Abstract
Acid rain is considered one of the most serious plant abiotic stresses. Photosynthesis is the basis of crop growth and development. The effect of acid rain on barley photosynthesis remains unclear. A glasshouse experiment was conducted, and the photosynthetic rate, chlorophyll (Chl) fluorescence, and pigment content of barley were measured in simulated acid rain (SAR) under pH 6.5, 5.5, 4.5, and 3.5. The results showed that net photosynthetic rate, maximal photosynthetic rate, and light saturation point decreased and the light compensation point, and dark respiration rate increased with increasing acidity. The results suggested that photosynthesis in barley plants was inhibited by SAR stress. The Chl content and stomatal conductance declined in parallel with the reduced net photosynthetic rate when barley plants were under SAR stress conditions. This indicated that non-stomatal factors may contribute to reduced photosynthesis under acid rain stress. Acid rain had greater effects on the photosynthesis of the acid rain-sensitive plant Zhepi 33 than on non-sensitive Kunlun 12. A significant difference in parameters such as the maximal fluorescence, variable fluorescence, and active PSII reaction centers was found among the SAR treatments and may be used to evaluate the sensitivity of plants to acid rain stress. The visualization model showed that the photosynthetic reaction centers were inactivated in acid rain stressed barley plants. These findings are valuable for the evaluation of the plant sensitivity to acid rain stress and may be used for the detection and monitoring of acid rain effects on plants in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain contains acidic components, such as sulfuric acid or nitric acid. It is a global environmental problem and has become more severe with the use of fossil fuels in recent decades (Yu et al. 2017; Debnath et al. 2020). Acid rain is one of the major abiotic stresses caused to plants and plants will die under serious acid rain growing conditions. Previous studies showed that acid rain has a strong influence on plant physiological and biochemical processes (Debnath and Ahammed 2020). In 2016, Sun et al. reported that acid rain at pH 4.0 or less decreased the rice leaf water content. The study of Yu et al. (2002) showed that acid rain significantly increased the activities of guaiacol peroxidase and superoxide dismutase, but decreased the activity of catalase. In addition, the chlorophyll (Chl) contents of perennial ryegrass and tomato, leaf area, and the yield of wheat were also adversely affected by acid rain stress (Kumari and Tomar 2009; Vicas et al. 2009; Debnath et al. 2018; Debnath et al. 2018; Singh and Agrawal 2004)
Photosynthesis is the basis of crop growth and development (Long et al. 2006; Zhu et al. 2012). It is very important to evaluate acid rain effects on crop photosynthetic activities. Previous studies showed that acid rain destroyed the chloroplast structure and decreased the crop photosynthetic rate, and the reduction in photosynthesis depended on the strength of acid in the rain (Yu et al. 2002; Sun et al. 2016; Qiu et al. 2001; Roberts 1990; Hu et al. 2016). There was a non-linear relationship between acid rain and plant photosynthesis (Dong et al. 2017).
Chl fluorescence released during plant photosynthesis is a rapid, non-destructive detection method to detect abiotic and biotic stress in plants (Pérez-Bueno et al. 2019; Melo et al. 2017; Stirbet et al. 2018). This type of measurement contains rich photosynthesis information and many studies have proved that it can be widely used to quickly monitor the state of leaf photosynthesis of plants under stress such as salt (Tsonev et al. 2011), nutrient elements (Santamaría et al. 2009), senescence (Kalaji et al. 2018), mutagenesis (Cordon et al. 2016), and pathogen infection (Boureima et al. 2012; Ivanov and Bernards 2016). Plant Chl fluorescence is strongly influenced by acid rain. In 2014, Hu et al. found that when soybean seedlings were treated with acid rain, the maximal photochemical efficiency (Fv/Fm) decreased, while the initial fluorescence (F0) increased. Similarly, Yu et al. (2002) also found that acid rain significantly reduced Fv/Fm of cucumber seedlings.
Barley (Hordeum vulgare L.) is the fourth most important global cereal crop behind maize, rice, and wheat, and is grown in many countries (Giraldo et al. 2019). Acid rain has a strong influence on the growth of barley plants. In 2018, Ding et al. (2018) showed that acid rain at pH 3.0 inhibited seed germination, increased foliar damage, decreased the Chl content, damaged roots, and delayed seedling’s growth in barley. Mersie and Foy (1986) found that Chl content of barley plants subjected to acidic rain at pH 2.5 was consistently lower than that at higher pH levels.
