal-regulated kinase (ERK) inhibition.
Secoiridoids as epidrugs against cancer
Oleacein
Oleacein is a polyphenol (Fig. 8) of the secoiridoid group found in Olea europaea L. Juli et al. (2019) studied in vitro the antitumor potential of oleacein against human multiple myeloma (MM) cell and its underlying bio-molecular sequelae. The results showed that oleacein was able to reduce the viability of primary human MM samples and cell lines, inhibit the clonogenicity of human MM, and induce cell cycle blockade and trigger apoptosis (Table 8). Oleacein has shown an effect in the epigenetic regulation by induced accumulation of acetylated histones and α-tubulin in a dose-dependent manner, as well as the downregulation of several class I/II HDACs both at the mRNA and protein level. However, this molecule had no effect on global DNA methylation. In terms of the mechanism of action, it was found that oleacein affected the downregulation of Sp1, the major transactivator of the HDAC promoter, via the activation of caspase-8, which inhibited HDAC. These results indicate that the oleacein inhibited the in vitro anti-MM effect of the proteasome inhibitor carfilzomib.
Oleosidedecarboxymethyl oleuropein aglycone (DOA)
In 2018, cancer stem cells (CSC) and female athymic nude mice were used by Corominas-Faja et al. (2018) to clarify the ability of DOA to suppress functional traits of CSC in breast cancer (BC). The results showed that DOA targets subpopulations of epithelial-like, mesenchymal-like, aldehyde dehydrogenase (ALDH)-positive, and CD44+/CD24−/low CSC, as well as blocking the formation of multicellular tumor spheres generated from single founder stem-like cells in a panel of genetically diverse BC models. An in vivo study also showed that mice treated with DOA reduced subsequent tumor-forming capacity and inhibited the tumor growth for several months in mice orthotopically injected with CSC-enriched BC cell populations. In order to determine the mechanism by which DOA acts, an in silico assay was used to subsequently find that DOA inhibits the ATP-binding kinase domain site of mTOR. The following year, Verdura et al. (2019) showed that DOA is a phenol-conjugated oleoside present in extra-virgin olive oil (EVOO). The aim of their study was to assess the ability of DOA in the inhibition of neomorphic activity of mutant IDH1 (R132H) and reducing of 2HG production, reversing 2HG-driven rewiring of epigenetic and immunological landscape, and restricting 2HG-enhanced tumor-initiating capacity. The results showed that DOA inhibited the production of 2HG (2-hydroxyglutaratye) by neomorphic IDH1 (isocitrate dehydrogenase 1) mutations. In silico studies and molecular dynamic analyses showed that DOA preferentially occupied the allosteric pocket of the IDH1 mutant and inhibited the enzymatic activity of the recombinant IDH1 mutant protein (R132H) in the low micromolar range. After enzymatic analyses of IDH1 activity/inhibition, it was found that DOA suppresses 2HG overproduction in engineered human cells expressing heterozygous IDH1-R132H mutation and restores the 2HG-suppressed activity of histone demethylases. Further tests conducted during this study showed that DOA reactivated the expression of PD-L1 (via epigenetic control through an immunosuppressive gene silenced in IDH1 mutant cells). In addition, DOA inhibited the formation of IDH1 mutant colony cells.
Steroids as epidrugs against cancer
Natural steroids isolated from some medicinal plants exhibited anticancer effects on different cancer cell lines. Indeed, guggulsterone, prostaglandin E2, and withaferin A (Fig. 9) presented remarkable anticancer activity by targeting epigenetic pathways involved in cell transformation and cancerization (Table 9).
Withaferin A and guggulsterone
The steroid compounds, withaferin A (WA) and guggulsterone, exhibit remarkable cytotoxic activity as they induce beneficial changes in gene expression through epigenetic mechanisms (Mirza et al. 2013; Szarc vel Szic et al. 2014). Therefore, at low concentrations, these two compounds showed a significant effect on the regulation of epigenetic marks involved in the activation of tumor suppressor genes, where WA compound was the most investigated. Mirza et al. (2013) detected the potential of these molecules to reverse the epigenetic changes caused by DNA hypermethylation, with a decrease in the transcript levels of the DNA methyltransferases (DNMT1, DNMT3a, and DNMT3b) and their associated proteins (DNMT1, HDAC1, and MeCP2) in two human breast cancer cell lines, MCF7 and MDA-MB-231. Moreover, WA used alone or in combination with sulforaphane (SFN) (1 and 5 μM, respectively) has also been shown to be able to regulate the epigenetic marks (DNMT, HDAC, HMT, and HAT) (Rodríguez et al. 2017; Royston et al. 2018). In fact, WA induced a decrease of DNMT1, DNMT3A, and DNMT3B mRNA expression in MCF-7 cells, while only a significant decrease in DNMT3B mRNA expression was observed in MDA-MB-231 cells. However, the combinatorial treatment (SFN + WA) showed a high significant decrease in DNMT1, DNMT3A, and DNMT3B mRNA and their protein expression in both breast cancer cell lines. Regarding the effects of WA and WA + SFN on HDAC, WA induced considerable downregulation of mRNA and protein expression of HDAC1 in both cell lines, with a potent effect in combination with SFN. Additionally, WA and combination therapy decreased histone methyltransferase (HMT) and increased histone acetyltransferase (HAT) activities in both cell lines, with significant impact on MDA-MB-231 cells (Royston et al. 2018). In contrast, Szarc vel Szic et al. (2014) revealed that WA induced a small change in expression of DNMT manifested by only decreased expression of DNMT3B in MCF-7 cells. These activities of WA and the combinatorial treatment (SFN + WA) have been associated with decreasing anti-apoptotic BCL-2 and an increasing proapoptotic BAX (Rodríguez et al. 2017). Furthermore, a significant downregulation of cyclin D1, CDK4, and p-RB, known by their various roles in the cell cycle, and an increase in the levels of E2F mRNA and tumor suppressor p21 were shown under the WA and SFN + WA treatments (Royston et al. 2018). Furthermore, both transcription activators, FOXM1 and E2F1, were inhibited and a cyclin-dependent kinase inhibitor 2A (CDKNA2A) was activated after WA treatment. While WA administration is predicted to repress histone demethylase JARID1B and activate histone acetylase p300 activities, in MDA-MB-231 and MCF-7 cell lines. On the other hand, WA could dramatically reduce cell motility, invasion, and epithelial-mesenchymal transition, all of which are key processes in breast cancer metastasis in MDA-MB-231 cells (Szarc vel Szic et al. 2017). Cell motility and invasion were inhibited both by the activation of the transcription factor SPDEF known by its inhibitory role in the migration of several types of cancer and by an increase in the expression of a well-established breast cancer metastasis suppressor 1 (BRMS1) following WA treatment (Szarc vel Szic et al. 2014). Moreover, the impact of WA treatment on MDA-MB-231 cells and their invasion is also mediated by the downregulation of tumor-promoting genes, such as urokinase-type plasminogen activator (PLAU), tumor necrosis factor (ligand) superfamily, ADAM metallopeptidase domain 8 (ADAM8), member 12 (TNFSF12), genes implicated in the detoxification (glutathione-S-transferase mu 1, GSTM1), and/or mitochondrial metabolism (malic enzyme 3, ME3) (Szarc vel Szic et al. 2014; Szarc vel Szic et al. 2017).
