Abstract
The biomass control potential of three metabolic uncouplers (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), and m-chlorophenol (m-CP)) was tested in biotrickling filters (BTFs) degrading toluene. The experiments employed two types of reactors: a traditional column design and a novel differential BTF (DBTF) reactor developed by De Vela and Gostomski (J Environ Eng 147:04020159, 2021). Uncouplers caused the toluene elimination capacity (EC) (~33 g/m3h for column reactors and ~600 g/m3h for DBTF) to decrease by 15–97% in a dose-dependent fashion. The EC completely recovered in the column reactor in 3 to 13 days, while only partial recovery happened in the DBTF. Short-term (1 to 3 days) true uncoupling was indicated by the 20–160% increase in %CO2 recovery, depending on concentration. FCCP and CCCP increased the pressure drop due to increased extracellular polymeric substances (EPS) production for protection against the uncouplers. The 4.0-mM m-CP weakened the biofilm in the BTF bed, as evidenced by the 130–500% increase in the total organic carbon in the liquid sump of the column and DBTF reactors. Moreover, a microbial shift led to the proliferation of genera that degrade uncouplers, further demonstrating that the uncouplers tested were not a sustainable biomass control strategy in BTFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two common biological air treatment technologies used to treat air emissions in industry are biofilters and biotrickling filters (BTFs). A biofilter employs humidification but normally lacks continuous liquid addition to the packed bed, and removal is driven by the natural biofilms present in the packing material (Devinny et al. 1999; Qian et al. 2018). On the other hand, a BTF typically recirculates an aqueous nutrient solution that stimulates microbial growth on a natural or synthetic packing material (Kim and Deshusses 2008; Martinez-Soria et al. 2009; Zhang et al. 2009). Recirculating the aqueous phase increases the complexity over the conventional biofilter. The aqueous phase in a BTF assists in the mass transfer of pollutants (Moussavi and Mohseni 2008; Cheng et al. 2016), as well as in controlling the thickness and water content of the biofilm (Ramirez et al. 2009), and enables suspended microorganisms to biodegrade volatile pollutants (Cox et al. 2000). Despite its relative complexity compared to biofilters, BTFs are generally more effective especially in handling acidic by-products of biodegradation (Diks et al. 1994) and are often more cost-effective due to their high VOC removal rates even at low empty bed residence time (EBRT) (Lebrero et al. 2014). These advantages result in BTFs being smaller than biofilters when treating the same gas stream.
The major challenge in the operation of a BTF is the excessive biomass accumulation which can be controlled using a variety of approaches (De Vela and Gostomski 2018; Dobslaw et al. 2018). These approaches are generally classified as physical, mechanical, biological, and chemical methods of biomass control and include backflushing, changes in flow pattern, manual cleaning, use of agitation systems, rotating contactors, and use of chemicals, to name a few (Dobslaw et al. 2018). Among these methods, chemical methods, which make use of NaOH, NaOCl, and NaCl, have the advantage of being easily integrated into the system (Flores-Valle et al. 2011; Mendoza et al. 2004; Yu et al. 2006). In addition to these chemicals, metabolic uncouplers as biomass control agents have shown promising results in activated sludge systems (Fang et al. 2015; Li et al. 2016; Han et al. 2017; Tian et al. 2013), but have not yet been tested for biomass control in BTFs. Metabolic uncouplers are lipophilic weak acids, which have the ability to transport H+ ions through the H+-impermeable cell membrane, thereby weakening the proton motive force that drives the ATP synthesis (Russell and Cook 1995; Madigan et al. 2003; Terada 1990). Some examples include chlorophenols (i.e., m-chlorophenol, p-chlorophenol, dichlorophenol, trichlorophenol) (Yang et al. 2003; Fang et al. 2015; Tian et al. 2013; Han et al. 2017), nitrophenols (i.e., dinitrophenol, paranitrophenol) (Aragon et al. 2009; Low et al. 2000), and 3,3′,4′,5-tetrachlorosalicylanilide (TCS) (Chen et al. 2002). Apart from the true metabolic uncoupling effect lowering the growth yield, two additional mechanisms by which metabolic uncouplers control biomass production/and or accumulation have been proposed: (1) uncouplers weaken biofilm stability through changes in EPS production and quorum signaling molecules; and (2) uncoupler toxicity causes an ecological shift to a biofilm with reduced biomass yield (Gostomski and De Vela 2018). More work is required to better understand these uncoupling mechanisms that lead to biomass control.
This work is the first attempt to use uncouplers in BTFs. Two of the well-known uncoupling mechanisms were tested: true metabolic uncoupling and weakening of biofilm stability. A preliminary test to determine if a microbial shift due to the uncoupler occurred was also conducted. The three metabolic uncouplers tested were carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), and m-chlorophenol (m-CP). CCCP and FCCP were chosen as they are classical uncouplers and previously demonstrated activity at concentrations as low as 0.1 μM (To et al. 2010), while m-CP demonstrated the highest biomass control potential among phenol-based uncouplers such as p-chlorophenol, m-nitrophenol, and o-nitrophenol (Yang et al. 2003). Nitrogen-containing uncouplers such as the nitrophenols were not tested due to the abundance of nitrophenol-mineralizing bacteria in the soil (Arora et al. 2014).
Materials and methods
The BTF reactors
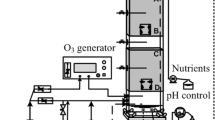
Three column BTF reactors consisting of an acrylic tube 60 mm in diameter, 5-mm thickness, and 400 mm in length were packed with 5-mm glass beads to give an effective bed volume of 0.45 L. The column BTF reactors employed a co-current downflow of trickling liquid and toluene-contaminated air. Nutrient medium (Online Resource 3) was added at a rate of 24 ± 1 mL/min through a four-port nozzle (Online Resource 4). Medium was recirculated through the system using a peristaltic pump employing a Masterflex 7018-20 pump head and L/S 18 Norprene tubing. The differential BTF (DBTF) reactor system (De Vela and Gostomski 2021) was used to further test the effect of m-CP. The operating conditions for the m-CP test in DBTF and the FCCP, CCCP, and m-CP tests in the column BTF are summarized in Table 1. In summary, three column BTFs were used: one for FCCP, one for CCCP, and another one for m-CP test. The fourth reactor was the DBTF reactor.
Overall description of the experimental setup
Compressed air (838 ± 11 mL/min) was metered by a mass flow controller and passed through the diffusion system (Detchanamurthy and Gostomski 2013) in a water bath controlled at 50 °C, thereby generating an inlet concentration of 140 ± 10 ppm toluene. A portion of the inlet or outlet gas flowed through the Vaisala CARBOCAP Carbon Dioxide Probe GMP 343 (Vaisala, Finland) for CO2 measurement (“Gas phase analysis” section) and then to the SRI Gas Chromatograph (GC) 8610C with a FID detector (SRI Instruments, USA) for toluene gas analysis (“Gas phase analysis” section) (Online Resource 1). The DBTF system is described in the work of De Vela and Gostomski (2021) and is illustrated in Online Resource 2. A backpressure regulator was integrated in both systems to regulate upstream pressure so that a reasonable amount of gas would flow to the GC when that location was being measured, while the majority of the gas was still exiting the main exhaust port but avoiding a complicated valve system. This was accomplished by allowing gas to bubble out at a certain level in a water column. The depth at which the tube was immersed in a water column influenced the backpressure.
Inoculation, startup, and long-term operation
A seed reactor was inoculated with 20 mL of garden soil filtrate in 200 mL of nutrient medium. The reactors were initially fed with 50 ppm toluene (0.20 g/m3), before eventually increasing to the desired operating inlet concentration (0.5–1 g/m3). Steady-state elimination capacity (EC) was assumed at ± 10% variability in EC for at least 5 days. Approximately 20 mL of deionized water was added daily to the liquid sump to compensate for evaporative losses.