However, limited information is available on the influence of acid rain on barley photosynthesis. The causes and the mechanisms behind the effect of the formation of acid rain remain unclear. This study aimed to investigate the changes in photosynthesis, Chl fluorescence, and the mechanisms involved in these changes. It was hypothesized that (i) photosynthetic functions would be depressed by acid rain, (ii) the reduced photosynthesis might be related to non-stomatal factors, and (iii) some Chl fluorescence parameters might be used to detect and monitor acid rain stressed plants. To test these hypotheses, the photosynthetic rate, Chl fluorescence, and pigment content in barley cultivars stressed by SAR were assessed.
Materials and methods
Glasshouse experiments were performed at the Zhejiang Academy of Agricultural Sciences (ZAAS), Zhejiang province, P.R. China. Two barley cultivar Zhepi 33 and Kunlun 12 were seeded in 10 L polyvinyl chloride (PVC) pots on November 27, 2018. Three plants were thinned each pot at the three-leaf stage. The cultivated soil physicochemical properties were as follows: the N content was 0.821 g/kg, the pH (soil: water = 1:5) was 6.50; the organic matter content was 12.23 g/kg; the available phosphorus and potassium contents were 24.28 g/kg and 51.41 g/kg, respectively. During the experiment in the glasshouse, the mean temperature was 25°C; the mean atmospheric relative humidity was 80%; and the mean light intensity was 620 μmol/m2/s.
Four acid rain simulated treatments with pH 6.5, pH 5.5, pH 4.5, and pH 3.5 were designed. The trial was set up as a randomized block design with nine replications. Acid rain at the abovementioned pH levels was simulated and created by mixed solutions of HNO3 and H2SO4 (1:3) and distilled water. Before the acid rain treatments were implemented, the plants were fully irrigated with distilled water, and soils in the pots were covered by plastic sheets. Barley plants were sprayed with simulated acid rain (SAR) at 91 DAS (days after seeding). At 95 DAS, 99 DAS, and 103 DAS, three uppermost fully expanded barley leaves of each treatment were selected for gas exchange, Chl fluorescence, and pigment measurements. Every measurement was repeated three times.
The net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) of the barley leaves were measured and recorded using a portable photosynthesis system (CIRAS-2, PP Systems, Amesbury, Mass, USA). Instantaneous Pn was measured at a photosynthetic photon flux density (PPFD) of 1000 μmol/m2/s. Pn to PPFD response curves was obtained using an LED light source coupled to a leaf chamber following the order of 2 000, 1 800, 1 500, 1 200, 1 000, 800, 500, 200, 100, 50, 20, and 0 μmol/m2/s. The photosynthetic chamber with a leaf area of 1.7 cm2 provided a leaf temperature of 25 °C, relative air humidity of 80%, and a CO2 concentration of 400 μmol/mol.
The Pn-PPFD response curves were fitted according to the exponential model of Iqbal et al. (1997);
The maximum photosynthetic rate (Pmax), apparent quantum yield (Φ), and dark respiration rate (Rd) were obtained. The light compensation point (LCP) was calculated when PPFD approached zero, and the light saturation point (LSP) was obtained as the PPFD value when photosynthesis reached Pmax.
Chl fluorescence transient (OJIP) was induced by a saturating red light and was measured using a Multi-Function Plant Efficiency Analyser (M-PEA, Hansatech, UK) after the leaves were adapted in the dark for about 30 min. The O, J, I, and P points in the curve represent the respective fluorescence intensities recorded at 20 μs, 2 ms, 30 ms, and the maximum. The biophysical parameters induced from the OJIP curves according to the references (Strasser et al. 2000; Strasser et al. 2004; Hassannejad et al. 2020; Tsimilli-Michael and Strasser 2008 ) used in our study were illustrated in Table 3.
Leaf samples were taken from the same leaves used in gas exchange and fluorescence measurements. The leaf disks were cleaned, ground, and extracted with 80% acetone. Absorption was measured at 663, 645, and 470 nm using an ultraviolet (UV)-visible spectrophotometer (Unico, UV-3802, China). Chl a, Chl b, and carotenoid (Car) contents were calculated according to reference (Arnon 1949). The unit of the photosynthesis pigment content was mg/g based on fresh mass.
Data analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, USA). The Pn-PPFD response was fitted by a nonlinear regression method. Differences in the parameters of Zhepi 33 and Kunlun 12 within various pH treatments were assessed with one-way ANOVA (analysis of variance), and multiple comparisons of means were tested by Fisher’s LSD (least significant difference) at the 0.05 level. All of the measurements were repeated three times, and the means and calculated standard errors were reported.