Tannins as epidrugs against cancer
Ellagic acid
Ellagic acid (EA) (Fig. 10), a ubiquitous polyphenol biosynthesized by many fruits and vegetables, showed potent cytotoxicity activity against MCF7 cells with a noticeable decrease in the activity of PARG (enzyme which might play a role in the anticytotoxic effect). In addition, EA significantly inhibited DNMT activity in MCF7 cells, without affecting the DNMT1 transcription or DNMT1 protein levels (Table 10). This compound, however, had no effect on the global methylation of histone H3 in MCF7 cells, as well as on the demethylation of RASSF1A, GSTP1, and HIN-1 genes, with only a slight increase in GSTP1 expression at the dose of 40 μM (Paluszczak et al. 2010). EA could also reduce the differentiation of adipocytes through the methylation of histone arginine and subsequent histone acetylation levels via epigenetic modification mediated by CARM1 inhibition (Kang et al. 2014). In fact, HDAC9 is characterized by a negative regulator role on adipogenesis, where in human adipogenic stem cells (hASCs), high levels of HDAC9 repress the transcriptional activation of adipogenic genes (Chatterjee et al. 2011). During an adipogenic stimulus, CARM1 enzyme facilitates the transfer of two methyl moieties to H3R17, which is accompanied by H3K9 acetylation and HDAC9 dissociation. In contrast, the EA repression activity against adipogenesis is mediated by the inhibition of CARM1 enzyme activity leading to the suppression of H3R17 methylation and consequently reduces H3K9 acetylation and HDAC9 dissociation (Kang et al. 2014). Furthermore, EA has a significant activity in the treatment of prostate cancer with antiproliferative and pro-differentiation properties. Indeed, treatment of two prostate cancer cell lines, LnCap and DU145, with EA showed an important decline of glycoprotein chromogranin A (CgA), where high levels of CgA are used as a marker of neuroendocrine tumors (NET). Besides, EA induced an increase in p75NGFR (NGF receptor) expression, which is also considered to be a marker of prostate cancer (p75NGFR levels decrease during tumorigenesis). Moreover, a decrease in p-Rb, Akt activation/phosphorylation and DNMT1 had been demonstrated under EA treatment (Vanella et al. 2013; Ebert et al. 2020).
Procyanidin B2
Procyanidin B2 (Fig. 10) was shown to be cytotoxic against MDA-MB231 cells. At low doses, it is able to inhibit DNMT activity and decrease DNMT expression in correlation with the upregulation of the expression of the E-cadherin, Maspin, and BRCA1 genes in MDA-MB-231 cells (Shilpi et al. 2015).
Tanshinones as epidrugs against cancer
Tanshinone IIA
Tanshinone IIA (TIIA) (Fig. 11), a compound detected in Salvia miltiorrhiza species, showed a considerable anticancer effect against mouse skin epidermal JB6 cells through an epigenetic mechanism (Table 11). This compound has been shown to be effective in activating the Nrf2 signaling pathway (regulator of the antioxidative stress response) by the demethylation of the CpGs of Nrf2. This activity was accompanied by a decrease in DNMT1, DNMT3a, DNMT3b, and HDAC3 protein levels and an inhibition of HDAC enzyme activity. These findings also confirmed the important role of oxidative stress in carcinogenesis and cancer progression and, therefore, the importance of antioxidant compounds in cancer prevention and therapy (Slattery et al. 2014; Wang et al. 2014).
Other bioactive compounds as epidrugs against cancer
Other natural bioactive compounds isolated from medicinal plants have also shown anticancer properties against various human cancers. These substances include curcumol, chlorogenic acid, ascorbic acid, folic acid, vitamins, and others (Fig. 12). Currently, several pharmacological investigations revealed that these molecules exhibit their anticancer effects via epigenetic mechanisms (Table 12).
Amarogentin, eugenol, and EGCG
Amarogentin (detected in chirata), eugenol (produced by clove), and epigallocatechin gallate (EGCG, found in green tea), used alone or in combination (EGCG with eugenol and EGCG with amarogentin) have shown their ability to block cell proliferation and colony formation, as well as induce apoptosis and epigenetic modification in the HeLa cell line (Pal et al. 2019). The combinatorial treatments were more effective than each of the compounds tested alone. In fact, these compounds produce a downregulation of DNMT1, with an increase in cell cycle inhibitors (LIMD1, RBSP3, and p16) and a decrease in cyclin D1 that plays a role in the regulation of cell cycle progression (Pal et al. 2019). The upregulation of cyclin D1 in cancer cells correlates with tumor differentiation and increased metastasis (Shan et al. 2017).
Chlorogenic acid
Chlorogenic acid was found to be a potent inhibitor of HDAC, M.SssIDNMT, and human DNMT1 (Lee and Zhu 2006; Bora-Tatar et al. 2009). The inhibition of DNMT1 is mainly due to the upregulation of S-adenosyl-L-homocysteine (SAH, a potent inhibitor of DNA methylation) (Lee and Zhu 2006). As well, chlorogenic acid could partially induce a hypomethylation of retinoic acid receptor beta (RARβ) gene promoter, a tumor suppressor gene encoding the RARβ receptor (Lee and Zhu 2006).
Butyric acid, nicotinamide, and calcium glucarate
Butyric acid (BA), calcium glucarate (CAG), and nicotinamide (NA), used alone or in combination, possess chemopreventive effects on mouse skin tumorigenesis by modification of genetic marks, where the combinatorial treatment was more effective. These compounds used alone or in combination have been shown to inhibit the epigenetic silencing of miR-203 and p16 tumor suppressor genes. This later is correlated with the reduction in DNMT1 and HDAC1 expression (Tiwari and Gupta 2014).
Proanthocyanidins and resveratrol
Both dietary components, grape seed proanthocyanidins (GSP) and resveratrol (Res) produced by many plants, were reported to be effective against MDA-MB-231 and MCF-7 human breast cancer cells, when used in combination. The combined treatment of GSP and Res induced decreased cell viability and proliferation as well as downregulation of DNMT and HDAC activity, with synergistic effects in both cell lines. In addition, the combinatorial treatment induced apoptosis in MDA-MB-231 cells correlating with an increase in proapoptotic Bax and a decrease in anti-apoptotic Bcl-2 expression (Gao and Tollefsbol 2018).