Application of metabolic uncouplers
Varying amounts of FCCP (5–200 μM), CCCP (50–800 μM), and m-CP (0.4–4.0 mM) were added in the reactors. FCCP addition involved removing the previous concentration of FCCP by passing fresh nutrient medium through the reactor for 15 min three times and eventually changing the liquid in the sump with fresh nutrient medium containing the desired FCCP concentration. Increasing CCCP and m-CP concentrations in the reactor was achieved by adding a predetermined amount of concentrated uncoupler solution to the liquid sump to achieve the desired working concentration. Uncoupler was added when the EC was stable (± 10%) to ensure that the changes were due to the uncoupler. A control BTF (no uncoupler) was run for 102 days and served as the seed reactor.
Analytical procedures
Gas phase analysis
Inlet and outlet gas streams from the reactor systems were sampled via a 22-port stream selector valve and then to a 10-port valve for sample injection and finally to the GC/FID (SRI-8610C, SRI Instruments). The gas sample (1 mL) was injected on to a 60-m MXT-1 capillary column (Restek Corporation) operated at 200 °C with a flame ionization detector (FID). Helium (15 psi) was used as a carrier gas, while hydrogen (20 psi) and air (5 psi) were used for the FID. Both the inlet and outlet lines of the reactor systems were measured in triplicate at least once a day, and data were processed using the Peak Simple software.
The CO2 content of both inlet and outlet gas lines were measured using a Vaisala CARBOCAP Carbon Dioxide Probe GMP 343 (Vaisala, Finland) and were logged through the MI170 Link software into the computer. The CO2 content of the inlet line was subtracted from the outlet CO2 and combined with toluene degradation to determine the %CO2 recovery in each run of gas analysis. This was defined as the molar flow rate of CO2 produced divided by the molar flow rate of CO2 if all the degraded toluene was converted to CO2 and H2O.
Pressure drop and total organic carbon (TOC) measurement
The change in pressure drop across the bed inferred the rate of biomass accumulation and was measured by establishing two pressure ports, (1) upstream and (2) downstream of the bed, and connecting them to a Zephyr II+ digital micromanometer (Graywolf Sensing Solutions, USA). Daily pressure measurements were obtained by taking a pressure reading every 10 min over a 60-min period and then averaged. Moreover, another means to estimate the biomass accumulation in the bed was through measurement of TOC. Since sample collection from the bed required disassembly and disturbance to the system, only the TOC of the liquid sump was measured. Nevertheless, the TOC of the liquid sump was indicative of the amount of biomass released from the bed and monitored by obtaining ~15 mL of liquid from the sump and measuring its TOC using a TOC-L analyzer (Shimadzu, USA) liquid module and Shimadzu SSM 5500 solid module for low and high solid-liquid samples, respectively. The TOC of the uncoupler solution was estimated and was deducted from actual TOC readings.
EPS quantification
EPS contained in the liquid sump were extracted using a heat extraction method (Zhang et al. 2013). The schedule of liquid sump sampling for EPS collection was not the same across all the reactors as the reactors were not run simultaneously. EPS sampling was synchronized with uncoupler addition. Protein (EPSPN) and polysaccharides (EPSPS), which were the main components of the EPS (Stewart et al. 2013), were quantified using the Bradford method (Bradford 1976) and the phenol-sulfuric acid method (Nielsen 2010), respectively. For the Bradford method of protein quantification, a Protein Quantification Kit-Rapid based on Coomassie Brilliant Blue (CBB) G was used. Approximately 1.5 mL of CBB solution was added to a 1.5 mL supernatant sample containing the EPS. The absorbance of the solution was measured at 600 nm using an Ultrospec 2100 pro UV/Visible spectrophotometer (Biochrom, UK), and the protein concentration was determined by comparison with the calibration curve (Online Resource 5).
Phenol-sulfuric acid method of polysaccharide quantification with glucose as the standard (Dubois et al. 1956) was employed by mixing 0.05 mL of 5% phenol, 5 mL concentrated (99%) sulfuric acid, and 2 mL of supernatant. The mixture was stirred for 10 s and was left to stand for 1 h at room temperature. Absorbance at 490 nm was determined using an Ultrospec 2100 pro UV/Visible spectrophotometer and was compared with the calibration curve (Online Resource 6).
Microscopic analysis
A Leica TCS SP5 confocal laser scanning microscope (CLSM) (Leica Microsystems, Germany) was used for observing live cells and dead cells from liquid sump samples. A Molecular Probes L7012 Live/Dead BacLight Bacterial Viability Kit was used to distinguish live (green) and dead cells (red) in the liquid sump before and after the addition of uncouplers. Excitation was at 488 and 561 nm, with emission windows from 500 to 550 and 600 to 700 nm. To confirm the presence of red and green fluorescence in the images captured via the CLSM, the images were processed using the Image Processing Toolbox of MATLAB (version 2017b).
Residual uncoupler quantification
The m-CP was quantified by injecting a 50-μL sample through a high-performance liquid chromatograph (HPLC) (Thermo Scientific, USA) employing a 150 × 3 mm C18 column (Restek Cat #917836E) maintained at 30 °C. The mobile phase gradient flow (0.5 mL/min) consisted of 75% of 0.1% H3PO4 and 25% of 99% acetonitrile, with a total run time of 50 min. A peak corresponding to m-CP was observed using a UV-VIS detector at 210 nm which was quantified using the Chromeleon 7.2 SR5 software. CCCP quantification employed the same HPLC column and temperature with a carrier comprising 50% 0.1% H3PO4 and 50% 99% acetonitrile but at a flow rate of 0.5 mL/min for 50 min. The detection was with a UV-VIS detector at 250 nm. Calibration curves for m-CP and CCCP were established (Online Resources 7–8).
Microbial community analysis
The microbial community was analyzed for changes in the microbial community due to the addition of the uncouplers. Bacterial samples from the liquid sump of the FCCP-treated BTF were collected before and during addition of varying amounts of FCCP. Biofilm samples were also obtained from the top, middle, and bottom portions of the BTF bed for comparison.
DNA from these samples were extracted using the DNeasy PowerBiofilm Kit (MO BIO Laboratories) and were sent to Auckland Genomics (Auckland, New Zealand) for sequencing. Libraries were prepared for sequencing according to Illumina protocols. Primers targeting the V3–V4 region of the 16S rRNA gene of each sample were used (515f (GTGYCAGCMGCCGCGGTAA) and 806rB (GGACTACNVGGGTWTCTAAT)) with overhang adapter sequences added (Lear et al. 2018). The uniquely indexed 16S amplicon libraries were sequenced (paired-end 250 bp sequencing) on the Illumina MiSeq 2500 platform. To analyze the sequencing results, adapters were trimmed from sequences using Cutadapt v1.16 (Martin 2011), and sequences were QCed using FastQC and MultiQC v1.7 (Ewels et al. 2016). Sequences with less than 150 reads were discarded, and remaining sequences were processed with Dada2 (Callahan et al. 2016), and SILVA rRNA database v132 was used to assign taxonomic identity to the sequences. The relative abundance of genera for each of the samples was calculated in R v3.5.0 (R Core Team 2015). Genera that had ≤ 1% relative abundance were removed. Unassigned genera that did not match anything in the database were labeled “unassigned” and included in the analysis. Shannon (H) and Simpson (D) diversity indices were also calculated in R v3.5.0.
Results and discussion
Effect of FCCP
Experiments showed that FCCP does not have a potential to cause sustained biomass reduction but instead caused a further increase in pressure drop due to the adaptive response of the microbes to produce more EPS as protection. The results revealed that although FCCP did not cause a significant drop in EC (25–30 g/m3h; Fig. 1), it did not reduce biomass accumulation as estimated from the continued pressure drop increase over the course of the experiment (0.8 kPa to 11 kPa; Fig. 2).