Results
Pigments
The effect of SAR on the pigments of Zhepi 33 and Kunlun 12 is shown in Table 1. It can be seen that Chl a and Chl (a+b) decreased with acid increasing acidity and there were significant differences between Chl a, Chl (a+b) in the pH 4.5 and pH 3.5 treatments and those at pH 6.5 across the two barley plants. Chl b, carotenoid (Car), and Chl a/b also decreased with increasing acidity; however, no significant differences among various the SAR treatments were found. With respect to Chl a in Zhepi 33, treatments with a pH than 4.5 differed significantly from that at pH 6.5. With respect to Chl a in Kunlun 12, treatments with a pH less than 3.5 differed significantly from that at pH 6.5. Similar results were obtained for Chl (a+b).
Leaf gas exchange
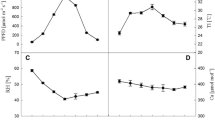
Figure 1 shows the changes in the net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) in Zhepi 33 and Kunlun 12 plants in response to SAR treatment. In both barley plants, Pn, Gs, and Tr significantly decreased with increasing acidity. No significant changes in Ci were found among the SAR treatments. Pn, Tr, and Gs measured in Zhepi 33 and Kunlun 12 at pH 4.5 and pH 3.5 showed significant differences from those at pH 6.5 (data not shown).
Effect of simulated acid rain (SAR) on net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular carbon dioxide concentration (Ci) in Zhepi 33 (a) and Kunlun 12 (b) plants. Each bar represents the mean ± SE (standard error) of three independent experiments data at 95 DAS (days after seeding), 99 DAS, and 103 DAS
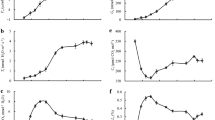
Pn to photosynthetic photon flux density (PPFD) curves of Zhepi 33 and Kunlun 12 under SAR stress are shown in Fig. 2. The photosynthetic parameters induced from the Pn to PPFD curves such as the light saturation point (LSP), light compensation point (LCP), apparent quantum yield (Φ), and dark respiration rate (Rd) are shown in Table 2. The maximum photosynthetic rate (Pmax), LSP decreased and Rd, LCP increased with increasing acidity. Pmax, Rd, LCP, and LSP at pH 4.5 and pH 3.5 showed significant differences from those at pH 6.5. Zhepi 33 had lower Pmax and LSP and higher Rd and LCP in comparison with Kunlun 12.
Chlorophyll fluorescence
Figure 3 shows that a difference in the Chl fluorescence transient measurement of Zhepi 33 and Kunlun 12 existed between SAR treatments. Based on the characteristic fluorescence transient curve shape (OJIP curve), there were differences between SAR stressed plants. On the whole, there was higher Chl fluorescence at pH 6.5 compared with the other treatments (pH 5.5, pH 4.5, and pH 3.5) across the two barley plants. Among the SAR treatments, small differences in the O step and large differences in the P step were observed. Except for the O step, the difference in the J, I, and P step in the OJIP curves increased with increasing acidity.
Effect of simulated acid rain (SAR) on OJIP transient curves of Zhepi 33 (a) and Kunlun 12 (b) plants. The O, J, I, and P points in the graph represent a fluorescence intensity recorded at 20 μs, 2 ms, and 30 ms and the maximum, respectively. Each value represents the mean of three independent experiments data at 95 DAS (days after seeding), 99 DAS, and 103 DAS
Parameters derived from the OJIP curves of Zhepi 33 and Kunlun 12 under SAR treatments are shown in spider plots (Fig. 4). It can be seen that Fv, Fv/F0, and Fm significantly decreased with increasing acidity in both barley plants. There were no significant differences in Fv/Fm among the SAR treatments. Figure 5 shows ABS/RC, TR0/RC, ET0/RC, DI0/RC, and RE0/RC calculated from the fluorescence transient curves. It can be seen that ABS/RC and DI0/RC gradually increased with increasing acidity in both barley plants, and the differences reached a significant level. Changes in the phenomenological energy fluxes per cross-section of Zhepi 33 and Kunlun 12 in the SAR treatments are shown in Fig. 6. ABS/CSm, RE0/CSm, and TR0/CSm gradually decreased with increasing acidity and the differences reached a significant level.