Curcumol
Curcumol showed a potent antitumor activity, in vitro and in vivo, on cancer stem-like cells (CSLCs) of choriocarcinoma, through epigenetic modifications. Curcumol is one of the major compounds of Curcuma zedoaria plant, mainly used as a spice and medicinal plant. This compound reduced the expression of DNMT1, DNMT3b, HDAC1, and HDAC3 that have been shown to be significantly upregulated in CSLCs (Peng et al. 2018). Further studies revealed that curcumol possesses the ability to upregulate cell cycle regulator p27 and p16 tumor suppressor (Cao et al. 2017).
Depispeptide
Depispeptide has been shown to be an effective compound capable of changing DNA methylation and histone modification. In prostate cell lines (PC-3, LNCaP, and BPH-1), this compound could reduce H3K9me2/3 and H3K27me2/3 (transcriptional repressor) expression and enhance that of H3K18Ac (transcriptional activator). These modifications were related to an upregulation of GSTP1 mRNA (Hauptstock et al. 2011). In fact, GSTP1, downregulated in prostate cancer, plays a key role in detoxification and reduction of oxidative damage in cells (Cui et al. 2006). In addition, depsipeptide has been shown to inhibit HDAC by suppressing histone methyltransferases (G9A and SUV39H1) and reducing DNMT1 activity and heterochromatin-associated proteins (HP1 and HP1β) in several genes. Consequently, these significant demethylation activities lead to increased expression of p16, GATA4, and SALL3 in human lung cancer cell lines (H719 and H23), pancreatic cancer cell line (PANC1), and colon cancer cell line (HT-29) (Wu et al. 2008). Depsipeptide (2 μM for 4 h) also induced a noticeable upregulation of HAT3 in three cell lines (HepG2, CL-48, and Hep3B) and a downregulation of HDAC3 expression in two cell lines (CL-48 and Hep3B). These epigenetic modifications were correlated with a considerable reactivation of growth arrest and DNA damage-inducible β (GADD45β) gene expression only in HepG2 cells (Jiang et al. 2007). GADD45 plays a role in the regulation of normal cell apoptosis. However, overexpression could inhibit apoptosis in many cells such as multiple myeloma cells (Zhang et al. 2020). In contrast, the combination of depsipeptide with 5-aza-2′-deoxycytidine or 5-azacytidine, known to be potent inhibitors of DNA methylation, produced important impacts on many epigenetic marks. In fact, a sequential treatment of depsipeptide (2 nM for 24 h) followed by 5-azacytidine (5 μM for 24 h) showed a synergistic effect on HAT3 and GADD4β gene expression in HepG2 cell lines. However, the simultaneous and sequential (5-azacytidine followed by depsipeptide) treatments had no impact on GADD4β gene reactivation (Jiang et al. 2007). Moreover, the combination of depsipeptide with 5-aza-2′-deoxycytidine showed a great upregulation of gelsolin and maspin (metastasis suppressor genes) in the MDA-MB-231 and MCF-7 cell lines as compared to depsipeptide alone (Primeau et al. 2003).
S-Allylcysteine
S-Allylcysteine has antitumor activity on human ovarian cancer A2780 cells. Epigenetically, this compound may decrease 5-methylcytosine levels and the expression of mRNA and DNMT1 protein. Consequently, due to these epigenetic modifications, the expression of proteins and mRNA of CDKN1A (tumor suppressor gene) was upregulated and the expression of cell division control 2 (CDC2) was decreased (Xu et al. 2018).
Selenite
Selenite could prevent prostate and colorectal oncogenesis through an epigenetic mechanism. Selenium used alone or in combination with green tea as a dietary supplement showed a preventive effect on colonic carcinogenesis in rats, with a great impact on the combinatorial diet. Thus, it has been shown that the treatments significantly inhibit the formation of aberrant crypt foci (ACF), the incidence and multiplicity of tumors, as well as the decrease in tumor size. Furthermore, the combined diet induced a remarkable decline of DNMT1 expression and an increase in histone H3 acetylation, with reactivation of SFRP5 mRNA expression and decrease in β-catenin nuclear and cyclin D1 expression, resulting in suppression of colorectal oncogenesis (Hu et al. 2013). In prostate cancer, selenite treatment produced partial demethylation of the glutathione-S-transferase P1 (GSTP1) promoter and re-expression of GSTP1 gene, which was shown to be hypermethylated in human prostate cancer. The epigenetic modifications caused by this treatment induced downregulation of DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B) mRNA expression, protein levels of DNMT1, histone deacetylase enzyme activity, and levels of methylated histone H3 on lysine 9 (H3-K9), with increased levels of acetylated H3-K9 (Xiang et al. 2008). On the other hand, it is also reported that selenium deficiency represents the main cause of Keshan’s disease (fatal dilated cardiomyopathy), where selenium deficiency is more probably related to DNA hypermethylation. Actually, selenium deficiency enhances the levels of mRNA and protein expression of TLR2, ICAM1 (inflammatory-related genes), and GADD45α. In this case, selenite treatment induces a downregulation of TLR2, ICAM1, and Gadd45α gene expression (Yang et al. 2014).
Vitamin C (ascorbate, ascorbic acid)
Vitamin C, mainly at pharmacological doses, showed promising action in cancer prevention and treatment via epigenetic modifications. In fact, vitamin C could induce DNA demethylation by downregulating DNMT activity and upregulating ten-eleven translocation (TET) expression in many cancer cell lines (Venturelli et al. 2014; Mikirova and C. Scimeca 2016; Amirabad et al. 2017; Shenoy et al. 201; Gerecke et al. 2020; Gerecke et al. 2018). Vitamin C increased the mRNA and protein expression of the tumor suppressor p16 and p21 in human epidermoid carcinoma A431 cells, as it may protect these cells against UV-induced apoptosis (Lin et al. 2014). Vitamin C treatment also produced modifications in the expression of 151 miRNAs, with the reactivation of 32 miRNAs involved in tumor suppression, metastasis, and drug resistance in BLM melanoma cells (Venturelli et al. 2014). The downregulation of DNMT1 expression in the mouse sarcoma 180 cell line following vitamin C treatment was accompanied by decreased expression of HIF (tumor-promoting gene) and an enhancement of p53 (tumor suppressor gene) and gene expression by the transcription factor Nrf2 (Mikirova and C. Scimeca 2016). On the other hand, increased TET activity by vitamin C treatment was correlated with the upregulation of SMAD1 (tumor suppressor gene) expression in DLBCL and PTCL cell lines (Shenoy et al. 2017) and also to the reactivation of the tumor suppressor p21 (CDKN1A) in human colorectal carcinoma (HCT11) cell line (Gerecke et al. 2018). Vitamin C could improve the activity of 5-aza-2′-deoxycytidine and azacytidine (DNA-demethylating agents), where the combined treatment induced a significant increase in CDKN1A expression (Gerecke et al. 2018). Moreover, although vitamin C has a low impact on 2-HG, oncometabolite caused by mutations in the enzyme isocitrate dehydrogenase 1 (IDH1), the combinatorial treatment of vitamin C with ML309 (inhibitor of mutant IDH enzymes) produced a significant decrease of 2-HG in the colon cancer cell line HCT116IDH1R132H/+ (Gerecke et al. 2020).
