Abstract
Epigenetic dysregulation through DNA methylation of the promoter, chromatin remodelling via histone modifying enzymes, and miRNA-mediated post-transcriptional gene silencing of target genes, for example, cell cycle regulators, signal transducers, transcription factors, nuclear receptors, and gene products for DNA repair and apoptosis, contribute to altered functions of the cells and ultimately cause disease or disorder. Numerous Himalayan medicinal plants have traditionally been recognised for treating various human diseases. Phytochemicals or bioactive agents isolated from these medicinal plants exhibit antioxidant activity, detoxification, anti-cancer activity, neuro-pharmacological activity, and immunity-potentiating properties. Thus, these phytochemicals effectively prevent various chronic diseases like cancer, diabetes and heart disease. This review paper highlights the potential roles and mode of action of Himalayan phytochemicals in targeting epigenetic alterations and discusses their potential for safety and clinical efficacy. Currently, very limited literature is available on the role of Himalayan phytochemicals and their role in epigenetic modulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epigenetics, in simple terms, implies differential regulation of gene expression with no alterations in DNA sequence that arise during development and are subsequently inherited. In mammalian cells, two main molecular mechanisms mediate these epigenetic effects via DNA methylation or histone modifications inducing chromatin remodelling that bring out changes in cellular phenotypes without the change in the genotype (Katiyar et al. 2012). Since these epigenetic effects on chromatin are inheritable, research has focused on developing novel therapeutics targeting epigenetic mechanisms for treating diseases such as cancer, thus improving conventional therapies (Tollefsbol 2014). Natural phytochemical compounds alone or in combination with existing treatments are one such alternative therapy that is the focus of research at the current time. Phytochemicals are plant-produced chemicals that have protective properties against several diseases (Chun et al. 2014). These are non-essential nutrients produced by plants to protect themselves as their defense mechanism (Pan et al. 2014). It was earlier believed that the human body did not require them, but several recent research demonstrate that they can also protect humans against various diseases (An et al. 2016). Dietary phytochemicals contain bioactive agents such as alkaloids, polyphenols, antioxidants, vitamins, minerals, and micronutrients. Through both genetic and epigenetic alterations mediated by activities of DNA methyltransferases and histone deacetylases, these bioactive agents have shown promising potential against a variety of disorders, including cancer (Tollefsbol 2014), diabetes (Chun et al. 2014), cardiovascular diseases, liver diseases (Pan et al. 2014), neurodegenerative diseases and osteoporosis (An et al. 2016). In this review article, we discuss the mechanism of epigenetic modification, including DNA methylation and histone modification, and their role in disease pathogenesis. We then describe the therapeutic roles of Himalayan plants, with emphasis on the phytochemical agents and effects. Lastly, we discuss the epigenetic regulation by the phytochemical agents in neurodegenerative diseases, cancers, and diabetes.
Search strategy
Search terms related to Himalayan phytochemicals and their role as epigenetic modulating agents in cancer, diabetes and neurodegenerative disorders were compiled in the following manner epigenetic mechanism in DNA methylation, histone modification, microRNAs, Himalayan plants and their therapeutic roles phytochemical agents and effects, epigenetic regulation in cancer, diabetes and neurodegenerative diseases treatment by using epigenetic modulators. The databases used for searching scientific literature include ScienceDirect, Google Scholar, ResearchGate, Elsevier, PubMed, SpringerLink, Wiley Online Library, and Taylor & Francis Online.
Epigenetic mechanism in DNA methylation
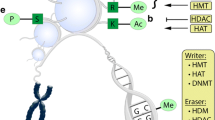
DNA methylation is a process of epigenetic modification that alters the gene expression without fluctuating the DNA sequence itself (Moore et al. 2013). This process involves the enzyme DNA methyltransferase (DNMT); during DNA methylation, a methyl group is added to cytosine in CpG islands located at gene regulatory regions via S-adenosyl methionine (SAM), such as promoter sequences, which can block DNA recognition and transcription factors, resulting in transcriptional repression. DNA methylation and demethylation pathways (Fig. 1A) illustrate the dynamic epigenetic mechanisms pivotal in regulating gene expression. At the top, the diagram depicts the conversion of cytosine to 5-methylcytosine, catalyzed by DNA methyltransferases (DNMTs), signifying the methylation process. However, in some cases, DNA methylation may activate transcription by preventing binding or limiting the expression of transcriptional repressors (Kiselev et al. 2021). DNA methylation helps regulate gene expression by hindering the binding of transcription factors to DNA or by engaging proteins that block transcription. DNA methylation is involved in critical processes such as X chromosome inactivation, genomic imprinting, and silencing of repetitive elements. Genomic imprinting involves the coordinated inhibition of gene clusters on a particular chromosome region by methylation of differentially methylated regions (DMRs), which often overlap with CpG islands (Moore et al. 2013). CpG sites within gene promoter regions are typically unmethylated, but increased methylation of these sites can silence tumour suppressors. Global DNA hypomethylation in CpG islands can induce genomic instability, often detected in cancerous cells (Kiselev et al. 2021).
Epigenetic pathways affected by plant phytochemicals. A DNA methylation and demethylation pathways: the figure illustrates the dynamic epigenetic mechanisms of DNA methylation and demethylation, pivotal in regulating gene expression. At the top, the diagram depicts the conversion of cytosine to 5-methylcytosine, catalyzed by DNA methyltransferases (dnmts), signifying the methylation process. B Post-transnational modifications: figure provides a detailed illustration of the various post-transnational modifications (ptms) that histones undergo, which play a critical role in regulating gene expression. The diagram depicts a nucleosome, the fundamental unit of chromatin, resembling a string of beads on a DNA strand. C microRNA biogenesis: in cancer cells, miRNAs are processed from primary to mature forms, influencing post-transcriptional regulation
Epigenetic regulation in cancer
Cancer is known to be a heterogeneous group of diseases involving uncontrolled cell proliferation and growth, which can spread to further parts of the body (Sarkar et al. 2013). Cancer-related global burden of deaths, 7.6 million deaths in 2008 and 9.6 million deaths in 2018, respectively, have been reported (Arora and Sharma 2019). Phytochemicals may also help in cancer prevention and its therapy through different epigenetic modifications like miRNA-mediated, DNA methylation or histone modification gene silencing in cancer progression. In terms of cancer, epigenetic modification works by altering gene expression of cancer-related genes, such as tumour-suppressive genes or oncogenes essential for cell proliferation and differentiation during carcinogenesis (Arora and Sharma 2019). Different mechanisms by which phytochemicals epigenetically alter these cancer-related gene expressions are mentioned (Table 1).
DNA methylation in cancer
Phytochemicals significantly impact the DNA methylation process by altering the DNMT enzyme level, directly or indirectly affecting cancer progression (Xie et al. 2014). For example, Genistein is a compound that is commonly found in soy. It reduces methylation activity when it forms a complex with the DNMT enzyme. This process leads to the activation of tumour suppressor genes, which can help prevent and treat cancer. Another compound that is helpful in cancer prevention and treatment is Resveratrol, which is found in grapes. Resveratrol acts as a DNMT inhibitor, which can help reduce cancer risk (Kala et al. 2015).