Effect of varying concentrations of FCCP on the performance of the BTF column reactor operating at 0.5 ± 0.05 g/m3 inlet toluene concentration. (Orange diamond) EC; (green triangle) % RE; (black square) %CO2 recovery; (1) 5 μM; (2) 10 μM; (3) 40 μM; (4) 80 μM; (5) 200 μM; (red line) addition of FCCP; (black line) removal of FCCP and replacement with fresh medium; (solid blue line) emptying of the bed for sampling, starvation, and GC troubleshooting operations; (broken blue line) end of starvation and fresh medium replacement
Effect of varying concentrations of FCCP on the pressure drop across the BTF reactor and TOC of the liquid sump. (Red diamond) TOC; (yellow triangle) pressure drop; (1) 5 μM; (2) 10 μM; (3) 40 μM; (4) 80 μM; (5) 200 μM; (red line) addition of FCCP; (black broken line) removal of the FCCP and replacement with fresh medium; (blue line) disruption due to sampling, starvation, and GC troubleshooting operations; (blue broken line) end of starvation and fresh medium replacement
The first FCCP concentration tested was 5 μM (Gould and Cramer 1977) and was 500 times higher than the rate tested by Detchanamurthy and Gostomski (2015) in a differential biofilter because the BTF in this study was expected to have a more robust bacterial community. The EC which was initially ~36 g/m3h and decreased to ~25 g/m3h in 24 h and did not recover with the application of 5 μM FCCP (Step 1). The pressure drop gradually decreased by 25% but eventually recovered even before FCCP was removed (Fig. 2). It should be noted that the high-pressure drop values shown in Fig. 2 are higher than that of a full-scale BTF due to the use of glass beads as packing material. As glass beads were chosen only as a research tool to study the effect of the uncouplers, the high-pressure drop was not a concern. The decrease in EC and pressure drop may have been due to death of some active biomass due to the initial addition of toxic FCCP. Dodd et al. (2007) postulated that sub-lethal stress caused by the uncoupling of growth from metabolism produced reactive oxygen species (ROS) which were more lethal than the stress itself. Excessive production of ROS was suspected to increase cell death in other reports (Chen et al. 2006; Wallach-Dayan et al. 2006; Simon et al. 2000), specifically in Enterococcus faecalis (Ong et al. 2017), Mycobacterium tuberculosis (Lamprecht et al. 2016), and Burkholderia cepacia complex bacteria (Van Acker et al. 2016). However, ROS production was not experimentally measured in this study.
After replacing the FCCP with fresh medium (day 48), the EC remained at ~25 g/m3h. The sudden drops in the TOC values (Fig. 2) (i.e., vertical lines) were due to the replacement of the liquid phase with a fresh medium each time FCCP was added or removed. The slope of the TOC line before (~20 mg/L-day, days 30–36) and after (~6 mg/L-day, days 37–47) adding 5 μM FCCP was 70% smaller indicating that less biomass was released with the uncoupler. This is potentially due to less biomass production with 5 μM FCCP, which is consistent with the slight decrease in EC and pressure drop. A decrease in biomass production from uncoupling was consistent with other studies (Aragon et al. 2009; Chen et al. 2002; Fang et al. 2015; Han et al. 2017; Li et al. 2016; Yang et al. 2003; Zheng et al. 2008). It should be noted that the TOC contribution of the FCCP solution alone was approximately 0.6 to 4.8% of the actual TOC readings (depending on the concentration) and hence was considered negligible.
With further increases in FCCP concentration caused short-term reductions in EC (up to 65% at 200 μM FCCP, day 186), but the EC eventually recovered correlated with the FCCP concentration (3–13 days). Higher concentrations of FCCP (10–200 μM) did not reduce the pressure drop but rather increased it significantly, potentially due to increased EPS production for protection against FCCP (Zhang et al. 2013; Rho et al. 2007). This is consistent with the rate of the TOC increase particularly with 10 μM FCCP addition. The contradicting effect of FCCP on the TOC increases at 5 μM and 10 μM concentrations suggested two potential effects of uncoupler: initially reduced biomass due to the death of cells in the bed followed by increased EPS production as the cells adapted to FCCP.
An increase in EPS production due to uncoupler addition is common in other uncoupler-treated systems and is a microbial response to toxicity (Ferrer-Polonio et al. 2017; Li et al. 2016). In the current work, there was no significant difference between the EPS content before and after adding 40 μM FCCP (Fig. 3a) which contradicted hypothesis (1) in the preceding paragraph. Therefore, in addition to hypothesis (1), the increased TOC on the last day of 40 μM FCCP treatment may be due to increased soluble microbial products (SMP) in the presence of FCCP. Barker and Stuckey (1999) surmised that SMP may increase in response to environmental stress and toxic substances. Increased SMP production was demonstrated in an activated sludge treated with TCS (Li et al. 2012).
With 40 and 80 μM FCCP addition, the %CO2 recovery briefly increased to > 100%. This suggested that FCCP caused uncoupling by increasing respiratory activities as observed in activated sludge systems treated with 2,4 DNP and TCS (Chen et al. 2007;Chen et al. 2004). However, if the uncoupling increased respiration in the current system, toluene degradation should have also increased but EC decreased. Moreover, the potential contribution of FCCP degradation to CO2 was estimated to be approximately 130,000 times smaller than that from toluene degradation and hence was too small to significantly increase the %CO2 recovery. Hence, excess CO2 may have been polyhydroxybutyrate degradation (PHB), which is a carbon and energy storage compound deposited inside microbial cells (Verlinden et al. 2007) which is released to the cell environment when microorganisms die and lyse (Jendrossek and Handrick 2002). With the death and lysis potentially caused by FCCP, these compounds may have been released and degraded by other microbes, resulting in more CO2 generation.
With continuous 80 μM FCCP treatment, the pressure drop increased by a 120% over a 40-day period. Similarly, the TOC continuously increased which was due to EPS production (Fig. 3a) but possibly SMP production (not tested). The sudden drop in TOC (approximately 25%) at day 122 was the dilution by fresh medium containing 80 μM FCCP. The long-term application of 80 μM FCCP increased the TOC of the liquid sump at a rate of ~14 mg/L day. Assuming the TOC increase in the liquid sump corresponded biomass increase in the bed, the slower TOC increase in FCCP-treated BTF compared with that of control BTF (40 mg/L day) (Online Resource 12) indicated a lower biomass production rate in the FCCP-treated BTF.
The addition of 200 μM FCCP (Step 5, day 187) reduced the EC by 65% in 24 h, while the %CO2 recovery peaked at 165% and exceeded a 100% for 2 additional days before returning to 80–90%. The pressure drop continued to rise, while the TOC initially dropped by 37% due to the dilution from fresh FCCP-containing medium. However, the TOC increase after adding 200 μM FCCP (~21 mg/L day) was approximately 4 times higher than the previous value of ~5 mg/L day, which indicated a potential increase in biomass production in the bed. The pressure drop increase may have also been due to EPS production which increased by 100% (Fig. 3a). At 200 μM FCCP, the EC recovery took ~13 days which exceeded the usual 3-day recovery period at lower FCCP concentrations.
In general, the EC of the BTF system decreased in a dose-dependent fashion potentially due to the death and lysis of microbes in the presence of FCCP which was most likely associated with increased ROS production (Dodd et al. 2007). Hence death of cells in a BTF treated with FCCP was possible. Moreover, the results of live/dead cell staining with CCCP-treated BTF (“Effect of CCCP” section) supported the hypothesis that FCCP caused bacterial cell death. Nevertheless, EC recovery after uncoupler addition was potentially due to the growth of new active biomass and/or production of EPS.