Vitamin D3/all-trans retinoic acid/resveratrol
In MCF-7 breast cancer cells, where RARβ2 was found to be partially methylated, treatments with vitamin D3 or all-trans retinoic acid (ATRA) improved the expression of RARβ2 gene. This action was more effective when these compounds were used in combination with 2-chloro-2′-deoxyadenosine (2CdA) (Stefanska et al. 2010). Furthermore, treatments with vitamin D3, ATRA, and resveratrol, used alone, showed a great ability to decline the promoter methylation of phosphatase and tensin homolog (PTEN) tumor suppressor gene in MCF-7 cell line. In addition, vitamin D3 and resveratrol could also increase PTEN and p21 activity, as well as reduce DNMT activity. The combinatorial treatment of vitamin D3 and 2CdA significantly increases the expression of PTEN in MCF-7 cells. However, only vitamin D3 has been shown to be able to decrease the PTEN methylation and enhance its expression in MDA-MB-231 cell line (Stefanska et al. 2012).
Folic acid (folate, vitamin B9) and vitamin E
Epigenetic modifications caused by DNA hypomethylation could also lead to the development of cancer as in the case of glioma tumor. Folic acid (vitamin B9) was found to be effective in increasing the DNA methylation by upregulating the expression of DNMT3a and DNMT3b proteins, which leads to enhanced methylation and then inactivation of numerous genes (PDGF-B, MGMT, survivin, and bcl-w) implicated in gliomagenesis (Hervouet et al. 2009). However, high folic acid levels, associated with excessive hypermethylation, could lead to the development of cancer by inactivating tumor suppressor genes (Lubecka-Pietruszewska et al. 2013). Vitamin E and folic acid supplements showed excellent results in regulating the disruption of uterine function and fertility caused by diabetes in rats via epigenetic pathway. These two compounds, used alone, could decline via hypermethylation process of the expression of TGF-β-1 and ESR-1 (upregulated due to diabetes disorder). While the expression of CDH-1, supposed to be downregulated by diabetes, was upregulated by vitamin E and folic acid supplementation in diabetic rats (Tabebordbar et al. 2020). Moreover, vitamin E showed a great impact on oxidative stress caused by obesity or diabetes by neutralizing the H2O2-induced lipid peroxidation. As well, vitamin E at lower concentrations could increase the global DNA methylation and DNMT1 gene expression in colorectal cancer cell line Caco-2, with an increase in MutL-homolog 1 (MLH1) gene expression, a protein implicated in the repair of DNA mismatches in humans (Zappe et al. 2018).
Arsenic trioxide
Arsenic trioxide (As2O3) is considered as a potent anticancer agent and DNA methylation inhibitor, with noticeable abilities to reactivate the silenced tumor suppressor genes, induce cell cycle arrest, and inhibit angiogenesis and metastasis (Cui et al. 2006; Du et al. 2012; Hassani et al. 2018). This compound also exhibits synergistic demethylation activity in correlation with 5-aza-2′-deoxycytidine (DAC) in some cancer cell lines (Peng et al. 2018; Ren et al. 2015). In liver cancer cell lines, the inhibition of DNMT activity by As2O3 was concomitant with CpG island demethylation of tumor suppressor genes, p16INK4a, RASSF1A, E-cadherin, and GSTP1, leading to reactivation of the expression of these genes (Cui et al. 2006). In the case of estrogen receptor-negative breast cancer, loss of ER expression is mainly due to hypermethylation of CpG islands in the promoter regions of the ER gene. Therefore, the upregulation of ER is among the effective strategies followed in the therapy of this type of cancer. As2O3 possesses a positive activity on the re-expression of estrogen receptor α (Erα) in ER-negative breast cancer cells in vitro and in vivo through demethylation of the Era promoter (Du et al. 2012; Zhang et al. 2011). This molecule induces cell cycle arrest through modifications of transcriptional activity of many cell cycle regulatory genes in colorectal cancer cell lines. Thus, As2O3 could induce an enhancement of cyclin E1, D1, GADD45A, and p21 and a decline of cyclin B1 gene expressions. Furthermore, As2O3 declined DNA demethylation of RBL1, CHFR, and p16 genes resulted in reactivation of their expression (Eyvani et al. 2016). As2O3 induces apoptosis and a significant reactivation of TMS1 expression, a tumor suppressor gene that was found to be completely methylated in the K562 cell line. In addition, As2O3 could decrease the expression of Bcl-2 (anti-apoptotic protein) and increase the expression of Bax (proapoptotic protein) (Li et al. 2015). Arsenic trioxide also showed excellent results in the treatment of acute promyelocytic leukemia (APL) via epigenetic mechanisms (Peng et al. 2010; Hassani et al. 2018). This compound induced demethylation of CpG island at promoter region of many cell cycle-related genes: CCND1, CCNE1, CCNF, CDKN1A, GADD45α, and RBL1 in the APL cell line NB4, which led to cell cycle arrest and apoptosis. Among these genes demethylated by this compound, the expression of mRNA of CCND1, CCNE1, and GADD45α was upregulated with an increase of the protein expression only in CCND1 and CCNE1. However, expression of the CCNF and CDKN1A genes was found to be suppressed (Hassani et al. 2018). Regarding the anti-angiogenesis activity of As2O3, this compound could attenuate angiogenic effect in human prostate cancer cells (in vitro and in vivo) by improving the expression of microRNA-155 (miR-155), resulting in inhibition of transforming growth factor beta (TGF-β)/SMAD signal pathway and vascular endothelial growth factor secretion (Ji et al. 2014). For the anti-metastasis activity, As2O3 effectively decreased the migration and invasive capacity of human breast cancer cell lines (MDA-MB-231 and BT-549) through the mesenchymal to epithelial transition process. This effect was related to the ability of this compound to increase miR-200c expression, then upregulate the expression of E-cadherin (epithelial marker) and downregulate N-cadherin and vimentin (mesenchymal markers) (Si et al. 2015). Moreover, arsenic trioxide could reverse the multiple drug resistance (MDR) in hepatocellular carcinoma (HCC). In fact, this compound enhances the expression of miR-148a and declines both NF-κB pathway and cancer stem-like cells (CSCs) properties in multiple drug resistant Bel-7402 cells (Wang et al. 2020). On the other hand, As2O3 showed a potent inhibitory effect against hMOF activity by decreasing global H4K16 acetylation, caused by arsenic, and subsequently increasing deacetyltransferase HDAC4 expression (Liu et al. 2015).
Conclusions and perspectives
The development of drugs acting on epigenetic pathways could offer major therapeutic avenues for cancer treatment, which could lead to definitive therapy by converting transformed cells into normal cells. In this review, the epigenetic modulatory effects against cancer of some natural compounds such as cannabinoids, carotenoids, fatty acids, lignans, polysaccharides, saponins, secoiridoids, steroids, tannins, and tanshinones are discussed. These compounds showed remarkable effects as epidrug modulators and can be therefore a source of anticancer epidrugs by targeting epigenetic modifications. However, these compounds should be investigated in clinical trials to confirm these pharmacokinetic effects. Moreover, toxicological studies should also be carried out to validate the safety of these natural epidrugs.