Histone post-translational modifications
Among epigenetic modifications, histone modifications have received particular attention due to their critical role in regulating gene expression. Himalayan plants are a rich source of phytochemicals that have been shown to modulate histone modifications and exert therapeutic effects against various diseases. Histone modifications play a crucial role in regulating gene expression by controlling the accessibility of chromatin to transcriptional machinery (Li 2012). Figure 1B gives a detailed illustration of the various post-translational modifications (PTMs) that histones undergo, which play a critical role in the regulation of gene expression. The diagram depicts a nucleosome, the fundamental unit of chromatin, resembling a string of beads on a DNA strand. This is achieved through acetylation, methylation, phosphorylation, ubiquitination, and SUMOylation. Acetylation and methylation of histone proteins are post-translational modifications that have been extensively studied. The dysregulation of these modifications is associated with various diseases, including cancer. Himalayan plants are a rich source of phytochemicals that have been shown to modulate histone modifications. For instance, curcumin, a polyphenol found in turmeric, has been shown to promote histone acetylation and inhibit histone deacetylases (HDACs), leading to increased expression of tumour suppressor genes and inhibition of cancer cell growth. Resveratrol, a polyphenol found in grapes and red wine, inhibits HDAC activity (Cutter and Hayes 2015). This, in turn, induces histone acetylation, leading to increased tumour suppressor gene expression and cancer cell growth inhibition. Histones are proteins that package and organize DNA in the nucleus of eukaryotic cells. The nucleosome is a basic unit of chromatin, consisting of an octamer of histone proteins around which DNA is wrapped. How DNA is packaged and organized by histones plays a crucial role in regulating gene expression (Biterge 2016). Histone acetylation is a process facilitated by histone acetyltransferases (HATs) and leads to neutralising the positive charge on the lysine residue of histones. This process results in a more open chromatin structure and increased transcriptional activity. On the other hand, histone deacetylases (HDACs) remove acetyl groups, resulting in a more condensed chromatin structure and decreased transcriptional activity. Histone methyltransferases (HMTs) catalyze the methylation of histones, influencing gene expression based on the site and degree of methylation (Shanmugam et al. 2018). Phosphorylation of histones is catalyzed by protein kinases and is involved in various cellular processes, including DNA damage response, mitosis, and meiosis. During mitosis, chromosome condensation is associated with H3S10ph, the phosphorylation of serine ten on histone H3. E3 ubiquitin ligases catalyse the ubiquitination of histones and can either activate or repress gene expression (Prakash and Fournier 2017). The simulation of histones is catalyzed by small ubiquitin-like modifier (SUMO) (Smith and Denu 2009) ligases and is involved in regulating chromatin structure and transcriptional activity (Lawrence et al. 2016).
Histone post-translational modifications in cancer
Histone modifications are frequently associated with the development of tumours and cancer and typically occur at the extended N-terminal domains of the histone core complex H3 and H4 (Füllgrabe et al. 2011). Lysine, serine, and threonine are the amino acid residues, present at the N-terminus domain, more prone to these modifications. There are various enzymes involved during the process of histone modification. Some enzymes play a role in modifying histones, which are proteins that package DNA in the nucleus of cells. These enzymes include HDACs, HATs, HMTs, and HDMs (Audia and Campbell 2016). Several phytochemical compounds are reported that induce change in the level of these enzymes that may either induce expression of tumour suppressive genes or silence expression of oncogenes, ultimately helping in epigenetic cancer treatment. One such example is sulforaphane, commonly found in broccoli, cauliflower and kale, which forms a complex with the HDAC enzyme active site to change its activity significantly (Okonkwo et al. 2018).
MicroRNAs
MicroRNAs, small RNA molecules of about 20–22 nucleotides, regulate critical biological processes and can also be affected by various diseases, including Cancer (Lu et al. 2005). Generating miRNAs involves proteins from the Argonaut family, RNA precursor structures, and ribonucleases such as Drosha and Dicer. MiRNAs can control multiple genes within related pathways by either imperfectly pairing with mRNA or influencing mRNA stability, and their dysregulation can play a role in the initiation and progression of Cancer (Lu et al. 2005). Epigenetic mechanisms, including DNA methylation and miRNA regulation, have been shown to interact and play essential roles in various biological processes and diseases. DNA methylation can regulate miRNA Biogenesis and expression, and alterations in DNA methylation patterns can lead to dysregulation of miRNA expression (Yao et al. 2019). However, recent research has shown that natural dietary compounds, such as phytochemicals, can reverse epigenetic changes before they cause disease, including Cancer (Fig. 1C). Phytochemicals can modify DNA methylation, histone modifications, and miRNA expression, making them potential candidates for chemoprevention. This article focuses on various dietary polyphenols that possess epigenomic-altering ability. Epigenetic mechanisms, including miRNAs, have been implicated in the modulation of gene expression and the interaction with internal and external factors (Mossman and Scott 2006).
MicroRNA mediated gene silencing
MicroRNAs (miRNAs) significantly contribute to epigenetic modifications and alter gene expression (Lu et al. 2005). miRNAs play a crucial role in controlling various biological processes such as cell death, cell proliferation, and cell differentiation. There are reports of a direct link between miRNA alteration and cancer. A single miRNA can regulate multiple genes involved in different cancer types' initiation and progression pathways, as evidenced by deregulated expression during carcinogenesis, mainly due to genetic and epigenetic changes (Brait and Sidransky 2011). Several studies have reported the ability of natural dietary phytochemicals to reverse the epigenetic changes of different miRNAs, thereby changing their function. Other epigenetic alterations like DNA methylation and histone acetylation can also cause altered microRNA expressions in different types of cancer (Weber et al. 2007).