The varying effect of FCCP on the TOC of the liquid sump demonstrated two potential effects of FCCP: (1) decreased TOC due to reduced biomass (i.e., active cells, EPS, SMP) production in the bed and (2) increased TOC due to increased EPS production in the bed as protection against the uncoupler. Although not investigated in this work, increased TOC may also be due to increased SMP production in response to FCCP treatment. The surge in %CO2 recovery immediately after treatment with 40 μM FCCP and higher could be potentially due to the degradation of PHB from dead cells.
Effect of CCCP
Overall, the addition of CCCP caused the EC to drift down from 50 to 30 g/m3h. Concentrations ranging from 100 to 200 μM CCCP showed potential for biomass control as pressure drop was relatively stable. However, the pressure drop increased when CCCP exceeded 400 μM.
Hiraishi and Kawagishi (2002) demonstrated that CCCP concentrations as low as 10 μM caused uncoupling, stimulated oxygen uptake, and decreased biomass production dramatically in an activated sludge system. However, this level of CCCP concentration (5 to 10 μM) was not tested in this experiment since FCCP, an analogue of CCCP (Zhang et al. 2016), had little effect at the same concentration.
The first addition of CCCP (50 μM) had little effect on the EC (Fig. 4) and the TOC of the liquid sump (Fig. 5) but reduced EPS production (Fig. 3b) by ~35%. Increasing the concentration of CCCP to 100 μM gradually decreased the EC by ~20% over a period of 12 days, while the TOC and pressure drop did not change significantly. Similarly, 200 μM caused fluctuations but generally decreasing EC, TOC, and pressure drop, demonstrating the ineffectiveness of these concentrations to control biomass accumulation. It should be noted that the contribution to the TOC of the CCCP solution was only about 1–7% and was considered negligible.
Increasing the CCCP concentration to 400 μM (Step 4, day 113) reduced the EC ~20%, which recovered within 2 days. The TOC was unaffected, while the pressure drop increased from 1.3 to 1.7 kPa (~30% increase) over the 15-day period potentially due to EPS production. 800 μM CCCP (Step 5, day 127) decreased the EC to ~5 g/m3h in 2 days (~83% drop), before it recovered (4 days after application) and increased by 33% as compared to 400 μM. The TOC increased by 25% for the first 3 days (from 560 to 700 mg/L) coinciding with the decrease in EC. This TOC increase, despite the EPS decrease (Fig. 3b), may be due to the release of live and dead cells (Online Resources 9 and 14) from the bed due to CCCP addition as well as increased SMP production.
The ratio of dead cells to live cells after addition of 800 μM CCCP increased by 50% relative to its value before the addition, hence suggesting that CCCP induced cell death. The release of live cells may have been associated with the increased production of ROS (“Effect of FCCP” section) which could inhibit biofilm formation (Guo et al. 2019). The recirculation of live cells back to the bed and the production of more EPS may have contributed to EC recovery for both CCCP- and FCCP-treated systems. The increase in EPS production coincided with the increasing TOC values following its decrease 4 days after 800 μM CCCP treatment. Depending on the time and concentration of CCCP and FCCP, cells can develop protective mechanisms against uncoupler-induced death (Kane et al. 2018).
Like FCCP, CCCP is also a nitrile (National Center for Biotechnology Information, n.d.) that could potentially be degraded by a number of bacteria (Bhalla et al. 2012). No distinct peak for CCCP was measured in the liquid samples collected 1 day after 200 and 400 μM CCCP application, suggesting that it became negligible within 24 h. This explains why CCCP did not cause a significant release of biomass from the bed. Being analogues of each other, the FCCP may have also been degraded like CCCP, hence explaining its ineffectiveness as a biomass control agent.
In general, the EC dropped by 20–80% at 400–800 μM CCCP, and this reduction was due to the ability of CCCP to induce cell death. The bactericidal effect of CCCP against multidrug-resistant bacterial strains was shown by Sinha et al. (2017) who observed a 1000-fold reduction in the colony count for all bacterial strains in 24 h. At 400 μM and 800 μM CCCP, the %CO2 recovery briefly exceeded 100% based on the toluene degraded, similar to that observed in a BTF treated with at least 40 μM FCCP. The potential CO2 generation from CCCP degradation was estimated to be about 73,000 times lesser than that from toluene degradation, hence too small to contribute to increase in %CO2 recovery. Hence, this increase may be due to the degradation of PHB in the system.
The above findings revealed that CCCP may have exhibited a potential biomass control ability at concentrations 100–200 μM but caused a considerable EC reduction at 800 μM. Moreover, it was potentially degraded and may have caused increased EPS production in the bed, which increased the pressure drop and was therefore not a promising biomass control agent.
Effect of m-CP
The addition of m-CP showed potential for biomass control in the column BTF as demonstrated by the fairly stable pressure drop over the course of the experiment. The EC drop and recovery period was a function of the m-CP concentration.
Addition of m-CP at concentrations 0.4 mM to 0.8 mM caused ~10% reduction in EC with no significant change on TOC and pressure drop (Figs. 6 and 7). Increasing the m-CP concentration to 1.6 mM (Step 3, day 120) decreased the EC by about 30% (32 to 22 g/m3h) on the first 3 days but increased it to ~40 g/m3h thereafter, while the TOC did not change significantly. Although the pressure drop increased by 28% over a period of 15 days, it was not significantly higher than prior to the start of the experiment. It should be noted from Fig. 7 that the 7–28% contribution of the m-CP solution to the TOC readings was already deducted.
(Orange diamond) EC; (green triangle) % RE; (black square) %CO2 recovery; (1) 0.4 mM; (2) 0.8 mM; (3) 1.6 mM; (4) 4 mM
The effects were more pronounced with 4 mM (Step 4, day 133) where the EC dropped dramatically from 30 to 9 g/m3h (~70% reduction) in 3 days, but it fully recovered in 7 days. This may be due to increase in cell death as shown by the 125% increase in the % dead cells to % live cells ratio after the addition of 4.0-mM m-CP (Online Resource 15).
The recirculation of the liquid sump, which also contained live cells, may have contributed to EC recovery. The detachment of the live cells from the biofilm may be due to the reduction in the EPS production (Fig. 3C) as shown by the 10% decrease on the first day of m-CP application. It then increased by 150% over the next 10 days, indicating the possibility of EPS production as a defense mechanism against m-CP.
Although studies have not demonstrated the specific effect of m-CP on EPS production, this kind of defense mechanism was similarly observed for a sequencing batch reactor treated with 2,6-DCP (Zhang et al. 2013) which is also a phenol derivative similar to m-CP. Similarly, Tian et al. (2013) observed that increased EPS production over the final 20 days of a 90-day period of 2,6-DCP application prevented the uncoupler from further affecting the sludge.
The shedding of cells from the bed may have also caused the TOC to continuously increase by approximately 130% (from 400 to 1100 mg/L), over the first 10 days of the 4-mM m-CP treatment. The pressure drop in the m-CP-treated BTF did not increase over the entire run as compared to that of FCCP, CCCP, and control BTF columns where the pressure drop increased by 800%, 45%, and 132%, respectively, over the entire runs. Although this required confirmation through replication, this result suggested that m-CP could control pressure drop increases in a BTF.
The sudden increase in %CO2 recovery observed for both FCCP and CCCP also occurred in the m-CP-treated BTF at 0.4, 1.6, and 4 mM concentration. From a typical 50–60% CO2 recovery, it increased to 80% for 0.4 mM and 1.6 mM and up to 150% for 4-mM m-CP. Although m-CP decreased the EC of the system, a big portion of the degraded carbon may have been released as CO2 as it could not be used for growth due to limited ATP production (due to uncoupling). This resulted in increased CO2 recovery which then exceeded 100%, potentially due to PHB degradation. The potential degradation of m-CP could generate CO2 which was 11,200 times lesser than that from toluene degradation and hence was less likely to significantly contribute to increase in %CO2 recovery.