Data availability
All data presented herein are constant with the published literature.
References
Amirabad LM, Massumi M, Alivand MR (2017) L-Ascorbic acid affect the DNA methytransferase expression in mouse embryonic fibroblasts, p 4
Bora-Tatar G, Dayangaç-Erden D, Demir AS, Dalkara S, Yelekçi K, Erdem-Yurter H (2009) Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: activity and docking studies. Bioorg Med Chem 17:5219–5228. https://doi.org/10.1016/j.bmc.2009.05.042
Cao F, Zhang C, Han W et al (2017) p-Akt as a potential poor prognostic factor for gastric cancer: a systematic review and meta-analysis. Oncotarget 8:59878
Ceccarelli V, Valentini V, Ronchetti S, Cannarile L, Billi M, Riccardi C, Ottini L, Talesa VN, Grignani F, Vecchini A (2018) Eicosapentaenoic acid induces DNA demethylation in carcinoma cells through a TET1-dependent mechanism. FASEB J 32:5990–6001. https://doi.org/10.1096/fj.201800245R
Ceccarelli V, Ronchetti S, Marchetti MC, Calvitti M, Riccardi C, Grignani F, Vecchini A (2020) Molecular mechanisms underlying eicosapentaenoic acid inhibition of HDAC1 and DNMT expression and activity in carcinoma cells. Biochim Biophys Acta BBA - Gene Regul Mech 1863:194481. https://doi.org/10.1016/j.bbagrm.2020.194481
Chatterjee TK, Idelman G, Blanco V, Blomkalns AL, Piegore MG Jr, Weintraub DS, Kumar S, Rajsheker S, Manka D, Rudich SM, Tang Y, Hui DY, Bassel-Duby R, Olson EN, Lingrel JB, Ho SM, Weintraub NL (2011) Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem 286:27836–27847
Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR (2014) Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp Biol Med 239:302–310. https://doi.org/10.1177/1535370213514927
Corominas-Faja B, Cuyàs E, Lozano-Sánchez J, Cufí S, Verdura S, Fernández-Arroyo S, Borrás-Linares I, Martin-Castillo B, Martin ÁG, Lupu R, Nonell-Canals A, Sanchez-Martinez M, Micol V, Joven J, Segura-Carretero A, Menendez JA (2018) Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis 39:601–613. https://doi.org/10.1093/carcin/bgy023
Cui X, Wakai T, Shirai Y, Yokoyama N, Hatakeyama K, Hirano S (2006) Arsenic trioxide inhibits DNA methyltransferase and restores methylation-silenced genes in human liver cancer cells. Hum Pathol 37:298–311. https://doi.org/10.1016/j.humpath.2005.10.013
Cui Y, Lu C, Liu L, Sun D, Yao N, Tan S, Bai S, Ma X (2008a) Reactivation of methylation-silenced tumor suppressor gene p16INK4a by nordihydroguaiaretic acid and its implication in G1 cell cycle arrest. Life Sci 82:247–255. https://doi.org/10.1016/j.lfs.2007.11.013
Cui Y, Lu C, Kang A, Liu L, Tan S, Sun D, Hu J, Ma X (2008b) Nordihydroguaiaretic acid restores expression of silenced E-cadherin gene in human breast cancer cell lines and xenografts. Anti-Cancer Drugs 19:487–494. https://doi.org/10.1097/CAD.0b013e3282fd5310
Du J, Zhou N, Liu H et al (2012) Arsenic Induces functional re-expression of estrogen receptor α by Demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS One 7:e35957. https://doi.org/10.1371/journal.pone.0035957
Ebert A, König J, Frommer L, Schuppan D, Kahaly GJ (2020) Chromogranin serves as novel biomarker of endocrine and gastric autoimmunity. J Clin Endocrinol Metab 105:2606–2615
Eyvani H, Moghaddaskho F, Kabuli M, Zekri A, Momeny M, Tavakkoly-Bazzaz J, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH (2016) Arsenic trioxide induces cell cycle arrest and alters DNA methylation patterns of cell cycle regulatory genes in colorectal cancer cells. Life Sci 167:67–77. https://doi.org/10.1016/j.lfs.2016.10.020
Feng S, De Carvalho DD (2021) Clinical advances in targeting epigenetics for cancer therapy. FEBS J. https://doi.org/10.1111/febs.15750
Fialova B, Luzna P, Gursky J, Langova K, Kolar Z, Trtkova KS (2016) Epigenetic modulation of AR gene expression in prostate cancer DU145 cells with the combination of sodium butyrate and 5′-aza-2′-deoxycytidine. Oncol Rep 36:2365–2374. https://doi.org/10.3892/or.2016.5000
Fu L-J, Ding Y-B, Wu L-X, Wen CJ, Qu Q, Zhang X, Zhou HH (2014) The effects of lycopene on the methylation of the GSTP1 promoter and global methylation in prostatic cancer cell lines PC3 and LNCaP. Int J Endocrinol 2014:1–9. https://doi.org/10.1155/2014/620165
Gao Y, Tollefsbol T (2018) Combinational Proanthocyanidins and resveratrol synergistically inhibit human breast cancer cells and impact epigenetic–mediating machinery. Int J Mol Sci 19:2204. https://doi.org/10.3390/ijms19082204
Gerecke C, Schumacher F, Edlich A et al (2018) Vitamin C promotes decitabine or azacytidine induced DNA hydroxymethylation and subsequent reactivation of the epigenetically silenced tumour suppressor CDKN1A in colon cancer cells. Oncotarget 9:32822–32840. https://doi.org/10.18632/oncotarget.25999
Gerecke C, Schumacher F, Berndzen A, Homann T, Kleuser B (2020) Vitamin C in combination with inhibition of mutant IDH1 synergistically activates TET enzymes and epigenetically modulates gene silencing in colon cancer cells. Epigenetics 15:307–322. https://doi.org/10.1080/15592294.2019.1666652
Griffiths C, Aikins J, Warshal D, Ostrovsky O (2021) Can cannabidiol affect the efficacy of chemotherapy and epigenetic treatments in cancer? Biomolecules 11:766. https://doi.org/10.3390/biom11050766
Hassani S, Khaleghian A, Ahmadian S, Alizadeh S, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH (2018) Redistribution of cell cycle by arsenic trioxide is associated with demethylation and expression changes of cell cycle related genes in acute promyelocytic leukemia cell line (NB4). Ann Hematol 97:83–93. https://doi.org/10.1007/s00277-017-3163-y
Hauptstock V, Kuriakose S, Schmidt D, Düster R, Müller SC, von Ruecker A, Ellinger J (2011) Glutathione-S-transferase pi 1(GSTP1) gene silencing in prostate cancer cells is reversed by the histone deacetylase inhibitor depsipeptide. Biochem Biophys Res Commun 412:606–611. https://doi.org/10.1016/j.bbrc.2011.08.007