Spider plot of parameters induced from OJIP transient curves in SAR stressed Zhepi 33 (a) and Kunlun 12 (b) plants. Area, Sm, F0, Fm, Fv, Fv/Fm, Fv/F0, Vj, Vi, and dV/dt0 represent the area between the fluorescence curve and Fm, normalized area, initial fluorescence, fluorescence at 50 μs, maximal fluorescence, variable fluorescence (Fm–F0), maximum quantum efficiency of PSII photochemistry, active PSII reaction centers, relative variable fluorescence at time 2 ms, relative variable fluorescence at time 30 ms, and slope at the origin of the relative variable fluorescence curves, respectively. Each value represents the mean of three independent experiments data at 95 DAS (days after seeding), 99 DAS, and 103 DAS
Energy pipeline models of specific fluxes (membrane model) per reaction center (RC) in SAR stressed Zhepi 33 (a) and Kunlun 12 (b) plants. ABS/RC, TR0/RC, ET0/RC, and DI0/RC indicate absorbed, trapped, electron transport, and dissipated energy flux per active reaction center, respectively. RE0/RC represents the reduction of end acceptors at PSI electron acceptor side per reaction center. Each value in the graph represents the mean of three independent experiments data at 95 DAS (days after seeding), 99 DAS, and 103 DAS
Energy pipeline models of phenomenological fluxes (leaf model) per excited leaf cross-section in SAR stressed Zhepi 33 (a) and Kunlun 12 (b) plants. ABS/CSm, DI0/CSm, TR0/CSm, ET0/CSm, and RE0/CSm indicate absorbed, trapped, electron transport, and dissipated energy flux per excited leaf cross-section, respectively. RE0/CSm represents the reduction of end acceptors at PSI electron acceptor side per excited leaf cross-section. Each value in the graph represents the mean of three independent experiments data at 95 DAS (days after seeding), 99 DAS, and 103 DAS
Discussion
SAR treatments with low pH levels had a negative influence on barley photosynthesis. The reduction in photosynthesis depended on the strength of the acid. In the present study, Pn, Pmax, and LSP of the two barley plants decreased and LCP, and Rd increased at pH values less than 6.5. These results are similar to many previous observations in cucumber, yellow poplar, rice, and soybean (Sun et al. 2016; Yu et al. 2002; Qiu et al. 2001; Roberts 1990). SAR at pH 3.0 or lower reduced the plant photosynthetic rate in these studies. This may be related to the resistance of plants to acid rain and soil physical-chemical properties. Plant with strong ability to withstand acid rain may maintain a high photosynthetic rate. In addition, acid rain may improve soil nutrient availability and photosynthetic rate of plants growing in this soil environment may not decrease.
The decrease in barley photosynthesis caused by acid rain may be mainly due to non-stomatal factors. The results are in accordance with the findings for cucumber seedlings (Yu et al. 2002; Roberts 1990). In our study, Gs declined in parallel with Pn, but Ci barely changed when the two barley plants were exposed to acid rain stress. Previous studies showed that Pn and Gs decreased by 29% and 33%, respectively, when yellow poplar was treated with SAR (Roberts 1990). Thus, mainly non-stomatal factors may contribute to the decrease in photosynthesis caused by acid rain. In addition, plant pigments are very sensitive to air pollutants and are identified as an indicator of the physiological states of plants stressed by acid rain (Sensor et al. 1990). Chl a and Chl (a+b) in the two barley plants in our experiments also decreased with Pn when acidity increased. These results are similar to the reports of acid rain on Chl content in soybean, trees, restinga, and Mentha piperata plants (Hu et al. 2014; Liu et al. 2011; Neves et al. 2009; Kumari and Tomar 2009). The Chl a/b ratio and the content of total carotenoids tended to decrease in acid rain stressed barley plants, but these changes were not significant. Pavel Šiffel et al. (Šiffel et al. 1996) showed a similar result for acid rain stressed spruce seedlings. Considering the close relationship between Chl a and Pn, it suggested that a reduced Chl a content may be one reason for the decreased Pn in acid rain stressed barley plants.
Chl fluorescence as a tool can reveal the photosynthetic status and the involved mechanisms in plants under stress (Maxwell and Johnson 2000). OJIP transient measurements are key indicators for studying the response of plants to environmental stress (Baker 2018). In our experiments, the I and P steps of the OJIP curves in two barley plants gradually decreased with SAR decreased. Parameters induced from the above OJIP curves may be used for valuable application to monitor plant stress (Kalaji et al. 2016). In the present study, Fv/Fm, which characterizes the maximum quantum efficiency of PSII in dark-adapted leaves, decreased with no significant differences between SAR treatments. The results partly agree with the findings of Yu et al. (2002). Fv/Fm used as an acid rain stress indicator may need further evaluation. The reduced Fv/F0 under SAR stress in our experiment is in accordance with the study conducted by Wang et al. (2017), who showed SAR dramatically decreased Fv/F0 by 31.53%. A decrease in Fv, observed in our experiment, was also reported by Mena-Petite et al. (2000). In the present study, Fv/F0, Fm, and Fv had differed significantly between SAR treatments in both barley plants. These parameters may be used to evaluate plant stress caused by acid rain.