In general, addition of m-CP also decreased the EC in a dose-dependent fashion. The results of live and dead cell staining further strengthened the hypothesis that uncoupling caused shedding of live cells. The recovery period (3–7 days) for EC was a function of the m-CP concentration as higher concentrations caused a greater reduction in the EC (10–70%) and hence a longer recovery period. This EC recovery was attributed to the recirculation of live cells back to the bed and the production of more EPS by the microbes.
Effect of m-CP in DBTF
Understanding the effect of uncouplers on EC and pressure drop in the column reactor was challenging since recirculating liquid made it difficult to determine whether the TOC released from the bed was uniform between sample collections. Hence, a DBTF reactor, which did not recycle the liquid to the bed, was a better tool to see the changes in the EPS content and composition. To better understand the effect of the uncouplers, m-CP, which was the only uncoupler that demonstrated biomass reduction and pressure drop stability in a column reactor (“Effect of m-CP” section), was tested in a DBTF operating at 0.9 ± 0.1 g/m3 inlet concentration, 20 L/min gas recycle rate, 0.25 ± 0.2 mL/min liquid trickling rate, 840 mL/min inlet flow rate, and 580 ± 75 g/m3h loading rate.
The EC and %CO2 recovery (Fig. 8) as well as the recycle gas flow rate and TOC of the liquid sump (Fig. 9) at varying concentrations of m-CP were monitored. The recycle gas flow rate was indicative of biomass accumulation in the bed. Increased biomass increased the pressure drop, which decreased the recycle gas flow as the recycle fan flow rate was pressure sensitive. For the DBTF, the recycle gas flow rate did not impact the EC calculation, as it was only directly dependent on the gas flow that passed through the system (0.84 L/min) (De Vela and Gostomski 2021).
Using the DBTF clarified some of the hypotheses concerning m-CP usage in the column reactors. Both reactor types have shown a dose-dependent reduction in EC causing at least 70% reduction at 4-mM m-CP and with EC recovery occurring after 4–5 days in both the reactors. However, the EC of the DBTF reactor only recovered by 40% prior to 4-mM m-CP treatment, where the column reactor EC fully recovered. The poor EC recovery in the DBTF was potentially due to the absence of liquid recirculation returning live cells to the bed or continuous exposure of the microbes to m-CP from the aerosol feed reservoir. The m-CP degraded slowly in the reservoir (i.e., 40% reduction in 20 days) (“Bacterial degradation of m-CP” section) as compared to the rapid disappearance in the column reactor.
The biomass reduction potential of m-CP was better demonstrated in the DBTF as shown by the 500% increase in TOC within 3 days as compared to only 130% increase in the column reactor. This translated to a 33% increase in the gas recycle rate. The increased EPS production (Fig. 3d) with longer exposure to m-CP in both reactors validated the hypothesis that the microorganisms produced more EPS to protect themselves from the uncouplers. The very low %CO2 recovery (~5%) was anomalous and could be due to incomplete degradation of toluene induced by co-metabolism with m-CP (“Bacterial degradation of m-CP” section).
Although the above results demonstrated that m-CP at a concentration of at least 0.8-mM might control biomass accumulation in the bed, its potential inhibition of toluene metabolism may make it an unsustainable technique (“Bacterial degradation of m-CP” section). Moreover, a chlorophenol may contribute to the total AOX value (adsorbable organic halogens) of the liquid phase (Xu et al. 2021). In addition, bacterial degradation of chlorophenols may result in the formation of toxic intermediates like protoanemonin and chloromuconic acid (Arora and Bae 2014; Solyanikova and Golovleva 2004). These issues will complicate water disposal.
Bacterial degradation of m-CP
The decreased impact of the m-CP on the biofilter may have been due to bacterial degradation, as indicated by the 40% reduction in its concentration in the aerosol reservoir after 20 days of treatment and rapid removal in the column reactor. No significant m-CP was measured in the DBTF bed biomass, thereby eliminating m-CP absorption to the biomass as one of the reasons for its decrease.
The degradation of m-CP occurs either via the formation of 3-chlorocatechol or 4-chlorocatechol (Solyanikova and Golovleva 2004). Bacteria such as Pseudomonas putida F1, Ralstonia pickettii, and Rhodococcus opacus can degrade m-CP degraders (Arora and Bae 2014; Spain and Gibson 1988) and are toluene degraders with chlorocatechol as an intermediate (Daugulis and Boudreau 2003; Duetz et al. 2001; Parales et al. 2000; Spain and Gibson 1988). Specifically, 3-methylcatechol is among the intermediates of toluene degradation via the ortho-cleavage pathway (Dobslaw and Engesser 2015), while 3-chlorocatechol is an intermediate via the meta-cleavage pathway (Göbel et al. 2004).
The change in color of the liquid phase in the aerosol reservoir from transparent to black (Online Resource 11), which was also observed in the column reactor with m-CP, indicated the possible accumulation of chlorocatechol in the system (Haller and Finn 1979). This accumulation was caused by the inactivation of catechol 2,3-dioxygenase enzyme (Bartels et al. 1984). These reports concluded that toluene was a primary substrate allowing co-metabolism of m-CP.
Meanwhile, the %CO2 recovery decreased from 60 to 40% and < 5% after 0.8-mM and 4-mM m-CP addition, respectively (Fig. 8). This meant that much of the carbon from toluene degradation ended up either in the solid phase (bed) or in the liquid phase. A crude carbon balance over the whole period of 4-mM m-CP application (20-day period) indicated that ~77% of the carbon from toluene degradation ended up in the bed (Online Resource 16). This was different from the carbon balance prior to m-CP addition (days 68–72) (Online Resource 17) which revealed that ~88.6% of the degraded toluene exited as CO2, while only 2.7% ended up in the bed (De Vela and Gostomski 2021). In addition, the decrease in %CO2 recovery meant that toluene was not fully mineralized to CO2 and water. It could be that the high percentage of carbon in the bed was associated with partially metabolized toluene, hence limiting energy production and growth. The limited energy available due to incomplete degradation could have been used by the microbes in producing more EPS as indicated by the gradual increase in EPS starting day 111 (4 days after adding 4-mM m-CP) (Fig. 3d).
Bartels et al. (1984) demonstrated that the meta-cleavage pathway of chlorophenol degradation inhibits 2,3-dioxygenase enzyme. This enzyme is found in Pseudomonas putida F1 (Parales et al. 2000), Burkholderia (Pseudomonas) cepacia G4 (Shields et al. 1995), and Pseudomonas putida mt-2 (Lee et al. 1992) which are all known toluene degraders. Inactivation of this enzyme therefore indicates the partial degradation of toluene which meant less energy available for growth and maintenance functions. The hypothesis concerning inefficient use of energy where microorganisms tend to prioritize production of EPS over growth functions may be correct as observed in the gradual increase of EPS from day 14 to day 20 (days 121 to 127 in Fig. 9) after a sudden drop due to 4.0-mM m-CP addition. The microorganism utilized the available energy for EPS production rather than production of new cells.
Microbial community analysis in FCCP-treated BTF
To investigate the potential shift in the microbial population caused by the uncouplers, biomass samples were collected from the column reactor treated with FCCP. Due to time constraints, only the FCCP-treated column was examined.