Epigenetics role in disease
Epigenetic alterations, such as DNA methylation and histone modifications, play an essential role in the development and progression of various diseases, especially cancer. Abnormal changes in epigenetic modifications have been found in different kinds of cancer. DNA methylation, whether hypo or hyper-methylation, is crucial in regulating cell proliferation, DNA repair, and apoptosis. It activates oncogenes and suppresses tumour suppressor genes, leading to carcinogenesis (Berdasco and Esteller 2010). For example, LY6K, SLC34A2, and RBBP6 oncogenes are commonly reported to be hypomethylated in glioblastoma, papillary thyroid carcinoma, and colorectal cancer, respectively (Amalraj and Gopi 2017). Hypomethylation of the LINE-1 gene is a common characteristic found in lung cancer, early breast cancer, and metastatic colorectal adenocarcinoma (Park et al. 2014). Similarly, hypomethylation of TTF-3, MUC4, and CT45 genes is often observed in pancreatic, prostate, and ovarian cancers (Nørgaard et al. 2017; Zhang et al. 2015). On the other hand, many tumour suppressor genes, such as RASSF10 in kidney cancer, SIX3 in glioblastoma, CDKN2A, and PTEN in melanoma, are hypermethylated. RASSF1A gene hypermethylation has been linked to an increased risk of ovarian cancer and a higher mortality rate in breast cancer patients and has also been identified as a diagnostic marker for lung cancer (El-Sherif et al. 2016; Rezk et al. 2018). Recent research has demonstrated that hypermethylation of the PDE3A gene is associated with treatment response in cisplatin-resistant non-small cell lung cancer (NSCLC) (Tian et al. 2017). Furthermore, hypermethylation of genes, such as NDN, stimulates the WNT signalling pathway, aiding colorectal cancer cell growth (Hu et al. 2017). Apart from aberrant DNA methylation, changes in histone modification patterns also occur in cancer cells. The activation or repression of transcription is linked to histone changes at specific amino acid sites. In the case of prostate cancer, the epigenetic marks H3K9me2 and H3Ac can be utilized to distinguish tumours from normal tissues. The H3K4me1 alteration has been recognized as a biomarker of tumour progression and can also be used to predict recurrence after treatment, which is radical prostatectomy. Medicinal plants phytochemicals are listed (Supplementary Table 2). Lower levels of H3K4me3 and higher levels of H3K9me3 and H4K20me3 correlate with good prognosis in early-stage colon cancer (Benard et al. 2014).
Himalayan plants and therapeutic roles
Around the globe, medicinal plants have played a significant role in treating and preventing various diseases. In some places, people still depend on medicinal plants. People also have general knowledge of these plants that are utilized in treating different ailments (Tag et al. 2012). A variety of Himalayan plants have been listed in (Supplementary Table 3) with their therapeutic roles.
Phytochemical agents and effects
Phytochemicals are plant chemicals that may provide health advantages beyond basic nutrition that may lessen the risk of major chronic illness (e.g., cancer and heart disease). Prominent phytochemicals present in various Himalayan plants are curcumin which is the principal natural polyphenol in turmeric rhizomes, and other Curcuma spp. is curcumin, commonly known as diferuloylmethane. Curcumin has long been recognized as a medicinal plant in Asia for its potent anti-inflammatory, anti-mutagenic, anti-bacterial, and cancer-fighting properties. Regarding curcumin’s capacity to fight free radicals, there are several unique processes in action (Sharifi-Rad et al. 2020). In addition to GSH, catalase, and SOD enzymes, which all neutralize free radicals, reactive oxygen and nitrogen species (ROS and RNS) can be scavenged. Lipoxygenase/cyclooxygenase and xanthine hydrogenase/oxidase can also be inhibited. Additionally, it can influence the activities of GSH, catalase, and SOD. Curcumin also inhibits NF-κB, which many inflammatory stimuli trigger. Resveratrol is the stilbenoid group of polyphenols that includes resveratrol (3,5,4′-trihydroxy-trans-stilbene) with two phenol rings connected by an ethylene bridge (Kala et al. 2015). When plants like grapevines, berries, and peanuts are injured, they release compounds like this. Red grapes, mulberries, peanuts, and pines also contain resveratrol in their skins. Resveratrol’s biological purpose is to defend plants from fungal diseases, particularly Botrytis cinerea infection. Resveratrol has been shown to have anti-cancer properties and is being studied as a possible cancer-preventative and therapy option. Some additional bioactive properties, such as analgesic, cardiovascular and vasorelaxant properties, have also been documented. A study in breast cancer cell line (HCC1806) found that on combined treatment with Resveratrol and Pterostilbene, Sirtuin 1 (SIRT1) was down-regulated through inhibiting telomerase activity and γ-H2AX expression. Through its ability to inhibit DNMT and HDAC, resveratrol demonstrates antioxidant, anti-inflammatory, anti-angiogenic, and anti-cancer effects through epigenetic control (Li et al. 2019). Apigenin, also known as 4′,5,7-trihydroxyflavone, is a polyphenol member of the flavonoid family of compounds. Celery, chamomile, and parsley are all examples of foods that contain it. Apigenin hindered cell cycle progression and triggered cell death in human prostate cancer cells (PC-3 and 22Rv1). According to the findings, apigenin treatment in PC-3 and 22Rv1 cells inhibited the expression of HDAC1 and HDAC3 because of global hyper-acetylation of histone H3 and H4 (Busch et al. 2015). EGCG is a green tea that contains a polyphenolic catechin called epigallocatechin-gallate. Breast and prostate cancer risk can be greatly reduced by regular ingestion of it (Negri et al. 2018). Cancer cell cycle disruption is linked to epigenetic changes induced by DNA methylation or methyltransferase, histone acetylation or deacetylase, and non-coding RNAs. Much research has linked EGCG to the control of DNMTs, HDACs and HATs in various tumours, and this has been supported by numerous investigations (Singh et al. 2011). Genistein is one of the bioactive isoflavones generated from soy. Genistein impacts cancer via epigenetic regulation. Genistein stimulates tumour suppressor genes and impacts cancer cell viability through changing chromatin architecture and DNA methylation. DNMTs, HDACs, mainly HDAC6, HMTs, demethylases, tyrosine kinases and histone phosphorylases, were all affected by genistein (Kedhari Sundaram et al. 2019). Furthermore, genistein lowered global DNA methylation levels by inhibiting the activity of DNMTs, HDACs, and HMTs (Drețcanu et al. 2021). Sulforaphane (SFN) is an epigenetic modulator of gene transcription, it may directly or indirectly regulate epigenetic modulations. As a competitive inhibitor, SFN and its metabolites (SFN-cysteine and SFN-N-acetylcysteine) interact with amino acid residues in the active site of HDAC enzymes. SFN likely regulates gene promoter activity via a direct decrease in its histone deacetylase activity, which is then followed by an indirect change in the methylation of the gene promoter. SFN, a natural substance found in cruciferous vegetables, has been shown to reduce cancer risk, decrease the progression of cancer, and enhance the efficiency of several traditional chemotherapeutics through epigenetic modification of gene transcription activity (Kaufman-Szymczyk et al. 2015). SFN has been shown to decrease the expression and activity of hTERT, the catalytic subunit of the telomerase enzyme, in two prostate cancer cell lines. Gene expression is lowered due to these changes in chromatin structure and composition. SFN-induced changes in the levels of histone post-translational modifications, comprising acetylation of lysine 18 and di-methylation of lysine 4 of histone H3, were linked to regulators in the hTERT promoter region (Abbas et al. 2016). Quercetin is a flavanol-rich in onion, berries, okra and apple peels. It modulates chromatin modifiers such as DNMTs, HDACs, HAT, and HMTs (Busch et al. 2015; Kedhari Sundaram et al. 2019). The oncogenic hamster buccal pouch (HBP) cancer model generated by 7, 12 dimethyl Benz[a]anthracene (DMBA) was used to investigate the antioxidant’s chemopreventive and therapeutic properties. In the HBP model, quercetin inhibited the transcription factors HDAC1 and DNMT1, resulting in cell cycle arrest and death and a decrease in angiogenesis and invasion. In