Energy pipeline models were used in our study to reveal the photosynthetic physiological changes when barley plants were subject to acid rain stress. They contain membrane and leaf models that can visualize the structure and function of the photosynthetic reaction centers (Hillier and Babcock 2001). In the membrane model, the energy fluxes affected by acid rain are shown by the width of the corresponding arrows (Fig. 5). DI0/RC increased under acid rain stress showing the increased quantity of dissipated energy. This energy reflects the excessive absorption of photons that could not be trapped by the RC that were released mainly in the form of heat (Castro et al. 2011; Strasser et al. 2000). Wang et al. (2019) also showed acid rain treatment reduced DI0/RC in tobacco leaves. ABS/RC and TR0/RC were the lowest at pH 6.5 and reached the maximum at pH 4.5 across the two barley plants. This suggested that acid rain can increase absorbed and trapped energy flux per reaction center of PSII in barley below pH 4.5.
In the present study, significantly decreased ABS/CSm, TR0/CSm, and RE0/CSm were observed at low pH level. This suggested that absorption, trapping in the photosynthetic reaction center, increased per excited leaf cross-section when the barley plants were exposed to acid rain stress. Lu et al. (2001) also reported that inactivation of some PSII reaction centers can lead to an increase in ABS/RC. These results were confirmed by our present experiments. As shown in Fig. 6, the number of inactive PSII reaction centers per cross-section indicated by closed circles increased with increasing acidity, indicating that acid rain inactivated the photosynthetic reaction center. The result is similar to that reported by Li et al. (2012), who showed that under acid rain stress, the curve of Chl fluorescence was quickly changed and the PSII reaction center was damaged.
Plant photosynthesis under stress may be affected by stress resistance which could help plants to minimize the negative impacts of the stress (Sharma et al. 2020; Lu et al. 2019). Liu et al. (2015) indicated that the effects of acid rain on the seedling photosynthesis of E. glabripetalus revealed resistance to acid deposition. In our experiment, Pn and Gs were lower in Zhepi 33 than in Kunlun 12 under acid rain stress. The Pn-PPFD response parameters Pmax, and LSP were lower and LCP, and Rd higher in Zhepi 33 than in Kunlun 12 (Table 2). Figure 3 also shows that the OJIP curves of Zhepi 33 had greater changes than those of Kunlun 12 under acid rain stress. These results indicated that Zhepi 33 photosynthesis was more sensitive to acid rain stress and was more easily negatively affected compared with Kunlun 12. Zhepi 33 had lower acid rain resistance in comparison with Kunlun 12. An increased area and decreased Sm were observed in SAR stressed Kunlun 12, while no such phenomena were observed in Zhepi 33. Reduced F0 and increased Vj, Vi, dV/dt0, and PIabs occurred in Zhepi 33 under SAR stress conditions, but these parameters exhibited no obvious changes in Kunlun 12. Changes in these parameters may be valuable for identifying plant resistance against SAR stress in application. Changes in Chl fluorescence energy flux induced by SAR stress were different between Zhepi 33 and Kunlun 12. ABS/RC and TR0/RC in Zhepi 33 had greater changes among SAR treatments than those in Kunlun 12 (Fig. 5). In addition, the number of closed circles for Zhepi 33 was higher than that for Kunlun 12 (Fig. 6). These suggested that the photosynthetic reaction center in Zhepi 33 was more seriously damaged than that in Kunlun 12.
There are some uncertainties in our study of the acid rain effect on plant photosynthesis. First, only barley seedlings were used in the existing acid rain exposure experiments. The response of other plants to acid rain may differ from the present result and this may lead to uncertainties in the application. Second, acid rain effect on plant photosynthesis caused by physiological changes involved in enzymatic and non-enzymatic antioxidant compounds was not studied in our experiments. In 2018b, Debnath et al. reported that the tomato seedlings in pH 3.5 SAR stress produced more reactive oxygen species (ROS) scavenging enzymatic and non-enzymatic antioxidant compounds than in pH 5.6. The study may reveal the physiological basement of SAR affected photosynthesis. In addition, the exposure frequency and time might affect the observed effects of acid rain and may produce uncertain results. For example, Velikova et al. (1999) reported that 3 h after SAR treatment, stomatal factors predominated in bean photosynthesis reduction, while during the next experimental period (5–24 h), mainly non-stomatal factors determined the decrease in photosynthetic rate. Therefore, experiments with more acid rain exposure frequencies and times on various plant species are needed to understand the effects of acid rain on plants.