The microbial samples were obtained from the liquid sump, not from the BTF bed itself. Hence, it was necessary to compare the microbial communities in the liquid sump and the BTF bed (Online Resource 18). Rhodococcus sp. was the dominant genera (40–60% relative abundance) in the BTF bed (i.e., top to bottom), followed by Achromobacter sp. (~20%) which are both notable toluene degraders (Woods et al. 2011; Kim et al. 2018; Hong et al. 2017; Liao et al. 2018). Achromobacter dominated the liquid sump. Other species of significance (4–12% relative abundance) in both the bed and the sump were Castellaniella sp., a facultative anaerobe associated with denitrification (Kämpfer et al. 2006), and Thermomonas sp. which was potentially a phenol degrader (Baek et al. 2003).
Based on the Shannon diversity index (H) which was primarily influenced by the microbial richness (Yeom and Kim 2011), only the top portion of the bed (i.e., the inlet side) significantly differed from the liquid sump (p = 0.04). However, based on the Simpson diversity index (D), the three portions of the bed (top, middle, and bottom) did not significantly differ from each other. However, the Simpson index in the liquid sump was statistically different from the bed positions. The liquid sump had lower microbial diversity (based on Shannon and Simpson indices) than the rest of the bed. This could indicate that not all species in the bed may have been released to the liquid sump. In these experiments, it was shown that the most abundant species in the bed are the ones most likely to be in the liquid sump. Hence, the analysis of the microbial samples in the liquid sump was appropriate for understanding the potential microbial shift in the bed caused by FCCP.
The temporal variation of the microbial community with increasing amounts of FCCP was examined in the liquid sump sample (Fig. 10). Significant differences in Shannon (H) (p = 0.01) and Simpson (D) (p = 0.01 to 0.03) indices before and after addition of FCCP were observed only after the addition of at least 80 μM FCCP. Specifically, the H decreased from 3.0 to 2.1 at 80 μM FCCP, while D decreased from 0.9 to 0.68 at 200 μM FCCP. Specifically, Rhodococcus sp. and Hydrocarboniphaga sp., both toluene degraders (Woods et al. 2011; Kim et al. 2018; Palleroni et al. 2004; Popp et al. 2006) dominated the community prior to the first addition of FCCP (5 μM). Rhodococcus sp. persisted with increasing amounts of FCCP, but Hydrocarboniphaga sp. vanished on addition of 5 μM FCCP. However, Achromobacter sp., a known toluene degrader (Hong et al. 2017; Liao et al. 2018), emerged and persisted even at 200 μM FCCP. The emergence of Achromobacter sp. may be due to its ability to degrade cyanide (Igeño et al. 2018), a major component of FCCP. Thermomonas sp, which can also degrade cyanide (Wang et al. 2015), also emerged in the presence of FCCP, while Castellaniella sp. which are facultative anaerobic degraders of hydrocarbons (Widdel et al. 2010) could persist in the inner depths of the biofilm even in the presence of FCCP. Other genera which vanished were Terrimonas sp. and Parapedobacter sp. Neither species are known to be cyanide degraders.
The change in the microbial community showed that FCCP addition shifted the population to one capable of adapting to its toxic effects. Although there are no reports on the specific biomass production rates of these different genera of toluene and/or FCCP degraders, the changed microbial community may have increased biomass production (i.e., EPS production) (Fig. 3a) to enable their survival against FCCP. Hence, the pressure drop in the bed continued to increase with constant exposure to FCCP (Fig. 2). However, this hypothesis on species shift and increased biomass yield requires further investigation.
Conclusion
FCCP, CCCP, and m-CP reduced the EC by 15–97% depending on the dose, potentially due to the death and lysis of microbes. EC recovered due to the recirculation of released live cells back to the bed and the potential bacterial degradation of the uncoupler. FCCP and CCCP resulted in 800% and 45% increase in pressure drop, respectively, due to the microbial response of producing more EPS for protection. This may have limited the ability of the uncouplers to weaken the biofilm as indicated by the temporary increase in the TOC of the sump liquid. Only m-CP at 4-mM concentration demonstrated potential to control biomass production, in both the column and DBTF systems. However, co-metabolism between m-CP and toluene may have resulted in their incomplete degradation. The temporal change in the microbial population with FCCP addition and the HPLC assays for CCCP and m-CP showed potential for incomplete bacterial degradation. Therefore, none of the uncouplers tested demonstrated potential for sustainable biomass control in a BTF.
Data availability
This manuscript contains Supplementary Information which may provide additional information about the study. Other datasets relative to this study are available from the corresponding author upon request.
References
Aragon C, Quiroga JM, Coello MD (2009) Comparison of four chemical uncouplers for excess sludge reduction. Environ Technol 30:707–714
Arora PK, Bae H (2014) Bacterial degradation of chlorophenols and their derivatives. Microb Cell Factories 13:17
Arora PK, Srivastava A, Singh VP (2014) Bacterial degradation of nitrophenols and their derivatives. J Hazard Mater 266:42–59
Baek SH, Kim KH, Yin CR, Jeon CO, Im WT, Kim KK, Lee ST (2003) Isolation and characterization of bacteria capable of degrading phenol and reducing nitrate under low-oxygen conditions. Curr Microbiol 47:462–466
Barker DJ, Stuckey DC (1999) A review of soluble microbial products (SMP) in wastewater treatment systems. Water Res 33:3063–3082
Bartels I, Knackmuss HJ, Reineke W (1984) Suicide inactivation of catechol 2,3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl Environ Microbiol 47:500–505
Bhalla T, Sharma N, Bhatia R (2012) Microbial degradation of cyanides and nitriles. In: Satyanarayana T, Johri BN, Prakash A (eds) Microorganisms in environmental management: microbes and environment, 1st edn, chap 25. Springer Science+Business Media B.V. https://doi.org/10.1007/978-94-007-2229-3
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Callahan BJ, Mcmurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Chen GH, Mo HK, Liu Y (2002) Utilization of a metabolic uncoupler, 3,3 ',4 ',5-tetrachlorosalicylanilide (TCS) to reduce sludge growth in activated sludge culture. Water Res 36:2077–2083
Chen YX, Ye FX, Feng XS (2004) The use of 3,3 ',4 ',5-tetrachlorosalicylanilide as a chemical uncoupler to reduce activated sludge yield. J Chem Technol Biotechnol 79:111–116
Chen YS, Lin FY, Lin CC, Tai CYD, Liu HS (2006) Packing characteristics for mass transfer in a rotating packed bed. Ind Eng Chem Res 45:6846–6853
Chen G-W, Yu H-Q, Xi P-G (2007) Influence of 2,4-dinitrophenol on the characteristics of activated sludge in batch reactors. Bioresour Technol 98:729–733
Cheng Y, He H, Yang C, Zeng G, Li X, Chen H, Yu G (2016) Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol Adv 34:1091–1102
Cox HHJ, Nguyen TT, Deshusses MA (2000) Toluene degradation in the recycle liquid of biotrickling filters for air pollution control. Appl Microbiol Biotechnol 54:133–137