a study in which quercetin was administered at the same time as DMBA, the number and size of the tumours decreased. Following DMBA exposure, therapy with quercetin significantly reduced the formation of tumors (Busch et al. 2015). Quercetin generally inhibits HDAC2, HDAC4, HDAC7, and HDAC8, restoring tumour suppressor genes (TSG). Quercetin restores TSG function by reducing the methylation of their promoters, which competes with DNMT families and decreases gene expression (Drețcanu et al. 2021). Melatonin is the mammalian pineal gland's principal indoleamine hormone and is recognised to have various neuroprotective and neuroregulatory effects. Although melatonin is not a major plant product, apples, barley, beans, cucumber, grapes, lupine, maize, potato, rice, and tomatoes grown in northwestern India have been found to contain melatonin. The mammalian central nervous system expresses two melatonergic-signalling GPCRs, MT1 and MT2. Melatonin levels and receptor expression frequently decline with age, a decline that may be exacerbated in certain illness situations (Bahna and Niles 2018). In several peripherals and CNS oxidative stress types, melatonin stimulates Nrf2 expression and is the downstream target of Nrf2ARE signalling (Hardeland 2014). Melatonin and its metabolites can scavenge free radicals to influence intracellular antioxidant pathways. A single melatonin molecule acts as a powerful forager and neutraliser to counteract the harmful effects of numerous reactive oxygen, nitrogen, and hydroxyl radicals. Below listed (Table 2) are phytochemical agents, their chemical structures and mechanisms. Recent investigations reveal that the transcriptional activation of the MT1 receptor gene by valproic acid (VPA) is mediated by an epigenetic mechanism, especially histone acetylation of the MT1 promoter caused by HDAC suppression (Bahna and Niles 2018). Experiments using VPA indicated that variations in mRNA levels of MeCP2, HDAC1, 2, and 3 were related to changes in MTNR1A expression in C6 glioma cells. It also shows a link between DNA methylation and chromatin remodelling via histone deacetylation and MT1 expression (Hardeland 2014).
Phytochemical agents as epigenetic modulators in disease
The recent area of research involves using dietary phytochemicals to treat diseases like diabetes, neurodegenerative diseases, cardiovascular diseases, and cancer (Kumar et al. 2023). The reason for increasing interest in these agents may be their natural origin, availability in abundance, minimal side effects, and ease of including them in routine diets (Salm et al. 2023).
Phytochemical agents as epigenetic modulators in cancer
Himalayan medicinal plants have traditionally been used for ages as they are rich in various polyphenols. These agents are also known to have some epigenetic role in preventing cancer. Some phytochemical compounds like green tea contain EGCG, turmeric has curcumin, garlic has organosulfur, and broccoli has sulforaphane, which are known to have chemo-preventive roles (Painuli et al. 2021). The ability of dietary phytochemicals to alter the expression of genes by epigenetic mechanisms via DNA methylation, histone modifications and miRNA expression suggests their role in chemo-preventive potential (Shankar et al. 2013). The role and mode of action of some known phytochemical agents in epigenetic mechanisms are shown in the figure (Supplementary Fig. 2). Several phytochemical agents have been reported to have a role in cancer prevention via epigenetic modification, and some of them are under clinical trial information elaborates on some extensively studied phytochemical agents and how they alter the expression of cancer-related genes in an epigenetic fashion (Thakur et al. 2014).
Phytochemical agents as epigenetic regulation in diabetes
Diabetes mellitus (DM) is a long-term metabolic disorder that results in high blood glucose levels due to either insulin production defects, insulin resistance, or both. It is considered one of the most complex health challenges worldwide, with studies showing that it has reached epidemic proportions in many countries (Atlas 2015). It is predicted that by 2045, 700.2 million grown-ups will be suffering from DM across the globe (Dandekar et al. 2021). Resistance of insulin and reduction in insulin secretion are the primary mechanisms to cause DM. In most cases, impairment in action and insulin secretion might occur simultaneously (Gandhi et al. 2017). However, the underlying molecular mechanism for high glucose is yet to be studied entirely (Ullah and Khan 2020). In DM pathogenesis, various factors overlap, like genetics, epigenetics, and environmental factors (Prasad and Groop 2015; Raman 2016). A patient’s life quality can be affected if DM is left untreated, as it can develop into serious complications. Acute complications develop suddenly and involve HSS (hyperosmolar hyperglycaemic state) and diabetic ketoacidosis (DKA). In contrast, chronic complications progress over time and involve cerebrovascular events, peripheral arterial disease (PAD), peripheral neuropathy, neuropathy, atherosclerotic cardiovascular disease, autonomic neuropathy, and retinopathy (Jung 2015; Papatheodorou et al. 2018; Umpierrez and Korytkowski 2016). In recent decades, the development and acceptance of novel agents have proved an incredible improvement in the treatment of DM and related problems (Fig. 2). The excessive pricing of existing therapeutic opportunities is the primary issue physicians face, as it surges the economic burden on patients and can affect obedience to the treatment (Enwere et al. 2010). The occurrence of DM among young people is also increasing continuously but for this group, the available therapeutic opportunities are minimal. Presently, only two drugs (metformin and insulin) for the treatment of diabetes have been permitted by the US FDA for utilization in youths and children (Zimmet and Shaw 2017). In such cases, plant extracts and isolated compounds may be used as a source of alternative treatment, as these are backed by the conventional utilization of plants for DM therapy (Ezuruike and Prieto 2014). Experimental studies proved the benefits of plant extracts against DM and other metabolic abnormalities (Belwal et al. 2020). The historical literature and conventional knowledge of medicine play a crucial role in discovering new leads from medicinal plants (Ayyanar and Subash-Babu 2012; Buenz et al. 2005). Lately, because of the effectiveness of medicinal plants in clinical trials for treating DM, research for new medicines has been focused on them (Suba et al. 2004). Evidence of some plant extract's effectiveness in DM from clinical trials is tabulated here (Supplementary Table 3).
The term epigenetics was first proposed by Conrad Waddington in the 1940s to describe the interaction of genes with their environment during development (Lacagnina 2020). Epigenetics is “the causal interactions between genes and their products, which bring the phenotype into being”. Epigenetic mechanisms are crucial for genomic stability and regulating gene transcription without causing variations to the underlying sequence of DNA (Barker 2007). The well-established viewpoint that noncommunicable disease (cardiovascular disease, diabetes mellitus) are the product of the gene-lifestyle interaction in adult life has undergone considerable evolution since the 1990s (Moskalev et al. 2014). A study conducted in Hertfordshire, United Kingdom, focusing on men born in the 1920s who weighed less than 2.5 kg, revealed a significant link between low birth weight and the likelihood of developing coronary heart disease, type 2 diabetes, and metabolic syndrome. (Barker 2004). It is proposed that the atmosphere persuades alterations in the initial stages of development and growth with lasting consequences on disease and health later on (Meseure et al. 2015). It is supposed that these modifications can be instigated by a change in a specific person's genetic expression (Shams and Müller 2014). Nowadays, researchers have recognized numerous biomarkers associated with genetic expression alteration linked with several chronic pathologies like diabetes, cancer, cardiovascular disorders, and autoimmune and inflammatory disorders (Ullah and Khan 2020). These biomarkers can aid as therapeutic targets (Cheng et al. 2018). Mechanisms such as histone modification, miRNAs, and DNA methylation function under the epigenetic umbrella, offering signs for the individual to respond to the signs of the environment.