Hervouet E, Debien E, Campion L, Charbord J, Menanteau J, Vallette FM, Cartron PF (2009) Folate supplementation limits the aggressiveness of glioma via the remethylation of DNA repeats element and genes governing apoptosis and proliferation. Clin Cancer Res 15:3519–3529. https://doi.org/10.1158/1078-0432.CCR-08-2062
Hu Y, McIntosh GH, Le Leu RK et al (2013) Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS One 8:e64362. https://doi.org/10.1371/journal.pone.0064362
Huang Q, Mo M, Zhong Y, Yang Q, Zhang J, Ye X, Zhang L, Cai C (2019) The anticancer role of omega-3 polyunsaturated fatty acids was closely associated with the increase in genomic DNA hydroxymethylation. Anti-Cancer Agents Med Chem- Anti-Cancer Agents 19:330–336. https://doi.org/10.2174/1871520618666181018143026
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen J, Yang X (2014) Inhibition of transforming growth factor beta/SMAD signal by MiR-155 is involved in arsenic trioxide-induced anti-angiogenesis in prostate cancer. Cancer Sci 105:1541–1549. https://doi.org/10.1111/cas.12548
Jiang C, Zhou B, Fan K et al (2007) A sequential treatment of depsipeptide followed by 5-azacytidine enhances Gadd45, expression in hepatocellular carcinoma cells. Anticancer Res 27:3783–3789
Juli G, Oliverio M, Bellizzi D, Gallo Cantafio ME, Grillone K, Passarino G, Colica C, Nardi M, Rossi M, Procopio A, Tagliaferri P, Tassone P, Amodio N (2019) Anti-tumor activity and epigenetic impact of the polyphenol oleacein in multiple myeloma. Cancers 11:990. https://doi.org/10.3390/cancers11070990
Kang KA, Kim HS, Kim DH, Hyun JW (2013) The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells. Int J Oncol 43:228–236. https://doi.org/10.3892/ijo.2013.1931
Kang I, Okla M, Chung S (2014) Ellagic acid inhibits adipocyte differentiation through coactivator-associated arginine methyltransferase 1-mediated chromatin modification. J Nutr Biochem 25:946–953. https://doi.org/10.1016/j.jnutbio.2014.04.008
Khan AA, Liu X, Yan X et al (2021) An overview of genetic mutations and epigenetic signatures in the course of pancreatic cancer progression. Cancer Metastasis Rev 40:1–28
Kim D, Kim Y, Kim Y (2019) Effects of β-carotene on expression of selected microRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J Cancer Prev 24:224–232
King-Batoon A, Leszczynska JM, Klein CB (2008) Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ Mol Mutagen 49:36–45. https://doi.org/10.1002/em.20363
Lee WJ, Zhu BT (2006) Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 27:269–277. https://doi.org/10.1093/carcin/bgi206
Lee C-C, Lin Y-H, Chang W-H, Lin PC, Wu YC, Chang JG (2011) Squamocin modulates histone H3 phosphorylation levels and induces G1 phase arrest and apoptosis in cancer cells. BMC Cancer 11:58. https://doi.org/10.1186/1471-2407-11-58
Lee M, Son M, Ryu E et al (2015) Quercetin-induced apoptosis prevents EBV infection, p 22
Li H, Wang Y, Xu W, Dong L, Guo Y, Bi K, Zhu C (2015) Arsenic trioxide inhibits DNA methyltransferase and restores TMS1 gene expression in K562 cells. Acta Haematol 133:18–25. https://doi.org/10.1159/000362683
Li L, Wu J, Zheng F, Tang Q, Wu WY, Hann SS (2016) Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res 35:112. https://doi.org/10.1186/s13046-016-0388-x
Liao C-H, Lai I-C, Kuo H-C, Chuang SE, Lee HL, Whang-Peng J, Yao CJ, Lai GM (2019) Epigenetic modification and differentiation induction of malignant glioma cells by oligo-fucoidan. Mar Drugs 17:525. https://doi.org/10.3390/md17090525
Lin J, Qin H, Wu W, He SJ, Xu JH (2014) Vitamin C protects against UV irradiation-induced apoptosis through reactivating silenced tumor suppressor genes p21 and p16 in a Tet-dependent DNA demethylation manner in human skin cancer cells. Cancer Biother Radiopharm 29:257–264. https://doi.org/10.1089/cbr.2014.1647
Liu D, Wu D, Zhao L, Yang Y, Ding J, Dong L, Hu L, Wang F, Zhao X, Cai Y, Jin J (2015) Arsenic trioxide reduces global histone H4 acetylation at lysine 16 through direct binding to histone acetyltransferase hMOF in human cells. PLoS One 10:e0141014. https://doi.org/10.1371/journal.pone.0141014
Lubecka-Pietruszewska K, Kaufman-Szymczyk A, Stefanska B, Fabianowska-Majewska K (2013) Folic acid enforces DNA methylation-mediated transcriptional silencing of PTEN, APC and RARbeta2 tumour suppressor genes in breast cancer. Biochem Biophys Res Commun 430:623–628. https://doi.org/10.1016/j.bbrc.2012.11.103
Mancarella D, Plass C (2021) Epigenetic signatures in cancer: proper controls, current challenges and the potential for clinical translation. Genome Med 13:1–12
Manso J, Censi S, Mian C (2021) Epigenetic in medullary thyroid cancer: the role of microRNA in tumorigenesis and prognosis. Curr Opin Oncol 33:9–15
Mikirova N, C. Scimeca R (2016) Gene expression response to ascorbic acid in mice implanted with sarcoma S180 cells. J Transl Sci 2:132. https://doi.org/10.15761/JTS.1000132
Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R (2013) Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteinS. J Breast Cancer 16:23–31. https://doi.org/10.4048/jbc.2013.16.1.23
Pal D, Sur S, Roy R, Mandal S, Kumar Panda C (2019) Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line: Pal et al. J Cell Physiol 234:825–836. https://doi.org/10.1002/jcp.26900
Paluszczak J, Krajka-Kuźniak V, Baer-Dubowska W (2010) The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett 192:119–125. https://doi.org/10.1016/j.toxlet.2009.10.010
Pandey M, Gupta K (2010) Epigenetics, an early event in the modulation of gene expression by inositol hexaphosphate in ethylnitrosourea exposed mouse lungs. Nutr Cancer 63:1–11. https://doi.org/10.1080/01635581.2010.516868
Peng C-Y, Jiang J, Zheng H-T, Liu X-S (2010) Growth-inhibiting effects of arsenic trioxide plus epigenetic therapeutic agents on leukemia cell lines. Leuk Lymphoma 51:297–303. https://doi.org/10.3109/10428190903486212