Daugulis AJ, Boudreau NG (2003) Removal and destruction of high concentrations of gaseous toluene in a two-phase partitioning bioreactor by Alcaligenes xylosoxidans. Biotechnol Lett 25:1421–1424
De Vela RJL, Gostomski PA (2018) Minimising biomass accumulation in biotrickling filters. Rev Environ Sci Biotechnol 17:417–430
De Vela RJL, Gostomski PA (2021) Design and performance of a toluene-degrading differential biotrickling filter as an alternative research tool to column reactors. J Environ Eng 147:04020159
Detchanamurthy S, Gostomski PA (2013) Development of a modified differential biofiltration reactor with online sample and carbon dioxide monitoring system. Asia Pac J Chem Eng 8:414–424
Detchanamurthy S, Gostomski PA (2015) Studies on the influence of different metabolic uncouplers on the biodegradation of toluene in a differential biofilter reactor. Biotechnol Bioprocess Eng 20:915–923
Devinny JS, Deshusses MA, Webster TS (1999) Biofiltration for air pollution control. Lewis Publishers, Boca Raton
Diks RMM, Ottengraf SPP, Van Den Oever AHC (1994) The influence of NaCl on the degradation rate of dichloromethane by Hyphomicrobium sp. Biodegradation 5:13
Dobslaw D, Engesser K-H (2015) Degradation of toluene by ortho cleavage enzymes in Burkholderia fungorum FLU100. Microb Biotechnol 8:143–154
Dobslaw D, Woiski C, Winkler F, Engesser K-H, Dobslaw C (2018) Prevention of clogging in a polyurethane foam packed biotrickling filter treating emissions of 2-butoxyethanol. J Clean Prod 200:609–621
Dodd CER, Richards PJ, Aldsworth TG (2007) Suicide through stress: a bacterial response to sub-lethal injury in the food environment. Int J Food Microbiol 120:46–50
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Duetz WA, Fjallman AH, Ren S, Jourdat C, Witholt B (2001) Biotransformation of D-limonene to (+) trans-carveol by toluene-grown Rhodococcus opacus PWD4 cells. Appl Environ Microbiol 67:2829–2832
Ewels P, Magnusson M, Lundin S, Kaller M (2016) MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048
Fang F, Hu HL, Qin MM, Xue ZX, Cao JS, Hu ZR (2015) Effects of metabolic uncouplers on excess sludge reduction and microbial products of activated sludge. Bioresour Technol 185:1–6
Ferrer-Polonio E, Fernandez-Navarro J, Alonso-Molina JL, Amoros-Munoz I, Bes-Pia A, Mendoza-Roca JA (2017) Changes in the process performance, sludge production and microbial activity in an activated sludge reactor with addition of a metabolic uncoupler under different operating conditions. J Environ Manag 203:349–357
Flores-Valle SO, Rios-Berny O, Chanona-Perez J, Fregoso-Aguilar T, Morales-Gonzalez JA, Prado-Rubianes OJ, Herrera-Bucio R, Lopez-Albaran P, Morales-Gonzalez A, Garibay-Febles V, Dominguez EG, Kennes C, Veiga-Barbazan MC, Mendoza-Perez JA (2011) A comparative study of physical and chemical processes for removal of biomass in biofilters. Molecules 16:6927–6949
Göbel M, Kranz OH, Kaschabek SR, Schmidt E, Pieper DH, Reineke W (2004) Microorganisms degrading chlorobenzene via a meta-cleavage pathway harbor highly similar chlorocatechol 2,3-dioxygenase-encoding gene clusters. Arch Microbiol 182:147–156
Gostomski PA, De Vela RJ (2018) Metabolic uncouplers for controlling biomass accumulation in biological waste treatment systems. Rev Environ Sci Biotechnol 17:1–18
Gould JM, Cramer WA (1977) Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem 252:5491–5497
Guo L, Zhang C, Chen G, Wu M, Liu W, Ding C, Dong Q, Fan E, Liu Q (2019) Reactive oxygen species inhibit biofilm formation of Listeria monocytogenes. Microb Pathog 127:183–189
Haller HD, Finn RK (1979) Biodegradation of 3-chlorobenzoate and formation of black color in the presence and absence of benzoate. Eur J Appl Microbiol Biotechnol 8:191–205
Han YP, Luo M, Chen H, Zhang WZ, Liu JX, Xiao BY (2017) Deterioration mechanisms of sludge settleability in sludge reduction systems with metabolic uncouplers. Int Biodeterior Biodegradation 123:296–303
Hiraishi A, Kawagishi T (2002) Effects of chemical uncouplers on microbial biomass production, metabolic activity, and community structure in an activated sludge system. Microbes Environ 17:197–204
Hong Y-H, Ye C-C, Zhou Q-Z, Wu X-Y, Yuan J-P, Peng J, Deng H, Wang J-H (2017) Genome sequencing reveals the potential of Achromobacter sp. HZ01 for bioremediation. Front Microbiol 8:1507–1507
Igeño M, Macías D, Guijo M, Sánchez Clemente R, Poblacion A, Merchán F, Blasco R (2018) Bacterial consortiums able to use metal-cyanide complexes as a nitrogen source. Proceedings 2:1284
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Kämpfer P, Denger K, Cook AM, Lee ST, Jäckel U, Denner EBM, Busse HJ (2006) Castellaniella gen. nov., to accommodate the phylogenetic lineage of Alcaligenes defragrans, and proposal of Castellaniella defragrans gen. nov., comb. nov. and Castellaniella denitrificans sp. nov. Int J Syst Evol Microbiol 56:815–819
Kane MS, Paris A, Codron P, Cassereau J, Procaccio V, Lenaers G, Reynier P, Chevrollier A (2018) Current mechanistic insights into the CCCP-induced cell survival response. Biochem Pharmacol 148:100–110
Kim S, Deshusses MA (2008) Determination of mass transfer coefficients for packing materials used in biofilters and biotrickling filters for air pollution control. 1. Experimental results. Chem Eng Sci 63:841–855
Kim D, Choi KY, Yoo M, Zylstra GJ, Kim E (2018) Biotechnological potential of Rhodococcus biodegradative pathways. J Microbiol Biotechnol 28:1037–1051
Lamprecht DA, Finin PM, Rahman MA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJC (2016) Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7:14
Lear G, Dickie I, Banks J, Boyer S, Buckley H, Buckley T, Cruickshank R, Dopheide A, Handley K, Hermans S, Kamke J, Lee C, Macdiarmid R, Morales S, Orlovich D, Smissen R, Wood J, Holdaway R (2018) Methods for the extraction, storage, amplification and sequencing of DNA from environmental samples. N Z J Ecol 42(1):10
Lebrero R, Gondim AC, Perez R, Garcia-Encina PA, Munoz R (2014) Comparative assessment of a biofilter, a biotrickling filter and a hollow fiber membrane bioreactor for odor treatment in wastewater treatment plants. Water Res 49:339–350
Lee J, Min KR, Kim Y (1992) Cloning and overexpression of methylcatechol 2,3-dioxygenase gene from toluene-degrading Pseudomonas putida mt-2 (pWWO). Arch Pharm Res 15:360–364
Li Y, Li AM, Xu J, Liu B, Fu LC, Li WW, Yu HQ (2012) SMP production by activated sludge in the presence of a metabolic uncoupler, 3,3',4',5-tetrachlorosalicylanilide (TCS). Appl Microbiol Biotechnol 95:1313–1321
Li P, Li HC, Li J, Guo XS, Liu JX, Xiao BY (2016) Evaluation of sludge reduction of three metabolic uncouplers in laboratory-scale anaerobic-anoxic-oxic process. Bioresour Technol 221:31–36
Liao D, Li E, Li J, Zeng P, Feng R, Xu M, Sun G (2018) Removal of benzene, toluene, xylene and styrene by biotrickling filters and identification of their interactions. PLoS One 13:e0189927
Low EW, Chase HA, Milner MG, Curtis TP (2000) Uncoupling of metabolism to reduce biomass production in the activated sludge process. Water Res 34:3204–3212
Madigan MT, Martinko JM, Parker J (2003) Brock biology of microorganisms. Pearson Education,Inc, New York
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 17:3
Martinez-Soria V, Gabaldon C, Penya-Roja JM, Palauj J, Alvarez-Hornos FJ, Sempere F, Soriano C (2009) Performance of a pilot-scale biotrickling filter in controlling the volatile organic compound emissions in a furniture manufacturing facility. J Air Waste Manage Assoc 59:998–1006