Medicinal plants are the main source of organic compounds such as polyphenols, tannins, alkaloids, carbohydrates, terpenoids, steroids and flavonoids. These organic compounds represent a source for the discovery and development of new types of antidiabetic molecules. Different phytochemicals target specific metabolic and physiological processes in diabetes mellitus. Many compounds isolated from plant sources have been reported to show antidiabetic activity (Firdous 2014). Chicoric acid isolated from Ocimum gratissimum L. reduced significantly the glycemic levels of diabetic mice (Casanova et al. 2014). Asiatic acid, showed antidiabetic activity with improvement in the lipid profile in rats (Ramachandran et al. 2014). The study was carried out in multi-low-dose streptozotocin-induced (MLDS) diabetic mice in which hypoglycaemic effect of Chamaemelum nobile (CN) aqueous extract (20 mg/kg) was shown after single and repeated oral administration (Abderrahmane et al. 2009). Berberine and Pectin compounds are associated with reduced fasting blood glucose, HbA1c, postprandial glucose levels, and increased insulin release, addressing key aspects of glycemic control. Glycans are involved in the basic synthesis promotion, and they play a role in the overall metabolism of glucose (Xie et al. 2022). Quercetin and Resveratrol are known for enhancing insulin sensitivity; these phytochemicals help mitigate insulin resistance, a common issue in type 2 diabetes (Shahwan et al. 2022). Inulin compound is linked to increased secretion from pancreatic β cells, crucial for insulin production and secretion (Fu et al. 2013). Silymarin extracted from thistle milk exhibits antioxidant properties, which are beneficial in preventing oxidative stress-related damage in diabetes. Sircupsin B acts as an inhibitor of α-amylase secreted from salivary glands or the pancreas, playing a role in carbohydrate digestion and subsequent glucose absorption (Surai 2015). GLP-1/GIP is secreted from intestine L-cells, and these incretins stimulate insulin secretion, contributing to postprandial glucose regulation (Nauck et al. 2021). Curcumin and Turmerosaccharides compounds increase insulin sensitivity due to their secretion from the brush border of the small intestine, highlighting their role in nutrient absorption and metabolism (Ghorbani et al. 2014). Illustration depicts the structures of phytochemical agents involved in preventing diabetes through epigenetic modification (Supplementary Fig. 4).
Cardiovascular disease treatment by using epigenetic modulators
Cardiovascular diseases remain a leading cause of health problems globally. In particular, China and India bear the highest burden of cardiovascular disease, which includes conditions such as coronary heart disease, hypertension, heart failure, and vascular calcification (Roth et al. 2020). In recent years, epigenetics has played a significant role in the historical development of cardiovascular diseases. The correlation of epigenetics with cardiovascular diseases has primarily been identified in the function and expression of epigenetic-related enzymes found in cardiovascular diseases (Farsetti et al. 2023). DNA methylation plays a critical role in cardiovascular diseases. Recent research has discovered that the expression of candidate genes associated with coronary heart disease, heart failure, hypertension, and other cardiovascular diseases is linked to DNA methylation (Shi et al. 2022). The abnormal methylation status of candidate genes is involved in the mechanism and development of cardiovascular disease and can be used as a marker to assess cardiovascular disease progression. A list of phytocompounds from medicinal plants reported for cardiovascular properties is provided in an additional file (Supplementary Table 5).
Neurodegenerative disease treatment by using epigenetic modulators
To control gene expression, epigenetic modifications regulate chromatin architecture and activate or suppress transcription machinery through DNA methylation, post-transcriptional, and RNA-based modifications. Epigenetic modulators control these mechanisms, like histone methyl transferases, histone demethylases, and other enzymes, along with various phytochemicals (Oppermann 2013). The brain requires a lot of energy and nutrients, and specific diets have been shown to improve brain function. Traditional medicines that contain nutrients such as resveratrol, theobromine, gallic acid, and catechin have been found to enhance cognitive function (Oppermann 2013). Phytochemicals such as cyanidin-3-glucopyranoside, resveratrol, curcumin, and flavonoids can penetrate the BBB and reverse the age-related cognitive decline by inducing neurotrophies through the Trk signalling pathway in the hippocampus. Plant phytochemicals also act as antioxidants, leading to neuronal regeneration, neuroprotection, and improved memory enhancement. Accumulating evidence indicates that dietary phytochemicals may prevent or reverse neurodegenerative diseases can be targeted by influencing neurotrophins, which play a crucial role in the survival, maintenance, and regeneration of specific neuronal populations in the brain. This can be achieved using plant-derived phytochemicals, and it is important to study their botanical sources, pharmacological effects, and medicinal applications. (Supplementary Table 5). Structures of phytochemical agents involved in neurodegenerative disease prevention via epigenetic modification have been illustrated (Supplementary Fig. 5).
Conclusion and future prospectus
The field of epigenetics and phytochemical therapeutics is rapidly expanding, with increasing interest in developing novel treatments for various diseases. Future research will focus on understanding epigenetic modification mechanisms and identifying new phytochemical agents that can target these mechanisms. The two main molecular mechanisms that mediate epigenetic effects are DNA methylation and histone modification, which induce chromatin remodelling and bring about changes in cellular phenotypes without altering the genotype. These epigenetic effects are inheritable and have been targeted for the development of novel therapeutics for diseases such as cancer. Phytochemical compounds, which are non-essential nutrients produced by plants as their defence mechanism, have shown promising potential as alternative therapies for various disorders. Through genetic and epigenetic alterations mediated by DNA methyltransferases and histone deacetylases, phytochemical agents have been found to have therapeutic roles in cancer, diabetes, cardiovascular diseases, neurodegenerative diseases, and osteoporosis. Further research on epigenetic regulation by phytochemical agents could lead to the development of effective treatments for these diseases. In addition, future research will focus on developing new delivery methods for these phytochemical agents, such as nanoparticle-based drug delivery systems, to improve their efficacy and reduce side effects. There will also be a focus on developing combination therapies that combine phytochemical agents with existing treatments to enhance their efficacy and reduce toxicity.
Data availability
All data supporting this review are available within the paper and its Supplementary Information.