Peng Z, Zhou W, Zhang C et al (2018) Curcumol controls choriocarcinoma stem-like cells self-renewal via repression of DNA methyltransferase (DNMT)- and histone deacetylase (HDAC)-mediated epigenetic regulation. Med Sci Monit 24:461–472. https://doi.org/10.12659/MSM.908430
Prasad R, Katiyar SK (2013) Prostaglandin E2 promotes UV radiation-induced immune suppression through DNA hypermethylation. Neoplasia 15:795–804. https://doi.org/10.1593/neo.13424
Prasad R, Katiyar S (2018) Abstract 1390: Honokiol, a bioactive component from Magnolia plant, promotes DNA demethylation and reactivates silenced tumor suppressors in pancreatic cancer cells through TET-dependent mechanism. Cancer Res 78:1390–1390. https://doi.org/10.1158/1538-7445.AM2018-1390
Prasad R, Singh T, Katiyar SK (2017) Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci Rep 7:1657. https://doi.org/10.1038/s41598-017-01774-5
Primeau M, Gagnon J, Momparler RL (2003) Synergistic antineoplastic action of DNA methylation inhibitor 5-AZA-2?-deoxycytidine and histone deacetylase inhibitor depsipeptide on human breast carcinoma cells. Int J Cancer 103:177–184. https://doi.org/10.1002/ijc.10789
Ren J, Yao J, Guo X, Guo X, Cai S (2015) The Effect of decitabine combined with arsenic trioxide on DAPK gene and HL-60 cell proliferation and apoptosis. J Cancer Ther 06:1229–1237. https://doi.org/10.4236/jct.2015.615134
Rodríguez M, Bajo-Santos C, Hessvik NP et al (2017) Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer 16:1–6
Royston KJ, Paul B, Nozell S, Rajbhandari R, Tollefsbol TO (2018) Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp Cell Res 368:67–74. https://doi.org/10.1016/j.yexcr.2018.04.015
Saldanha SN, Kala R, Tollefsbol TO (2014) Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp Cell Res 324:40–53. https://doi.org/10.1016/j.yexcr.2014.01.024
Sarabi MM, Naghibalhossaini F (2018) The impact of polyunsaturated fatty acids on DNA methylation and expression of DNMTs in human colorectal cancer cells. Biomed Pharmacother 101:94–99. https://doi.org/10.1016/j.biopha.2018.02.077
Shan Y-S, Hsu H-P, Lai M-D, Hung YH, Wang CY, Yen MC, Chen YL (2017) Cyclin D1 overexpression correlates with poor tumor differentiation and prognosis in gastric cancer. Oncol Lett 14:4517–4526
Shenoy N, Bhagat T, Nieves E, Stenson M, Lawson J, Choudhary GS, Habermann T, Nowakowski G, Singh R, Wu X, Verma A, Witzig TE (2017) Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J 7:e587–e587. https://doi.org/10.1038/bcj.2017.65
Shilpi A, Parbin S, Sengupta D, Kar S, Deb M, Rath SK, Pradhan N, Rakshit M, Patra SK (2015) Mechanisms of DNA methyltransferase–inhibitor interactions: procyanidin B2 shows new promise for therapeutic intervention of cancer. Chem Biol Interact 233:122–138. https://doi.org/10.1016/j.cbi.2015.03.022
Si L, Jiang F, Li Y, Ye X, Mu J, Wang X, Ning S, Hu C, Li Z (2015) Induction of the mesenchymal to epithelial transition by demethylation-activated microRNA-200c is involved in the anti-migration/invasion effects of arsenic trioxide on human breast cancer cells: AS INDUCES MET THROUGH miR-200c. Mol Carcinog 54:859–869. https://doi.org/10.1002/mc.22157
Sido JM, Yang X, Nagarkatti PS, Nagarkatti M (2015) Δ9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J Leukoc Biol 97:677–688. https://doi.org/10.1189/jlb.1A1014-479R
Slattery ML, John EM, Torres-Mejia G, Lundgreen A, Lewinger JP, Stern MC, Hines L, Baumgartner KB, Giuliano AR, Wolff RK (2014) Angiogenesis genes, dietary oxidative balance and breast cancer risk and progression: the Breast Cancer Health Disparities Study. Int J Cancer 134:629–644
Stefanska B, Rudnicka K, Bednarek A, Fabianowska-Majewska K (2010) Hypomethylation and induction of retinoic acid receptor beta 2 by concurrent action of adenosine analogues and natural compounds in breast cancer cells. Eur J Pharmacol 638:47–53. https://doi.org/10.1016/j.ejphar.2010.04.032
Stefanska B, Salamé P, Bednarek A, Fabianowska-Majewska K (2012) Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br J Nutr 107:781–790. https://doi.org/10.1017/S0007114511003631
Sukirman AM (2018) Molecular docking study of lycopene on reducing the DNA-methyltransfer in prostate cancer. Bioinform Biomed Res J 1:40–44
Szarc vel Szic K, Op de Beeck K, Ratman D et al (2014) Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS One 9:e87850. https://doi.org/10.1371/journal.pone.0087850
Szarc vel Szic K, Declerck K, Crans RAJ et al (2017) Epigenetic silencing of triple negative breast cancer hallmarks by Withaferin A. Oncotarget 8:40434–40453. https://doi.org/10.18632/oncotarget.17107
Tabebordbar M, Moradi Sarabi M, Vakili S, Zare R, Zal F (2020) Effect of folic acid and vitamin E on promoter DNA methylation and expression of TGF-β1, ESR-1 and CDH-1 in the uterus of STZ-induced diabetic rats. Arch Physiol Biochem:1–7. https://doi.org/10.1080/13813455.2020.1770798
Tiwari P, Gupta KP (2014) Modulation of miR-203 and its regulators as a function of time during the development of 7, 12 dimethylbenz [a] anthracene induced mouse skin tumors in presence or absence of the antitumor agents. Toxicol Appl Pharmacol 278:148–158. https://doi.org/10.1016/j.taap.2014.04.020
Vanella L, Barbagallo I, Acquaviva R, di Giacomo C, Cardile V, G. Abraham N, Sorrenti V (2013) Ellagic acid: cytodifferentiating and antiproliferative effects in human prostatic cancer cell lines. Curr Pharm Des 19:2728–2736
Venturelli S, Sinnberg TW, Berger A, Noor S, Levesque MP, Böcker A, Niessner H, Lauer UM, Bitzer M, Garbe C, Busch C (2014) Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front Oncol 4:227. https://doi.org/10.3389/fonc.2014.00227