Mendoza JA, Prado OJ, Veiga MC, Kennes C (2004) Hydrodynamic behaviour and comparison of technologies for the removal of excess biomass in gas-phase biofilters. Water Res 38:404–413
Moussavi G, Mohseni M (2008) The treatment of waste air containing phenol vapors in biotrickling filter. Chemosphere 72:1649–1654
Nielsen SS (2010) Phenol-sulfuric acid method for total carbohydrates. In: Nielsen SS (ed) Food Analysis Laboratory Manual. Boston, Springer US
Ong KS, Cheow YL, Lee SM (2017) The role of reactive oxygen species in the antimicrobial activity of pyochelin. J Adv Res 8:393–398
Palleroni NJ, Port AM, Chang HK, Zylstra GJ (2004) Hydrocarboniphaga effusa gen. nov., sp. nov., a novel member of the gamma-Proteobacteria active in alkane and aromatic hydrocarbon degradation. Int J Syst Evol Microbiol 54:1203–1207
Parales RE, Ditty JL, Harwood CS (2000) Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl Environ Microbiol 66:4098–4104
Popp N, Schlömann M, Mau M (2006) Bacterial diversity in the active stage of a bioremediation system for mineral oil hydrocarbon-contaminated soils. Microbiology 152:3291–3304
Qian H, Cheng Y, Yang C, Wu S, Zeng G, Xi J (2018) Performance and biofilm characteristics of biotrickling filters for ethylbenzene removal in the presence of saponins. Environ Sci Pollut Res 25:30021–30030
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramirez M, Gomez JM, Aroca G, Cantero D (2009) Removal of ammonia by immobilized Nitrosomonas europaea in a biotrickling filter packed with polyurethane foam. Chemosphere 74:1385–1390
Rho S, Nam GN, Shin JY, Jahng D (2007) Effect of 3,3',4',5-tetrachlorosalicylanilide on reduction of excess sludge and nitrogen removal in biological wastewater treatment process. J Microbiol Biotechnol 17:1183–1190
Russell JB, Cook GM (1995) Energetics of bacterial-growth - balance of anabolic and catabolic reactions. Microbiol Rev 59:48–62
Shields MS, Reagin MJ, Gerger RR, Campbell R, Somerville C (1995) TOM, a new aromatic degradative plasmid from Burkholderia (Pseudomonas) cepacia G4. Appl Environ Microbiol 61:1352–1356
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Sinha D, Pandey S, Singh R, Tiwari V, Sad K, Tandon V (2017) Synergistic efficacy of bisbenzimidazole and carbonyl cyanide 3-chlorophenylhydrazone combination against MDR bacterial strains. Sci Rep 7:44419
Solyanikova IP, Golovleva LA (2004) Bacterial degradation of chlorophenols: pathways, biochemica, and genetic aspects. J Environ Sci Health B 39:333–351
Spain JC, Gibson DT (1988) Oxidation of substituted phenols by Pseudomonas-putida F1 and Pseudomonas sp strain-js6. Appl Environ Microbiol 54:1399–1404
Stewart TJ, Traber J, Kroll A, Behra R, Sigg L (2013) Characterization of extracellular polymeric substances (EPS) from periphyton using liquid chromatography-organic carbon detection-organic nitrogen detection (LC-OCD-OND). Environ Sci Pollut Res Int 20:3214–3223
Terada H (1990) Uncouplers of oxidative-phosphorylation. Environ Health Perspect 87:213–218
Tian Y, Zhang J, Wu D, Li ZP, Cui YN (2013) Distribution variation of a metabolic uncoupler, 2,6-dichlorophenol (2,6-DCP) in long-term sludge culture and their effects on sludge reduction and biological inhibition. Water Res 47:279–288
To M-S, Aromataris EC, Castro J, Roberts ML, Barritt GJ, Rychkov GY (2010) Mitochondrial uncoupler FCCP activates proton conductance but does not block store-operated Ca2+ current in liver cells. Arch Biochem Biophys 495:152–158
Van Acker H, Gielis J, Acke M, Cools F, Cos P, Coenye T (2016) The role of reactive oxygen species in antibiotic-induced cell death in Burkholderia cepacia complex bacteria. PLoS One 11:20
Verlinden RJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R (2006) Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol 290:L790–L796
Wang Z, Liu L, Guo F, Zhang T (2015) Deciphering cyanide-degrading potential of bacterial community associated with the coking wastewater treatment plant with a novel draft genome. Microb Ecol 70(3):701–709. https://doi.org/10.1007/s00248-015-0611-x
Widdel F, Knittel K, Galushko A (2010) Anaerobic hydrocarbon-degrading microorganisms: an overview. In: Timmis KN (ed) Handbook of Hydrocarbon and Lipid Microbiology. Springer Berlin Heidelberg, Berlin, Heidelberg
Woods A, Watwood M, Schwartz E (2011) Identification of a toluene-degrading bacterium from a soil sample through H(2)(18)O DNA stable isotope probing. Appl Environ Microbiol 77:5995–5999
Xu R, Xie Y, Tian J, Chen L (2021) Adsorbable organic halogens in contaminated water environment: a review of sources and removal technologies. J Clean Prod 283:124645
Yang XF, Xie ML, Liu Y (2003) Metabolic uncouplers reduce excess sludge production in an activated sludge process. Process Biochem 38:1373–1377
Yeom D-J, Kim JH (2011) Comparative evaluation of species diversity indices in the natural deciduous forest of Mt. Jeombong. For Sci Technol 7:68–74
Yu JM, Chen JM, Wang JD (2006) Removal of dichloromethane from waste gases by a biotrickling filter. J Environ Sci (China) 18:1073–1076
Zhang LH, Meng XL, Wang Y, Liu LD (2009) Performance of biotrickling filters for hydrogen sulfide removal under starvation and shock loads conditions. J Zhejiang Univ Sci B 10:595–601
Zhang J, Tian Y, Zuo W, Chen L, Yin LL (2013) Inhibition of nitrification by the metabolic uncoupler, 2,6-dichlorophenol (2,6-DCP) in a sequencing batch reactor. Chem Eng J 233:132–137
Zhang YQ, Shen X, Xiao XL, Liu MY, Li SL, Yan J, Jin J, Gao JL, Zhen CL, Hu N, Zhang XZ, Tai Y, Zhang LS, Bai YL, Dong DL (2016) Mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone induces vasorelaxation without involving KATP channel activation in smooth muscle cells of arteries. Br J Pharmacol 173:3145–3158
Zheng GH, Li MN, Wang L, Chen ZY, Qian YF, Zhou Q (2008) Feasibility of 2,4,6-trichlorophenol and malonic acid as metabolic uncoupler for sludge reduction in the sequence batch reactor for treating organic wastewater. Appl Biochem Biotechnol 144:101–109
Acknowledgements
The authors acknowledge the New Zealand ASEAN Scholarship provided by the Ministry of Foreign Affairs and Trade for the PhD scholarship of RJDV.
Author information
Authors and Affiliations
Contributions
The following are the contributions of the authors:
RJDV was involved in the conceptualization, data curation, formal analysis, investigation, methodology, and writing of the original draft of this manuscript. KW helped RJDV in the methodology for microbial community and in the analysis of such results. KB was involved in the conceptualization and supervision. PAG was the main supervisor and was involved in the conceptualization, provision of resources, data interpretation, and project administration. KW, KM, and PG all contributed to the review and editing of the manuscript prepared by RJDV.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2097 kb).
Rights and permissions
About this article
Cite this article
De Vela, R.J., Wigley, K., Baronian, K. et al. Effect of metabolic uncouplers on the performance of toluene-degrading biotrickling filter. Environ Sci Pollut Res 28, 41881–41895 (2021). https://doi.org/10.1007/s11356-021-13708-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13708-w