References
Abbas A, Hall JA, Patterson WL, Ho E, Hsu A, Al-Mulla F, Georgel PT (2016) Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem Cell Biol 94(1):71–81. https://doi.org/10.1139/bcb-2015-0038
Abderrahmane M, Ahmed L, Farid O, Zegzouti Y, Hajji L, Eddouks M (2009) Mechanistic study of antidiabetic effect of Chamaemelum nobile in diabetic mice. In: Eddouks M (ed) Advances in phytotherapy research. Research Signpost, India, pp 161–173
Amalraj A, Gopi S (2017) Medicinal properties of Terminalia arjuna (Roxb.) Wight & Arn.: a review. J Tradition Complement Med 7(1):65–78. https://doi.org/10.1016/j.jtcme.2016.02.003
An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, Chen B (2016) Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci 147:46–58. https://doi.org/10.1016/j.lfs.2016.01.024
Arora I, Sharma M (2019) Combinatorial epigenetics impact of polyphenols and phytochemicals in cancer prevention and therapy. Int J Mol Sci 20(18):4567. https://doi.org/10.3390/ijms20184567
Atlas D (2015) International diabetes federation. IDF diabetes atlas, 7th edn. IDF, Brussels, Belgium
Audia JE, Campbell RM (2016) Histone modifications and cancer. Cold Spring Harb Perspect Biol 8(4):a019521. https://doi.org/10.1101/cshperspect.a019521
Ayyanar M, Subash-Babu P (2012) Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2(3):240–246. https://doi.org/10.1016/S2221-1691(12)60050-1
Bahna SG, Niles LP (2018) Epigenetic regulation of melatonin receptors in neuropsychiatric disorders. Br J Pharmacol 175(16):3209–3219. https://doi.org/10.1111/bph.14058
Barker DJP (2004) The developmental origins of well–being. Philos Trans R Soc Lond Ser B Biol Sci 359(1449):1359–1366. https://doi.org/10.1098/rstb.2004.1518
Barker DJP (2007) The origins of the developmental origins theory. J Intern Med 261(5):412–417. https://doi.org/10.1111/j.1365-2796.2007.01809.x
Belwal T, Bisht A, Devkota HP, Ullah H, Khan H, Pandey A, Bhatt ID, Echeverría J (2020) Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front Pharmacol. https://doi.org/10.3389/fphar.2020.00041
Benard A, Goossens-Beumer IJ, van Hoesel AQ, de Graaf W, Horati H, Putter H, Zeestraten EC, van de Velde CJ, Kuppen PJ (2014) Histone trimethylation at H3K4, H3K9 and H4K20 correlates with patient survival and tumor recurrence in early-stage colon cancer. BMC Cancer 14(1):531. https://doi.org/10.1186/1471-2407-14-531
Berdasco M, Esteller M (2010) Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 19(5):698–711. https://doi.org/10.1016/j.devcel.2010.10.005
Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO (2008) Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem 103(2):509–519. https://doi.org/10.1002/jcb.21417
Biterge B (2016) A mini review on post-translational histone modifications. MOJ Cell Sci Rep. https://doi.org/10.15406/mojcsr.2016.03.00047
Brait M, Sidransky D (2011) Cancer epigenetics: above and beyond. Toxicol Mech Methods 21(4):275–288. https://doi.org/10.3109/15376516.2011.562671
Buenz EJ, Johnson HE, Beekman EM, Motley TJ, Bauer BA (2005) Bioprospecting rumphius’s ambonese herbal: volume I. J Ethnopharmacol 96(1–2):57–70. https://doi.org/10.1016/j.jep.2004.08.016
Busch C, Burkard M, Leischner C, Lauer UM, Frank J, Venturelli S (2015) Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics 7(1):64. https://doi.org/10.1186/s13148-015-0095-z
Casanova LM, da Silva D, Sola-Penna M, de Magalhães Camargo LM, de Moura Celestrini D, Tinoco LW, Costa SS (2014) Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study. Fitoterapia 93:132–141. https://doi.org/10.1016/j.fitote.2013.12.024
Cheng Z, Zheng L, Almeida FA (2018) Epigenetic reprogramming in metabolic disorders: nutritional factors and beyond. J Nutr Biochem 54:1–10. https://doi.org/10.1016/j.jnutbio.2017.10.004
Chun SW, Cha BY, Ko KS, Ryu AJ, Kim YJ, Kim SJ (2014) PO227 gender differences of diabetic peripheral neuropathy in Korea. Diabetes Res Clin Pract 106:S164–S165. https://doi.org/10.1016/S0168-8227(14)70521-2
Cutter AR, Hayes JJ (2015) A brief review of nucleosome structure. FEBS Lett 589(20 Part A):2914–2922. https://doi.org/10.1016/j.febslet.2015.05.016
Dandekar P, Ramkumar S, RaviKumar A (2021) Structure–activity relationships of pancreatic α-amylase and α-glucosidase as antidiabetic targets. Bioactive natural products. Elsevier, Amsterdam, pp 381–410
Drețcanu G, Iuhas CI, Diaconeasa Z (2021) The involvement of natural polyphenols in the chemoprevention of cervical cancer. Int J Mol Sci 22(16):8812. https://doi.org/10.3390/ijms22168812
El-Sherif WT, Sayed SK, Galal SH, Makhlouf HA, Hassan AT, Yousef HA (2016) Diagnostic role of RASSF1A and p16INK4a promoter gene hypermethylation in serum DNA of lung cancer patients: clinicopathological significance. Egypt J Immunol 23(2):1–16
Enwere OO, Salako BL, Falade CO (2010) Prescription and cost consideration at a diabetic clinic in Ibadan, Nigeria: a report. Ann Ibadan Postgrad Med. https://doi.org/10.4314/aipm.v4i2.55232
Ezuruike UF, Prieto JM (2014) The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol 155(2):857–924. https://doi.org/10.1016/j.jep.2014.05.055
Farsetti A, Illi B, Gaetano C (2023) How epigenetics impacts on human diseases. Eur J Intern Med 114:15–22. https://doi.org/10.1016/j.ejim.2023.05.036
Firdous SM (2014) Phytochemicals for treatment of diabetes. EXCLI J 13:451–453
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev 9(1):25–53
Füllgrabe J, Kavanagh E, Joseph B (2011) Histone onco-modifications. Oncogene 30(31):3391–3403. https://doi.org/10.1038/onc.2011.121
Gandhi J, Dagur G, Warren K, Smith NL, Khan SA (2017) Genitourinary complications of diabetes mellitus: an overview of pathogenesis, evaluation, and management. Curr Diabetes Rev. https://doi.org/10.2174/1573399812666161019162747
Ghorbani Z, Hekmatdoost A, Mirmiran P (2014) Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metab. https://doi.org/10.5812/ijem.18081
Hardeland R (2014) Melatonin, noncoding RNAs, messenger RNA stability and epigenetics—evidence, hints, gaps and perspectives. Int J Mol Sci 15(10):18221–18252. https://doi.org/10.3390/ijms151018221
Hu Y-H, Chen Q, Lu Y-X, Zhang J-M, Lin C, Zhang F, Zhang W-J, Li X-M, Zhang W, Li X-N (2017) Hypermethylation of NDN promotes cell proliferation by activating the Wnt signaling pathway in colorectal cancer. Oncotarget 8(28):46191–46203. https://doi.org/10.18632/oncotarget.17580