Conclusions
Acid rain had a negative effect on barley photosynthesis. Chl a and Chl (a+b) decreased with acid increasing acidity and there were significant differences between Chl a, Chl (a+b) in the pH 4.5 and pH 3.5 treatments and those at pH 6.5. Decreased Pn, Pmax, and LSP and increased LCP and Rd with increasing acidity were found, which indicated that photosynthesis in barley plants may be depressed by SAR stress. The reduced Chl content and Gs in parallel with lower Pn suggested that non-stomatal factors may contribute to the decreased barley photosynthesis under acid rain stress. Zhepi 33 had lower Pmax and LSP and higher Rd and LCP in comparison with Kunlun 12. It suggested that acid rain may have greater effects on the photosynthesis of acid rain-sensitive plants compared with non-sensitive ones in our experiment. Chl fluorescence parameters Fv/F0, Fm, and Fv in both barley plants differed significantly between SAR treatments and may be used to evaluate acid rain stress affected plants. Energy pipeline models were used to reveal the photosynthetic damage caused by acid rain stress and the results showed that the photosynthetic reaction centers in barley were inactivated. These findings are valuable for the evaluation of plant sensitivity to acid rain stress and may be used for the detection and monitoring plants in acid rain stress conditions in the future.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Baker NR (2018) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Boureima S, Oukarroum A, Diouf M, Cisse N, Van Damme P (2012) Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ Exp Bot 81:37–43. https://doi.org/10.1016/j.envexpbot.2012.02.015
Castro FA, Campostrini E, Torres Netto A, Viana LH (2011) Relationship between photochemical efficiency (JIP-Test Parameters) and portable chlorophyll meter readings in papaya plants. Braz J Plant Physiol 23:295–304. https://doi.org/10.1590/S1677-04202011000400007
Cordon G, Lagorio MG, Paruelo JM (2016) Chlorophyll fluorescence, photochemical reflective index and normalized difference vegetative index during plant senescence. J Plant Physiol 99:100–110. https://doi.org/10.1016/j.jplph.2016.05.010
Debnath B, Ahammed GJ (2020) Effect of acid rain on plant growth and development: physiological and molecular interventions. In: Naeem M, Ansari A, Gill S (eds) Contaminants in Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-030-41552-5
Debnath B, Hussain M, Irshad M, Mitra S, Li M, Liu S, Qiu D (2018) Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 23:388. https://doi.org/10.3390/molecules23020388
Debnath B, Irshad M, Mitra S, Li M, Rizwan HM, Liu S, Pan T, Qiu D (2018) Acid rain deposition modulates photosynthesis, enzymatic and non-enzymatic antioxidant activities in tomato. Int J Environ Res 12:203–214. https://doi.org/10.1007/s41742-018-0084-0
Debnath B, Li M, Liu S, Pan T, Ma C, Qiu D (2020) Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol Environ Saf 200:110720. https://doi.org/10.1016/j.ecoenv.2020.110720
Ding F, Wang R, Wang TF (2018) Enhancement of germination, seedling growth, and oxidative metabolism of barley under simulated acid rain stress by exogenous trehalose. Crop Sci 58:783–791. https://doi.org/10.2135/cropsci2017.08.0491
Dong D, Du E, Sun Z, Zeng X, de Vries W (2017) Non-linear direct effects of acid rain on leaf photosynthetic rate of terrestrial plants. Environ Pollut 231:1442–1445. https://doi.org/10.1016/j.envpol.2017.09.005
Giraldo P, Benavente E, Manzano-Agugliaro F, Gimenez E (2019) Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 9:352. https://doi.org/10.3390/agronomy9070352
Hassannejad S, Lotfi R, Ghafarbi SP, Oukarroum A, Abbasi A, Kalaji HM, Rastogi A (2020) Early identification of herbicide modes of action by the use of chlorophyll fluorescence measurements. Plants 9:529. https://doi.org/10.3390/plants9040529
Hillier W, Babcock GT (2001) Photosynthetic reaction centers. Plant Physiol 125:33–37. https://doi.org/10.1104/pp.125.1.33
Hu HQ, Wang LH, Liao CY, Fan CX, Zhou Q, Huang XH (2014) Combined effects of lead and acid rain on photosynthesis in soybean seedlings. Biol Trace Elem Res 161:136–142. https://doi.org/10.1007/s12011-014-0088-3