Verdura S, Cuyàs E, Lozano-Sánchez J, Bastidas-Velez C, Llorach-Parés L, Fernández-Arroyo S, Hernández-Aguilera A, Joven J, Nonell-Canals A, Bosch-Barrera J, Martin-Castillo B, Vellon L, Sanchez-Martinez M, Segura-Carretero A, Menendez JA (2019) An olive oil phenolic is a new chemotype of mutant isocitrate dehydrogenase 1 (IDH1) inhibitors. Carcinogenesis 40:27–40. https://doi.org/10.1093/carcin/bgy159
Wang L, Zhang C, Guo Y, Su ZY, Yang Y, Shu L, Kong ANT (2014) Blocking of JB6 cell transformation by tanshinone IIA: epigenetic reactivation of Nrf2 antioxidative stress pathway. AAPS J 16:1214–1225. https://doi.org/10.1208/s12248-014-9666-8
Wang N, Wang Z, Wang Y et al (2015) Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 6:9854–9876. https://doi.org/10.18632/oncotarget.3396
Wang X, Cheng Y, Wang K, Liu R, Yang XL, Wen HM, Chai C, Liang JY, Wu H (2016a) Peperomin E reactivates silenced tumor suppressor genes in lung cancer cells by inhibition of DNA methyltransferase. Cancer Sci 107:1506–1519. https://doi.org/10.1111/cas.13029
Wang X, Li D, Ghali L, Xia R, Munoz LP, Garelick H, Bell C, Wen X (2016b) Therapeutic potential of delivering arsenic trioxide into HPV-infected cervical cancer cells using liposomal nanotechnology. Nanoscale Res Lett 11:94. https://doi.org/10.1186/s11671-016-1307-y
Wang Y, Jiang F, Jiao K, Ju L, Liu Q, Li Y, Miao L, Li Z (2020) De-methylation of miR-148a by arsenic trioxide enhances sensitivity to chemotherapy via inhibiting the NF-κB pathway and CSC like properties. Exp Cell Res 386:111739. https://doi.org/10.1016/j.yexcr.2019.111739
Wanga X, Gu J, Gao M, Bian Y, Liang JY, Wen HM, Wu H (2018) Peperomin E induces promoter hypomethylation of metastatic-suppressor genes and attenuates metastasis in poorly differentiated gastric cancer. Cell Physiol Biochem 50:2341–2364. https://doi.org/10.1159/000495096
Wippermann A, Rupp O, Brinkrolf K, Hoffrogge R, Noll T (2017) Integrative analysis of DNA methylation and gene expression in butyrate-treated CHO cells. J Biotechnol 257:150–161. https://doi.org/10.1016/j.jbiotec.2016.11.020
Wu L-P, Wang X, Li L, Zhao Y, Lu S, Yu Y, Zhou W, Liu X, Yang J, Zheng Z, Zhang H, Feng J, Yang Y, Wang H, Zhu WG (2008) Histone deacetylase inhibitor depsipeptide activates silenced genes through decreasing both CpG and H3K9 methylation on the promoter. Mol Cell Biol 28:3219–3235. https://doi.org/10.1128/MCB.01516-07
Xia D, Wang D, Kim S-H, DuBois RN (2011) Abstract 89: Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Cancer Res 71:89–89. https://doi.org/10.1158/1538-7445.AM2011-89
Xiang N, Zhao R, Song G, Zhong W (2008) Selenium modifies GSTP1 and histone methylation and acetylation in human prostate cancer cells. AACR
Xiang S, Zou P, Tang Q, Zheng F, Wu JJ, Chen ZQ, Hann SS (2018) HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta Gen Subj 1862:589–599. https://doi.org/10.1016/j.bbagen.2017.12.001
Xu Y, Su D, Zhu L, Zhang S, Ma S, Wu K, Yuan Q, Lin N (2018) S-Allylcysteine suppresses ovarian cancer cell proliferation by DNA methylation through DNMT1. J Ovarian Res 11:39. https://doi.org/10.1186/s13048-018-0412-1
Yahya EB, Alqadhi AM (2021) Recent trends in cancer therapy: a review on the current state of gene delivery. Life Sci 269:119087
Yan M-D, Yao C-J, Chow J-M, Chang CL, Hwang PA, Chuang SE, Whang-Peng J, Lai GM (2015) Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar Drugs 13:6099–6116. https://doi.org/10.3390/md13106099
Yang G, Zhu Y, Dong X, Duan Z, Niu X, Wei J (2014) TLR2-ICAM1-Gadd45α axis mediates the epigenetic effect of selenium on DNA methylation and gene expression in Keshan disease. Biol Trace Elem Res 159:69–80. https://doi.org/10.1007/s12011-014-9985-8
Yang Y, Fuentes F, Shu L, Wang C, Pung D, Li W, Zhang C, Guo Y, Kong AN (2017) Epigenetic CpG methylation of the promoter and reactivation of the expression of GSTP1 by astaxanthin in human prostate LNCaP cells. AAPS J 19:421–430. https://doi.org/10.1208/s12248-016-0016-x
Yang Y, Yang I, Cao M, Su ZY, Wu R, Guo Y, Fang M, Kong AN (2018) Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J 20:32. https://doi.org/10.1208/s12248-018-0197-6
Yu W, Liu C, Li X, Yang F, Cheng L, Liu C, Song Y (2017) Inositol hexaphosphate suppresses colorectal cancer cell proliferation via the Akt/GSK-3β/β-catenin signaling cascade in a 1,2-dimethylhydrazine-induced rat model. Eur J Pharmacol 805:67–74. https://doi.org/10.1016/j.ejphar.2017.03.011
Zappe K, Pointner A, Switzeny OJ, Magnet U, Tomeva E, Heller J, Mare G, Wagner KH, Knasmueller S, Haslberger AG (2018) Counteraction of oxidative stress by vitamin E affects epigenetic regulation by increasing global methylation and gene expression of MLH1 and DNMT1 dose dependently in Caco-2 cells. Oxidative Med Cell Longev 2018:1–13. https://doi.org/10.1155/2018/3734250
Zhang W, Wang L, Fan Q, Wu X, Wang F, Wang R, Ma Z, Yang J, Lu SH (2011) Arsenic trioxide re-sensitizes ERα-negative breast cancer cells to endocrine therapy by restoring ERα expression in vitro and in vivo. Oncol Rep 26:621–628
Zhang Y, Zhen C, Yang Q, Ji B (2020) Mathematical modelling of the role of GADD45β in the pathogenesis of multiple myeloma. R Soc Open Sci 7:192152
Zheng N-G, Wang J-L, Yang S-L, Wu J-L (2014) Aberrant epigenetic alteration in Eca9706 cells modulated by nanoliposomal quercetin combined with butyrate mediated via epigenetic-NF-κB signaling. Asian Pac J Cancer Prev 15:4539–4543. https://doi.org/10.7314/APJCP.2014.15.11.4539
Author information
Authors and Affiliations
Contributions
NO, MB, HI, FG, AB, GZ, and AB equally contributed to all stages of preparing, drafting, and writing of this review article. All authors read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Ethics statement
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Omari, N., Bakha, M., Imtara, H. et al. Anticancer mechanisms of phytochemical compounds: focusing on epigenetic targets. Environ Sci Pollut Res 28, 47869–47903 (2021). https://doi.org/10.1007/s11356-021-15594-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15594-8