Jung HS (2015) Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab 30(2):167. https://doi.org/10.3803/EnM.2015.30.2.167
Kala R, Shah HN, Martin SL, Tollefsbol TO (2015) Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 15(1):672. https://doi.org/10.1186/s12885-015-1693-z
Katiyar SK, Singh T, Prasad R, Sun Q, Vaid M (2012) Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets. Photochem Photobiol 88(5):1066–1074. https://doi.org/10.1111/j.1751-1097.2011.01020.x
Kaufman-Szymczyk A, Majewski G, Lubecka-Pietruszewska K, Fabianowska-Majewska K (2015) The role of sulforaphane in epigenetic mechanisms, including interdependence between histone modification and DNA methylation. Int J Mol Sci 16(12):29732–29743. https://doi.org/10.3390/ijms161226195
Kedhari Sundaram M, Hussain A, Haque S, Raina R, Afroze N (2019) Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J Cell Biochem 120(10):18357–18369. https://doi.org/10.1002/jcb.29147
Kiselev IS, Kulakova OG, Boyko AN, Favorova OO (2021) DNA methylation as an epigenetic mechanism in the development of multiple sclerosis. Acta Nat 13(2):45–57. https://doi.org/10.32607/actanaturae.11043
Kumar A, Nirmal P, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, Sneha K, Oz F (2023) Major phytochemicals: recent advances in health benefits and extraction method. Molecules 28(2):887. https://doi.org/10.3390/molecules28020887
Lacagnina S (2020) The developmental origins of health and disease (DOHaD). Am J Lifestyle Med 14(1):47–50. https://doi.org/10.1177/1559827619879694
Lawrence M, Daujat S, Schneider R (2016) Lateral thinking: how histone modifications regulate gene expression. Trends Genet 32(1):42–56. https://doi.org/10.1016/j.tig.2015.10.007
Lee WJ, Shim J-Y, Zhu BT (2005) Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol 68(4):1018–1030. https://doi.org/10.1124/mol.104.008367
Li S (2012) Implication of posttranslational histone modifications in nucleotide excision repair. Int J Mol Sci 13(10):12461–12486. https://doi.org/10.3390/ijms131012461
Li S, Chen M, Li Y, Tollefsbol TO (2019) Prenatal epigenetics diets play protective roles against environmental pollution. Clin Epigenetics 11(1):82. https://doi.org/10.1186/s13148-019-0659-4
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838. https://doi.org/10.1038/nature03702
Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, Hirata H, Li LC, Zhao H, Okino ST, Place RF, Pookot D, Dahiya R (2008) Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Can Res 68(8):2736–2744. https://doi.org/10.1158/0008-5472.CAN-07-2290
Meeran SM, Patel SN, Tollefsbol TO (2010) Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 5(7):e11457. https://doi.org/10.1371/journal.pone.0011457
Meseure D, Drak Alsibai K, Nicolas A, Bieche I, Morillon A (2015) Long noncoding RNAs as new architects in cancer epigenetics, prognostic biomarkers, and potential therapeutic targets. Biomed Res Int 2015:1–14. https://doi.org/10.1155/2015/320214
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38(1):23–38. https://doi.org/10.1038/npp.2012.112
Moskalev A, Aliper A, Smit-McBride Z, Buzdin A, Zhavoronkov A (2014) Genetics and epigenetics of aging and longevity. Cell Cycle 13(7):1063–1077. https://doi.org/10.4161/cc.28433
Mossman D, Scott RJ (2006) Epimutations, inheritance and causes of aberrant DNA methylation in cancer. Hered Cancer Clin Pract 4(2):75. https://doi.org/10.1186/1897-4287-4-2-75
Nauck MA, Quast DR, Wefers J, Pfeiffer AFH (2021) The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab 23(S3):5–29. https://doi.org/10.1111/dom.14496
Negri A, Naponelli V, Rizzi F, Bettuzzi S (2018) Molecular targets of epigallocatechin—gallate (EGCG): a special focus on signal transduction and cancer. Nutrients 10(12):1936. https://doi.org/10.3390/nu10121936
Nørgaard M, Haldrup C, Storebjerg T, Vestergaard E, Wild P, Høyer S, Borre M, Ørntoft T, Sørensen K (2017) Comprehensive evaluation of TFF3 promoter hypomethylation and molecular biomarker potential for prostate cancer diagnosis and prognosis. Int J Mol Sci 18(9):2017. https://doi.org/10.3390/ijms18092017
Okonkwo A, Mitra J, Johnson GS, Li L, Dashwood WM, Hegde ML, Yue C, Dashwood RH, Rajendran P (2018) Heterocyclic analogs of sulforaphane trigger DNA damage and impede DNA repair in colon cancer cells: interplay of HATs and HDACs. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201800228
Oppermann U (2013) Why is epigenetics important in understanding the pathogenesis of inflammatory musculoskeletal diseases? Arthritis Res Ther 15(2):209. https://doi.org/10.1186/ar4186
Painuli S, Semwal P, Cruz-Martins N, Bachheti RK (2021) Medicinal plants of Himalayan forests. In: Husen A, Bachheti RK, Bachheti A (eds) Non-timber forest products. Springer International Publishing, Cham, pp 175–212
Pan M, Lai C, Tsai M, Ho C (2014) Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res 58(1):147–171. https://doi.org/10.1002/mnfr.201300522
Pandey M, Shukla S, Gupta S (2010) Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer 126(11):2520–2533. https://doi.org/10.1002/ijc.24988
Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M (2018) Complications of diabetes 2017. J Diabetes Res 2018:1–4. https://doi.org/10.1155/2018/3086167
Park SY, Seo AN, Jung HY, Gwak JM, Jung N, Cho N-Y, Kang GH (2014) Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS ONE 9(6):e100429. https://doi.org/10.1371/journal.pone.0100429
Prakash K, Fournier D (2017) Histone code and higher-order chromatin folding: a hypothesis. Genomics Comput Biol 3(2):41. https://doi.org/10.18547/gcb.2017.vol3.iss2.e41
Prasad R, Groop L (2015) Genetics of type 2 diabetes—pitfalls and possibilities. Genes 6(1):87–123. https://doi.org/10.3390/genes6010087
Ramachandran V, Saravanan R, Senthilraja P (2014) Antidiabetic and antihyperlipidemic activity of asiatic acid in diabetic rats, role of HMG CoA: in vivo and in silico approaches. Phytomedicine 21(3):225–232. https://doi.org/10.1016/j.phymed.2013.08.027
Raman PG (2016) Environmental factors in causation of diabetes mellitus. In: Larramendy ML, Soloneski S (eds) Environmental health risk—hazardous factors to living species. InTech, London