Hu HQ, Wang LH, Zhou Q, Huang XH (2016) Combined effects of simulated acid rain and lanthanum chloride on chloroplast structure and functional elements in rice. Environ Sci Pollut Res 23:8902–8916. https://doi.org/10.1007/s11356-015-5962-9
Iqbal RM, Rao AR, Rasul E, Wahid A (1997) Mathematical models and response functions in photosynthesis: an exponential model. In: Pessarakli M (ed) Handbook of Photosynthesis. Marcel Dekker Inc., New York
Ivanov DA, Bernards MA (2016) Chlorophyll fluorescence imaging as a tool to monitor the progress of a root pathogen in a perennial plant. Planta 243:263–279. https://doi.org/10.1007/s00425-015-2427-9
Kalaji HM, Bąba W, Gediga K, Goltsev V, Samborska IA, Cetner MD, Dimitrova S, Piszcz U, Bielecki K, Karmowska K, Dankov K, Kompała-Bąba A (2018) Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res 136:329–343. https://doi.org/10.1007/s11120-017-0467-7
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:1–11. https://doi.org/10.1007/s11738-016-2113-y
Kumari P, Tomar YS (2009) Effect of simulated acid rain on chlorophyll and ascorbic acid contents of Mentha piperita L. (Peppermint). Agric Sci Dig 29:1–6
Li YY, Pan TF, Yu D, Qiu DL (2012) Effects of simulated acid rain stress on the PSII reaction center and free radical metabolism in leaves of longan. Acta Ecol Sin 32:7866–7873. https://doi.org/10.5846/stxb201203080315
Liu TW, Wu FH, Wang WH, Chen J, Li ZJ, Dong XJ, Patton J, Pei ZM, Zheng HL (2011) Effects of calcium on seed germination, seedling growth and photosynthesis of six forest tree species under simulated acid rain. Tree Physiol 31:402–413. https://doi.org/10.1093/treephys/tpr019
Liu MH, Yi LT, Yu SQ, Yu F, Yin XM (2015) Chlorophyll fluorescence characteristics and the growth response of Elaeocarpus glabripetalus to simulated acid rain. Photosynthetica 53:23–28. https://doi.org/10.1007/s11099-015-0071-z
Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29:315–330. https://doi.org/10.1111/j.1365-3040.2005.01493.x
Lu T, Yu HJ, Li Q, Chai L, Jiang WJ (2019) Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum L.) seedlings. Front. Plant Sci 10:490. https://doi.org/10.3389/fpls.2019.00490
Lu CM, Zhang JH, Zhang QD, Li LB, Kuang TY (2001) Modification of photosystem II photochemistry in nitrogen deficient maize and wheat plants. J Plant Physiol 158:1423–1430. https://doi.org/10.1078/0176-1617-00501
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jxb/51.345.659
Melo HF, Souza ER, Cunha JC (2017) Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev Bras de Eng Agricola e Ambient 21:232–237. https://doi.org/10.1590/1807-1929/agriambi.v21n4p232-237
Mena-Petite A, Begoña GM, Gonzalez-Murua C, Lacuesta M, Muñoz-Rueda A (2000) Sequential effects of acidic precipitation and drought on photosynthesis and chlorophyll fluorescence parameters of Pinus radiata D Don seedlings. J Plant Physiol 156:84–92. https://doi.org/10.1016/S0176-1617(00)80276-X
Mersie W, Foy CL (1986) Effects of acidity of simulated rain and its influence on the phytotoxicity of chlorsulfuron on velvetleaf and barley. Environ Exp Bot 26:341–347. https://doi.org/10.1016/0098-8472(86)90021-3
Neves NR, Oliva MA, da Cruz CD, Costa AC, Ribas RF, Pereira EG (2009) Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ 407:3740–3745. https://doi.org/10.1016/j.scitotenv.2009.02.035
Pérez-Bueno ML, Pineda M, Barón M (2019) Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front Plant Sci 10:1135. https://doi.org/10.3389/fpls.2019.01135
Qiu DL, Liu XH, Guo SZ (2001) Effects of simulated acid rain on longan photosynthesis. Acta Hortic 558:301–304. https://doi.org/10.17660/ActaHortic.2001.558.49
Roberts BR (1990) Physiological response of yellow-poplar seedlings to simulated acid rain, ozone fumigation, and drought. Forest Ecol Manag 31:215–224. https://doi.org/10.1016/0378-1127(90)90069-N
Santamaría JM, Hernández-Portilla D, Chi-Manzanero B, Espadas F, Castaño E, Iturriaga G, Rodríguez-Zapata LC (2009) Incorporation of two trehalose biosynthetic genes in banana increases trehalose levels and protects the photosynthetic apparatus from salt-stress damage. J Hortic Sci Biotechnol 84:665–671. https://doi.org/10.1080/14620316.2009.11512583