Rezk NA, Mohamed RH, Alnemr AA, Harira M (2018) Promoter methylation of RASSF1A gene in Egyptian patients with ovarian cancer. Appl Biochem Biotechnol 185(1):153–162. https://doi.org/10.1007/s12010-017-2648-4
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J et al (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol 76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010
Salm S, Rutz J, van den Akker M, Blaheta RA, Bachmeier BE (2023) Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1234701
Sarkar S, Horn G, Moulton K, Oza A, Byler S, Kokolus S, Longacre M (2013) Cancer development, progression, and therapy: an epigenetic overview. Int J Mol Sci 14(10):21087–21113. https://doi.org/10.3390/ijms141021087
Shahwan M, Alhumaydhi F, Ashraf GMd, Hasan PMZ, Shamsi A (2022) Role of polyphenols in combating type 2 diabetes and insulin resistance. Int J Biol Macromol 206:567–579. https://doi.org/10.1016/j.ijbiomac.2022.03.004
Shams TA, Müller DJ (2014) Antipsychotic induced weight gain: genetics, epigenetics, and biomarkers reviewed. Curr Psychiatry Rep 16(10):473. https://doi.org/10.1007/s11920-014-0473-9
Shankar S, Kumar D, Srivastava RK (2013) Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther 138(1):1–17. https://doi.org/10.1016/j.pharmthera.2012.11.002
Shanmugam MK, Arfuso F, Arumugam S, Chinnathambi A, Jinsong B, Warrier S, Wang LZ, Kumar AP, Ahn KS, Sethi G, Lakshmanan M (2018) Role of novel histone modifications in cancer. Oncotarget 9(13):11414–11426. https://doi.org/10.18632/oncotarget.23356
Sharifi-Rad J, Rayess Y. El, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC et al (2020) Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. https://doi.org/10.3389/fphar.2020.01021
Shi Y, Zhang H, Huang S, Yin L, Wang F, Luo P, Huang H (2022) Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal Transduct Target Ther 7(1):200. https://doi.org/10.1038/s41392-022-01055-2
Singh BN, Shankar S, Srivastava RK (2011) Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 82(12):1807–1821. https://doi.org/10.1016/j.bcp.2011.07.093
Smith BC, Denu JM (2009) Chemical mechanisms of histone lysine and arginine modifications. Biochim Biophys Acta (BBA) Gene Regul Mech 1789(1):45–57. https://doi.org/10.1016/j.bbagrm.2008.06.005
Suba V, Murugesan T, Arunachalam G, Mandal SC, Saha BP (2004) Anti-diabetic potential of Barleria lupulina extract in rats. Phytomedicine 11(2–3):202–205. https://doi.org/10.1078/0944-7113-00316
Surai P (2015) Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants 4(1):204–247. https://doi.org/10.3390/antiox4010204
Tag H, Kalita P, Dwivedi P, Das AK, Namsa ND (2012) Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. J Ethnopharmacol 141(3):786–795. https://doi.org/10.1016/j.jep.2012.03.007
Tan S, Wang C, Lu C, Zhao B, Cui Y, Shi X, Ma X (2009) Quercetin is able to demethylate the p16INK4a gene promoter. Chemotherapy 55(1):6–10. https://doi.org/10.1159/000166383
Thakur VS, Deb G, Babcook MA, Gupta S (2014) Plant phytochemicals as epigenetic modulators: role in cancer chemoprevention. AAPS J 16(1):151–163. https://doi.org/10.1208/s12248-013-9548-5
Tian F-M, Zhong C-Y, Wang X-N, Meng Y (2017) PDE3A is hypermethylated in cisplatin resistant non-small cell lung cancer cells and is a modulator of chemotherapy response. Eur Rev Med Pharmacol Sci 21(11):2635–2641
Tili E, Michaille J-J, Alder H, Volinia S, Delmas D, Latruffe N, Croce CM (2010) Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem Pharmacol 80(12):2057–2065. https://doi.org/10.1016/j.bcp.2010.07.003
Tollefsbol TO (2014) Dietary epigenetics in cancer and aging. In: Zappia V, Panico S, Russo GL, Budillon A, Ragione FD (eds) Advances in nutrition and cancer. Springer, Berlin, pp 257–267
Ullah H, Khan H (2020) Epigenetic drug development for autoimmune and inflammatory diseases. Histone modifications in therapy. Elsevier, Amsterdam, pp 395–413
Umpierrez G, Korytkowski M (2016) Diabetic emergencies—ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 12(4):222–232. https://doi.org/10.1038/nrendo.2016.15
Wang R-H, Zheng Y, Kim H-S, Xu X, Cao L, Lahusen T, Lee M-H, Xiao C, Vassilopoulos A, Chen W, Gardner K, Man Y-G, Hung M-C, Finkel T, Deng C-X (2008) Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis. Mol Cell 32(1):11–20. https://doi.org/10.1016/j.molcel.2008.09.011
Weber B, Stresemann C, Brueckner B, Lyko F (2007) Methylation of human microRNA genes in normal and neoplastic cells. Cell Cycle 6(9):1001–1005. https://doi.org/10.4161/cc.6.9.4209
Xie Q, Bai Q, Zou L, Zhang Q, Zhou Y, Chang H, Yi L, Zhu J, Mi M (2014) Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom Cancer 53(5):422–431. https://doi.org/10.1002/gcc.22154
Xie W, Su F, Wang G, Peng Z, Xu Y, Zhang Y, Xu N, Hou K, Hu Z, Chen Y, Chen R (2022) Glucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front Pharmacol. https://doi.org/10.3389/fphar.2022.1015045
Yao Q, Chen Y, Zhou X (2019) The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol 51:11–17. https://doi.org/10.1016/j.cbpa.2019.01.024
Zhang W, Barger CJ, Link PA, Mhawech-Fauceglia P, Miller A, Akers SN, Odunsi K, Karpf AR (2015) DNA hypomethylation-mediated activation of cancer/testis antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer. Epigenetics 10(8):736–748. https://doi.org/10.1080/15592294.2015.1062206
Zimmet P, Shaw J (2017) Rising incidence of diabetes mellitus in youth in the USA. Nat Rev Endocrinol 13(7):379–380. https://doi.org/10.1038/nrendo.2017.59
Acknowledgements
The authors thank the University Institute of Biotechnology, Chandigarh University, for providing the infrastructure to conduct reviews and research.
Funding
N/A.
Author information
Authors and Affiliations
Contributions
SK: “Original draft preparation”. JS: “Conceptualization, review and editing”. VD: “Original draft preparation”. SS: “Writing review”. DS: “Review and editing”. SS: “Review and editing”. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
N/A.
Informed consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Singh, J., Dwibedi, V. et al. Himalayan phytochemicals and their role as epigenetic modulating agents in cancer, diabetes and neurodegenerative disorders. Vegetos (2024). https://doi.org/10.1007/s42535-024-01015-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-024-01015-x