Sensor M, Kloos M, Lütz C (1990) Influence of soil substrate and ozone plus acid mist on the pigment content and composition of needles from young Norway spruce trees. Environ Pollut 64:295–314. https://doi.org/10.1016/0269-7491(90)90052-E
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu GPS, Bali AS, Handa N, Kapoor D, Yadav P, Khanna K, Bakshi P, Rehman A, Kohli SK, Khan EA, Parihar RD, Yuan HW, Thukral AK, Bhardwaj R, Zheng BS (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39:509–531. https://doi.org/10.1007/s00344-019-10018-x
Šiffel P, Braunová Z, Šindelková E, Cudlin P (1996) The effect of simulated acid rain on chlorophyll fluorescence spectra of spruce seedlings (Picea abies L. Karst.). J Plant Physiol 148:271–275. https://doi.org/10.1016/S0176-1617(96)80253-7
Singh B, Agrawal M (2004) Impact of simulated acid rain on growth and yield of two cultivars of wheat. Water Air Soil Pollut 152:71–80. https://doi.org/10.1023/B:WATE.0000015331.02874.df
Stirbet A, Lazár D, Kromdijk J, Govindjee (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 56:86–104. https://doi.org/10.1007/s11099-018-0770-3
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor and Francis, London
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. Advances in photosynthesis and respiration. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-3218-9_12
Sun JW, Hu HQ, Li YL, Wang LH, Zhou Q, Huang XH (2016) Effects and mechanism of acid rain on plant chloroplast ATP synthase. Environ Sci Pollut Res Int 23:18296–18306. https://doi.org/10.1007/s11356-016-7016-3
Tsimilli-Michael M, Strasser R (2008) In vivo assessment of stress Impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A (ed) Mycorrhiza. Springer, Berlin. https://doi.org/10.1007/978-3-540-78826-3_32
Tsonev T, Velikova V, Yildiz-Aktas L, Gürel A, Edreva A (2011) Effect of water deficit and potassium fertilization on photosynthetic activity in cotton plants. Plant Biosyst 145:841–847. https://doi.org/10.1080/11263504.2011.560199
Velikova V, Tsonev T, Yordanov I (1999) Light and CO2 responses of photosynthesis and chlorophyll fluorescence characteristics in bean plants after simulated acid rain. Physiol Plant 107:77–83. https://doi.org/10.1034/j.1399-3054.1999.100111.x
Vicas SI, Grosu E, Laslo V (2009) The effects of simulated acid rain on growth and biochemistry process in grass (Lolium perenne). Lucrări Ştiinţifice 52:277–283
Wang T, Yang WH, Xie YF, Shi DW, Ma YL, Sun X (2017) Effects of exogenous nitric oxide on the photosynthetic characteristics of bamboo (Indocalamus barbatus McClure) seedlings under acid rain stress. Plant Growth Regul 82:69–78. https://doi.org/10.1007/s10725-016-0239-y
Wang YJ, Zhang XL, Hu YB, Teng ZY, Zhang SB, Chi Q, Sun GY (2019) Phenotypic response of tobacco leaves to simulated acid rain and its impact on photosynthesis. Int J Agric Biol 21:391–398. https://doi.org/10.17957/IJAB/15.0000
Yu HL, He NP, Wang QF, Zhu JX, Gao Y, Zhang YH, Jia YL, Yu GR (2017) Development of atmospheric acid deposition in China from the 1990s to the 2010s. Environ Pollut 231:182–190. https://doi.org/10.1016/j.envpol.2017.08.014
Yu JQ, Ye S, Huang L (2002) Effects of simulated acid precipitation on photosynthesis, chlorophyll fluorescence, and antioxidative enzymes in Cucumis sativus L. Photosynthetica 40:331–335. https://doi.org/10.1023/A:1022658504882
Zhu XG, Song QF, Ort DR (2012) Elements of a dynamic systems model of canopy photosynthesis. Curr Opin Plant Biol 15:237–244. https://doi.org/10.1016/j.pbi.2012.01.010
Acknowledgements
The authors would like to thank the editor and two anonymous reviewers for their helpful comments on an earlier draft of this paper.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2018YFD0200507.
Author information
Authors and Affiliations
Contributions
HH and AS conceived and planned the experiments. HH, WH, HZ, LS, WL, and GZ carried out the experiments. HH and AS analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, H., Hua, W., Shen, A. et al. Photosynthetic rate and chlorophyll fluorescence of barley exposed to simulated acid rain. Environ Sci Pollut Res 28, 42776–42786 (2021). https://doi.org/10.1007/s11356-021-13807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13807-8